Introduction
Arterial diseases associated with atherosclerosis
are the leading cause of morbidity and mortality worldwide. Despite
the development of anti-inflammatory treatments and agents to lower
lipid levels, the use of which can reduce the incidence of acute
coronary syndromes (ACSs), preventive strategies that specifically
target the mechanisms causing plaque destabilization remain elusive
(1). It is widely known that type 2
diabetes is considered a ‘risk equivalent’ for cardiovascular
disease. Rosiglitazone, a thiazolidinedione, has been investigated
as a potential therapeutic agent for the prevention of
cardiovascular disease, such as atherosclerosis (2–4).
Collagen is the main component of the extracellular
matrix (ECM) in atherosclerotic plaques. It is not only a simple
supporting structure but also exerts numerous bioactivities, such
as storing lipids, secreting cellular factors and promoting smooth
muscle cell proliferation (5–7), and is
associated with the progression of atherosclerosis (8). Additionally, collagen can be found as
the main component of fibrous caps, providing tensile strength. The
loss of collagen can result in structural weakness and reduces the
resistance to the mechanical stresses associated with systole
(9). The consequence of this
structural weakness is plaque rupture, which is the key event in
the initiation of coronary thrombosis and, therefore, ACSs, such as
unstable angina and myocardial infarction (10). In vulnerable atherosclerotic plaques
(VAPs), types I and III collagen are the most evident collagen
categories, and the former is the most important collagen to endure
the loading in the plaque fibrous cap.
In human atherosclerosis, it is believed that the
increased activity of matrix metalloproteinase 9 (MMP-9) can lead
to the upregulation of collagen deposition, possibly through
transforming growth factor-β activation (11). In addition, MMP-9 can degrade
collagen fractions in atherosclerotic plaques to promote the
formation and rupture of the plaques. MMP-9 is specifically
inhibited by tissue inhibitor of metalloproteinase-1 (TIMP-1)
outside the cell. When the increasing expression of MMP-9 surpasses
that of TIMP-1, the rate of collagen degradation will surpass that
of collagen synthesis; therefore, the MMP-9/TIMP-1 ratio can be
used to evaluate the stability of atherosclerotic plaques (12,13).
Our previous results showed that rosiglitazone could
promote the stability of atherosclerotic plaques in fat-fed
ApoE-knockout mice by modifying the plaque composition, as well as
by decreasing the number of buried fibrous caps (14); however, the effect of rosiglitazone
on collagen deposition and metabolism in the plaque is unknown. The
aim of this study, therefore, was to determine the effect of
rosiglitazone on collagen metabolism in the plaques of fat-fed
ApoE-knockout mice.
Materials and methods
Animals
Male ApoE-knockout mice (n=30; age, 8 weeks; weight,
18–20 g) with a C57BL/6J background were introduced and bred by the
Laboratory Animal Center of Peking University Health Science Center
(Beijing, China). All animals were housed, cared for and used in
procedures in accordance with the guidelines and regulations of the
University of Bristol (Bristol, UK) and the United Kingdom Home
Office.
Husbandry
The ApoE-knockout mice were sustained on a high-fat
diet that contained 21% (wt/wt) fat from lard supplemented with
0.15% (wt/wt) cholesterol (Special Diet Services, Witham, UK)
(15) for 26 weeks. All mice were
inspected on a regular basis, with at least one inspection every 24
h.
Drug treatment
After the first 13 weeks of being fed the high-fat
diet, the ApoE-knockout mice were randomly assigned to one of three
groups (10 mice/group) and treated intragastrically with
rosiglitazone (0.60 mg/kg per day), simvastatin (9.01 mg/kg per
day), both purchased from GlaxoSmithKline Pharmaceutical Co., Ltd.
(Tianjin, China), or distilled water (control group) for the
remaining 13 weeks of the high-fat diet. The dose selection was
based on the equivalent clinical doses in humans, using the
conversion coefficient of 9.01; therefore the doses were calculated
using the following formula: Dose in mice = clinical dose in human
× 9.01. In clinical practice, rosiglitazone and simvastatin are
administered to patients at doses of 0.067 and 1 mg/kg per day,
respectively; applying these doses to the above formula gave mouse
doses of 0.60 mg/kg per day for rosiglitazone and 9.01 mg/kg per
day for simvastatin. The drugs were diluted using distilled water.
