Introduction
Due to a rapid increase in the aging population, the
amount of surgery that is now performed in elderly patients
accounts for 25–30% of all surgical procedures (1). Postoperative cognitive dysfunction
(POCD) is a common postoperative neurological complication in
elderly patients with an incidence of 5–15% (2). POCD is a subtle impairment of memory,
concentration and information processing with clinical
manifestations of delirium, anxiety, personality changes and
impaired memory, which is associated with prolonged
hospitalization, a reduced quality of life and an increase in
morbidity and mortality (3–7). Previous studies have suggested that
age, surgery itself and anesthesia may be the possible causes of
POCD (6,8); therefore, POCD has become a major
concern for the anesthetist. Evidence has shown that an
inflammatory increase, particularly the inflammatory response in
the central nervous system, may serve as a predictive parameter for
the occurrence of POCD (3,9–14). A
meta-analysis has indicated that interleukin (IL)-6 may serve as a
biomarker to provide guidance in the prevention and treatment of
POCD (15).
Dexmedetomidine is a highly selective α-2 adrenergic
receptor agonist with a dose-dependent sedative hypnotic effect. A
conventional treatment dose of dexmedetomidine has analgesic
properties and inhibits sympathetic activity without causing
respiratory depression (16–18). It has also been reported that
dexmedetomidine has a potential role in preventing POCD due to its
neuroprotective effects both in vitro and in vivo
(19).
In the present study, it was hypothesized that
dexmedetomidine reduces the incidence of POCD by decreasing the
inflammatory response. In order to validate the hypothesis, the
incidence of POCD was investigated and the inflammatory cytokines
were analyzed during combined intravenous dexmedetomidine general
anesthesia in elderly patients undergoing laparoscopic
cholecystectomy (LC).
Subjects and methods
Ethics considerations
The protocol was approved by the Ethics Committee of
Shaoxing People's Hospital (Shaoxing, China). The study was
conducted in accordance with the guidelines of Good Clinical
Practice and the principles expressed in the Declaration of
Helsinki. Each subject gave his or her consent for participation
after being informed of the study orally and in writing prior to
the surgery.
Patient recruitment
Between March 2010 and July 2012, 120 patients were
recruited to participate in the study. The patients were randomly
assigned to either the experimental group (DEX group, n=60) or the
normal saline group (control group, n=60) by a statistician.
Inclusion criteria were: i) Patients with American Society of
Anesthesiology (ASA) status of I–III; ii) aged >60 years old;
and iii) scheduled for elective LC under intravenous general
anesthesia. Exclusion criteria were: i) Patients were aged <60
or >75 years; ii) patients with accompanying medical conditions
that may affect the level of consciousness, such as stroke, stupor
or dementia, or patients with abnormalities in hepatic or renal
function; iii) patients suffering from preoperative bradycardia
[heart rate (HR) <45 bpm] or hypotension [mean arterial blood
pressure (MAP) <60 mmHg]; iv) patients who had recently received
a sedative or opioid drug; and v) patients with a Mini Mental State
Examination (MMSE) score <24.
Study design
This was a prospective, randomized,
placebo-controlled, double-blind clinical trial. The patients
recruited according to the inclusion criteria were distributed to
one of the groups according to computer-generated random
assignment. The personnel involved in the study, including
statisticians, investigators, anesthetists, surgeons and the
patients were blinded to the specific experimental scheme
implementation.
Procedures
Patients were fasted overnight and no premedication
was given. When the patients entered the operating theater,
catheterization of the left radical artery and a large vein in the
right forearm was performed. Anesthesia was induced with fentanyl
(2–3 µg/kg), midazolam (0.04–0.05 mg/kg) and target-controlled
infusion (TCI)-guided propofol targeting a plasma concentration of
3–4 µg/ml. Tracheal intubation was facilitated with 0.15 mg/kg
cis-atracurium. The patients were mechanically ventilated
and end-tidal CO2 was held constant between 35–40 mmHg.
Following the induction of general anesthesia, patients in the DEX
group were administered dexmedetomidine at a dose of 1 mg/kg over
10 min, followed by a continuous infusion at a rate of 0.4 mg/kg/h
until the end of surgery. The control group received a placebo
infusion of normal saline. Anesthesia was maintained with 1–2%
end-tidal sevoflurane together with TCI of propofol (target plasma
concentration, 2–3 µg/ml), continuous infusion of remifentanil
(0.10–0.20 µg/kg/min) and cis-atracurium intermittently as
required. Propofol and remifentanil were discontinued 5 min prior
to the end of surgery, while the infusion of dexmedetomidine or
normal saline was stopped at the end of surgery. The patients were
transported to the post-anesthesia care unit and administered
neostigmine (0.03 mg/kg) and atropine (0.015 mg/kg) to reverse
neuromuscular blockade subsequent to swallowing reflex recovery.
Tracheal extubation criteria were: i) Spontaneous breathing with
tidal volume >6 ml/kg and breathing rate <30 bpm; ii) partial
pressure of end-tidal carbon dioxide (PETCO2) was
maintained at 35–45 mmHg with presence of a normal shape of
capnographic curve (elance 7c, Spacelabs Medical Inc, USA); iii)
pulse oximetry (SpO2) was maintained at >92% under
air inspiration; and iv) recovery of consciousness and the ability
to form a strong fist.
Measurements and blood samples
During the investigation, HR, electrocardiography
(ECG), MAP, SpO2 and PETCO2 were continuously
monitored. The depth of anesthesia was monitored and recorded by a
Bispectral Index™(BIS) sensor (BIS monitor Model A-2000; Aspect
Medical System, Norwood, MA, USA) applied to the forehead. The
anesthesia was guided to reach a BIS value of 40–60. Body
temperature was maintained at ≥35.5°C. In the case of hypotension,
which was defined as a reduction of systolic blood pressure by
>30% of the pre-anesthetic value, <90 mmHg ephedrine was
administered, while 0.5 mg atropine was given if the HR fell to
<50 bpm. The treatments were repeated if necessary. Cognitive
function was evaluated with the MMSE immediately prior to the
induction of anesthesia (T0), and at 6 h (T1), 