The distilled water consumption of the mice was monitored twice
weekly, and the drug concentration was adjusted when necessary.
Histology
At the end of the 13-week drug treatment period, the
mouse hearts were removed and embedded in paraffin. Six serial 5-µm
sections were cut at 50-µm intervals from the cross section of the
cardiac base until the ascending aorta appeared. For the
quantitative analysis of the atherosclerotic lesions, four 5-µm
sections were selected and quantified using a previously described
method (16). Up to six of the 5-µm
sections per mouse were morphometrically and immunohistochemically
analyzed. The collagen and foam cells in the plaques were stained
using a modified Movat pentachrome stain. The area of lipid content
within the atherosclerotic plaque was calculated using the
following formula: Area of lipid content = area of extracellular
lipid core + area of foam cells in the atherosclerotic plaque.
Determination of collagen
category
The category of collagen was evaluated using the
picro-Sirius red polarization method. Following staining, type I
collagen appears red or yellow and type III collagen appears green
under a BH-2 polarimicroscope (Olympus Corporation, Tokyo,
Japan).
Immunohistochemistry
The serial 5-µm paraffin sections were dewaxed and
rehydrated, and the endogenous peroxidase activity was terminated
by incubation with 3% hydrogen peroxide. The sections were
subsequently blocked using 20% (v/v) goat serum in
phosphate-buffered saline, prior to being incubated overnight at
4°C with mouse MMP-9 monoclonal antibody (1:100; sc-21773; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and rabbit TIMP-1
polyclonal antibody (1:200; 10753-1-AP; Proteintech Group, Inc.,
USA). The sections were then incubated with the polyclonal
mouse/rabbit secondary antibodies (k5007; 1:200; Dako, Glostrup,
Denmark). Areas that were found to be positive for the target
protein expression were counted and expressed as a percentage of
the whole plaque area. Negative controls were established by
replacing the primary antibody with either mouse or rat IgG at the
same dilution. The analysis of the positive sections was conducted
in a blinded manner using Image-Pro Plus image analysis software
(Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation,
and comparisons between the groups were conducted using one-way
analysis of variance. For the analysis of the correlation between
the lipid to collagen, type III to type I collagen and MMP-9 to
TIMP-1 ratios in the rosiglitazone-treated group, the data were
assessed using correlation analysis. In all cases, P<0.05 was
considered to indicate a statistically significant difference.
Results
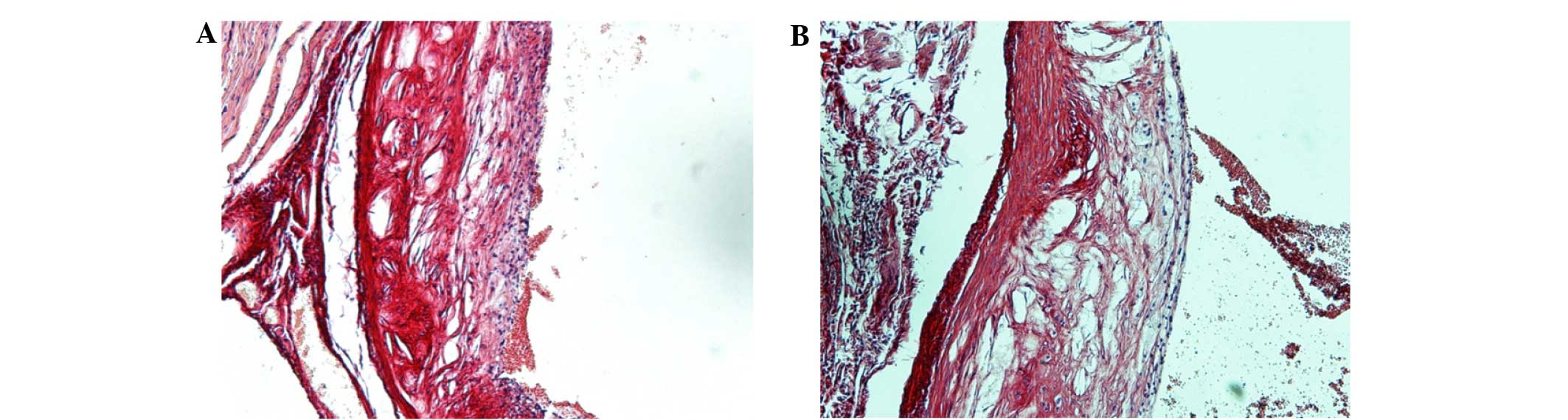
A high-fat diet induces the formation
of VAPs
Atherosclerotic plaques could be clearly observed in
the aortic roots of the ApoE-knockout mice after 13 weeks of the
high-fat diet (Fig. 1A). After a
further 13 weeks of the high-fat diet, the plaques exhibited the
typical morphological features of VAP, including a large lipid core
and thin fibrous cap (Fig. 1B).
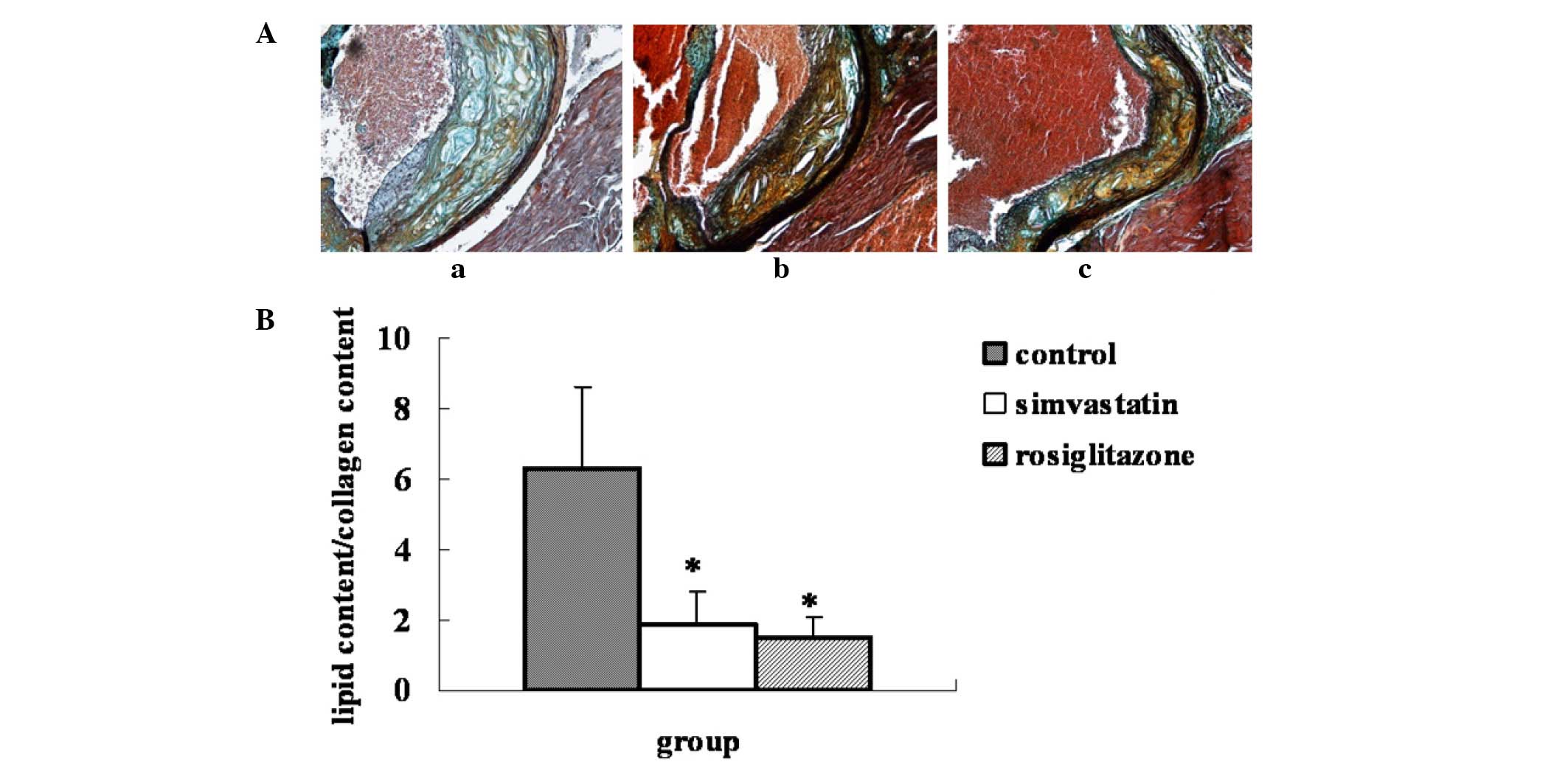
Effect of rosiglitazone on plaque
stability
Compared with the plaques of the control group, the
ratio of lipid to collagen content in the plaques of the
rosiglitazone-treated group was significantly decreased by 75.8%
(P=0.0004 vs. control), while in the simvastatin group the ratio
was decreased by 70.6% (P=0.001 vs. control). No statistical
difference in plaque stability was observed between the two
drug-treated groups (Fig. 2).
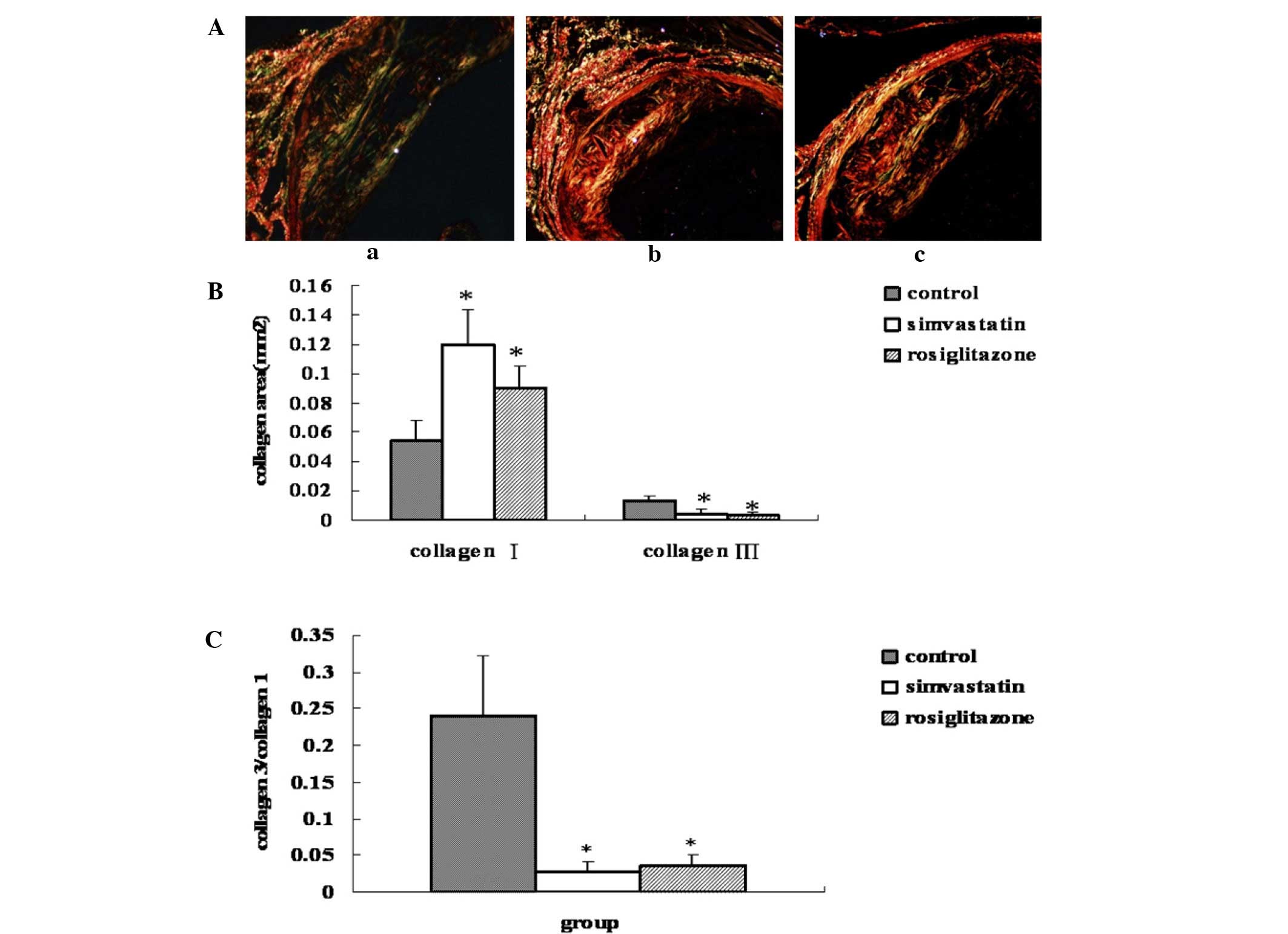
Effect of rosiglitazone on the
category of collagen in the plaque
As shown in Fig. 3,
rosiglitazone treatment for 13 weeks increased the type I collagen
content in the plaque by 66.8% (P=0.01 vs. control), while
simvastatin treatment led to a 1.2-fold increase (P=0.016 vs.
control) compared with the control group. In addition, treatment
with rosiglitazone and simvastatin decreased the type III collagen
content in the plaque by 76.6% (P=0.0005 vs. control) and by 69.4%
(P=0.0006 vs. control), respectively. The ratio of type III to type
I collagen in the rosiglitazone treated group was, therefore,
significantly decreased by 85% (P=0.0007 vs. control), while in the
simvastatin-treated group the ratio was decreased by 88.6% (P=0.01
vs. control). No statistically significant differences were
observed between the two drug-treated groups (P>0.05) (Fig. 3).
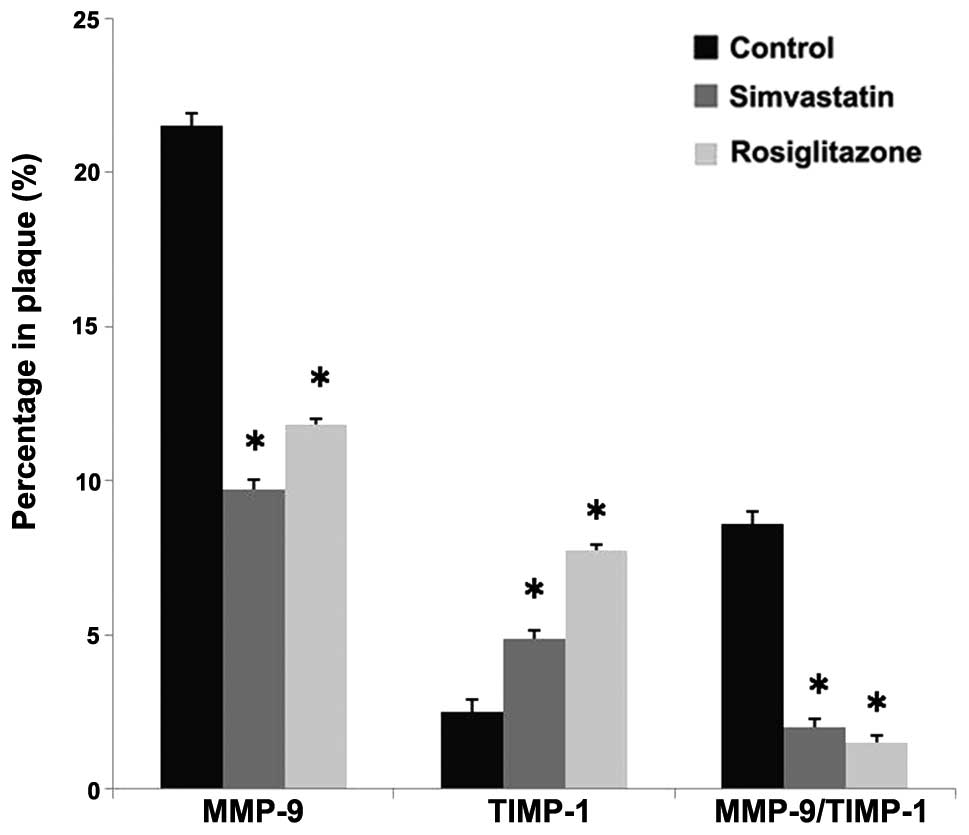
Effect of rosiglitazone on enzymes
modulating collagen metabolism
As in Fig. 4,
rosiglitazone and simvastatin treatment decreased the expression of
MMP-9 by 45.5% (P=0.0003 vs. control) and 54.7% (P=0.0005 vs.
control), respectively. By comparison, after rosiglitazone
treatment for 13 weeks the expression of TIMP-1 in the plaque was
increased by 2.1-fold compared with the control group (P=0.0003 vs.
control), while the TIMP-1 expression in the simvastatin group was
increased by 94.5% (P=0.0004 vs. control). Rosiglitazone treatment
therefore decreased the MMP-9 to TIMP-1 ratio by 82.3% (P=0.0002
vs. control) and simvastatin treatment decreased the ratio by 76.7%
(P=0.0003 vs. control) compared with the control group. No
statistically significant differences were observed between the two
drug-treated groups (P>0.05).
Correlation analysis for ratios of
enzyme expression, collagen category and plaque content in the
rosiglitazone group
The MMP-9 to TIMP-1 ratio in the
rosiglitazone-treated group was found to be significantly
correlated with the ratio of lipid to collagen content in the
plaque (γ=0.957, P<0.01). In addition, the ratio of MMP-9 to
TIMP-1 in the rosiglitazone-treated group was significantly
correlated with the ratio of type III to type I collagen in the
atherosclerotic plaque (γ=0.863, P<0.01).
Discussion
As a member of the thiazolidinedione class of drugs,
rosiglitazone can reduce the plasma levels of C-reactive protein
and soluble cluster of differentiation 40 ligand (17,18), and
pre- or post-treatment with rosiglitazone has been shown to reduce
aortic expansion and rupture in an angiotensin II-induced
hypercholesterolemic mouse model (19). Furthermore, the reduction in the
rupture of lesions in the mice pretreated with rosiglitazone was
coincident with a downregulation in the expression of inflammatory
mediators (19). Our previous study
showed that rosiglitazone could promote the stability of
atherosclerotic plaques in fat-fed ApoE-knockout mice by reducing
the vulnerability index, as well as the average quantity of buried
fibrous caps, which may have been associated with its
anti-inflammatory effects (14);
however, the effect of rosiglitazone on collagen deposition and
metabolism in atherosclerotic plaque was unclear. The present study
indicated that rosiglitazone could modulate collagen deposition and
metabolism in the atherosclerotic plaques of fat-fed ApoE-knockout
mice, which may represent an important mechanism underlying the
rosiglitazone-induced stability of atherosclerotic plaques.
Plaque stability has been suggested to be dependent
upon the plaque composition and the state of the fibrous cap
(20). Collagen is the main
component of the ECM and plays a crucial role in keeping the
atherosclerotic plaque intact and stable. Collagen is responsible
for the majority of the tensile strength of the intima, which is
significant as the rupture of the fibrous cap is believed to be the
event that immediately precedes atherosclerosis-related arterial
thrombosis. The ability of the atherosclerotic plaque to resist
mechanical tensile strength is reduced by an increase in the lipid
component, particularly cholesterol ester, of the plaque; however,
an increase in the collagen component of the plaque, particularly
in the fibrous cap, can keep the fibrous cap intact and promote the
resistance of mechanical tensile strength. The ratio of lipid to
collagen content is therefore an important index for the evaluation
of plaque stability (21). In the
present study, the results showed that rosiglitazone could reduce
the ratio of lipid to collagen, as well as the lipid core area, in
the atherosclerotic plaque.
In VAPs, the category of collagen deposited in the
plaque is another important factor. Types I and III collagen are
the main collagen categories. Type I collagen, a type of mature
collagen, is the important collagen for enduring the loading on the
plaque fibrous cap due to its enhanced ability to resist mechanical
strength; type I collagen gene expression is focal and particularly
prevalent in the fibrous cap (22).
By comparison, type III collagen, a type of immature collagen, can
reduce the stability of atherosclerotic plaques due to its poor
resilience. The ratio of type III to type I collagen in an
atherosclerotic plaque may therefore, to some degree, reflect the
pathological atherosclerotic changes. The present results indicated
that rosiglitazone, in a clinically relevant dose, could modulate
the collagen deposition in atherosclerotic plaques by reducing the
ratio of type III to type I collagen, which is favorable for
promoting plaque stability.
In the fibrous cap of an atherosclerotic lesion,
collagen represents the ECM component that is responsible for
maintaining the structural integrity; therefore, the balance
between the synthesis and degradation of this collagen appears to
be a critical factor in plaque stability. Excessive collagen
deposition can result in stenosis of the vasculature, whereas too
little collagen in an atherosclerotic plaque can render the plaque
vulnerable; therefore, maintaining the appropriate levels of
collagen deposition and metabolism in atherosclerotic plaques is of
particular importance.
MMPs form a large family of enzymes, which can
collectively degrade all components of the ECM. Types I and III
collagen are degraded by MMPs, the activity of which plays an
important role in the inflammatory reaction. There is an increased
rate of MMP formation in ruptured atherosclerotic plaques (23). MMP-9 is an important MMP secreted by
inflammatory cells, such as macrophages. Although it cannot
directly degrade types III and I collagen, it can thoroughly
decompose the collagen fragments of the fibrous cap in order to
promote the formation and rupture of the VAP. Previous studies have
suggested that MMP-9 is the key factor in the induction of plaque
rupture, and its expression strongly correlates with lesional
instability and the clinical manifestations of atherosclerosis
(24–26). The effect of MMP-9 overexpression on
plaque rupture was originally selected as a focus of study due to
the ability of MMP-9 to degrade elastin and cleaved collagen, both
of which abound in the ECM of fibrous caps of advanced
atherosclerotic lesions.
MMP activity is, at least partially, controlled by a
family of endogenous inhibitors known as TIMPs. TIMP-1 is believed
to be the key member in the TIMP family with regard to the
regulation of MMP-9 activity, and MMP-9 is secreted as a complex
with TIMP-1 when expressed by macrophages (13). The MMP-9 to TIMP-1 ratio also affects
the reactivity of MMPs, to a certain extent, so it reflects the
balance or imbalance between the degradation and synthesis of
collagen. MMP-9 to TIMP-1 ratios can therefore be used to evaluate
the stability of atherosclerotic plaques (12,13). The
present results showed that rosiglitazone could reduce the MMP-9 to
TIMP-1 ratio in atherosclerotic plaques and that the reduction was
significantly correlated with the reduction in the ratios of lipid
to collagen content and type III to type I collagen in the plaques.
We therefore concluded that rosiglitazone could modulate collagen
deposition to promote the stability of the atherosclerotic plaque
by reducing the MMP-9 to TIMP-1 ratio.
In conclusion, rosiglitazone, as an insulin
sensitizer, can stabilize atherosclerotic plaques by modulating
collagen deposition and metabolism in the plaques of fat-fed
ApoE-knockout mice. Further studies are required to elucidate the
precise mechanism.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81303086) and Beijing Nova
Programme (no. Z131107000413026).
References
|
1
|
Silvestre Roig, de Winther MP, Weber C, et
al: Atherosclerotic plaque destabilization mechanisms, models and
therapeutic strategies. Circ Res. 114:214–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ratner RE, Cannon CP, Gerstein HC, et al:
APPROACH Study Group: Assessment on the prevention of progression
by rosiglitazone on atherosclerosis in diabetes patients with
cardiovascular history (APPROACH): Study design and baseline
characteristics. Am Heart J. 156:1074–1079. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gerstein HC, Ratner RE, Cannon CP, et al:
Effect of rosiglitazone on progression of coronary atherosclerosis
in patients with type 2 diabetes mellitus and coronary artery
disease: The Assessment on the Prevention of Progression by
Rosiglitazone On Atherosclerosis in diabetes patients with
Cardiovascular History trial. Circulation. 121:1176–1187. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
García-García HM, Garg S, Brugaletta S, et
al: APPROACH Study Group: Evaluation of in-stent restenosis in the
APPROACH trial (Assessment on the Prevention of Progression by
Rosiglitazone On Atherosclerosis in diabetes patients with
Cardiovascular History). Int J Cardiovasc Imaging. 28:455–465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rekhter MD: Collagen synthesis in
atherosclerosis: Too much and not enough. Cardiovasc Res.
41:376–384. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raines EW: The extracellular matrix can
regulate vascular cell migration proliferation and survival:
Relationships to vascular disease. Int J Exp Path. 81:173–182.
2000. View Article : Google Scholar
|
|
7
|
Hou G, Mulholland D, Gronska MA and
Bendeck MP: Type VIII collagen stimulates smooth muscle cell
migration and matrix metalloproteinase synthesis after arterial
injury. Am J Pathol. 156:467–476. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orlandi A, Francesconi A, Marcellini M, et
al: Role of ageing and coronary atherosclerosis in the development
of cardiac fibrosis in the rabbit. Cardiovasc Res. 64:544–552.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee RT, Schoen FJ, Loree HM, et al:
Circumferential stress and matrix metalloproteinase 1 in human
atherosclersis: Implications for plaque rupture. Artherioscler
Thromb Vasc Biol. 16:1070–1073. 1996. View Article : Google Scholar
|
|
10
|
Davies MJ: The pathophysiology of acute
coronary syndromes. Heart. 83:361–366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lemaître V, Kim HE, Forney-Prescott M, et
al: Transgenic expression of matrix metalloproteinase-9 modulates
collagen deposition in a mouse model of atherosclerosis.
Atherosclerosis. 205:107–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng M, Hashmi S, Mao X and Zeng QT:
Relationships of adiponectin and matrix metalloproteinase-9 to
tissue inhibitor of metalloproteinase-1 ratio with coronary plaque
morphology in patients with acute coronary syndrome. Can J Cardiol.
24:385–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nenseter MS, Narverud I, Græsdal A, et al:
Elevated serum MMP-9/TIMP-1 ratio in patients with homozygous
familial hypercholesterolemia: Effects of LDL-apheresis. Cytokine.
61:194–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou M, Xu H, Pan L, et al: Rosiglitazone
promotes atherosclerotic plaque stability in fat-fed ApoE-knockout
mice. Eur J Pharmacol. 590:297–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson J, Carson K, Williams H, et al:
Plaque rupture after short periods of fat feeding in the
apolipoproteinE knockout mouse: Model characterization and effects
of pravastatin treatment. Circulation. 111:1422–1430. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki H, Kurihara Y, Takeya M, et al: A
role for macrophage scavenger receptors in atherosclerosis and
susceptibility to infection. Nature. 386:292–296. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong TY, Szeto CC, Chow KM, et al:
Rosiglitazone reduces insulin requirement and C-reactive protein
levels in type 2 diabetic patients receiving peritoneal dialysis.
Am J Kidney Dis. 46:713–719. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spinelli SL, O'Brien JJ, Bancos S, et al:
The PPAR-platelet connection: Modulators of inflammation and
potential cardiovascular effects. PPAR Res. 2008:3281722008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jones A, Deb R, Torsney E, et al:
Rosiglitazone reduces the development and rupture of experimental
aortic aneurysms. Circulation. 119:3125–3132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ambrose JA and Martinez EE: A new paradigm
for plaque stabilization. Circulation. 105:2000–2004. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naghavi M, Libby P, Falk E, et al: From
vulnerable plaque to vulnerable patient: A call for new definition
and risk assessment strategies: part I. Circulation. 108:1664–1672.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rekhter MD, Zhang K, Narayanan AS, et al:
Type I collagen gene expression in human atherosclerosis
localization to specific plaque regions. Am J Pathol.
143:1634–1648. 1993.PubMed/NCBI
|
|
23
|
Théroux P and Fuster V: Acute coronary
syndromes. Circulation. 97:1195–1206. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loftus IM, Naylor AR, Goodall S, et al:
Increased matrix metalloproteinase-9 activity in unstable carotid
plaques. A potential role in acute plaque disruption. Stroke.
31:40–47. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pöllänen PJ, Karhunen PJ, Mikkelsson J, et
al: Coronary artery complicated lesion area is related to
functional polymorphism of matrix metalloproteinase 9 gene: An
autopsy study. Arterioscler Thromb Vasc Biol. 21:1446–1450. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blankenberg S, Rupprecht HJ, Poirier O, et
al: Plasma concentrations and genetic variation of matrix
metalloproteinase 9 and prognosis of patients with cardiovascular
disease. Circulation. 107:1579–1585. 2003. View Article : Google Scholar : PubMed/NCBI
|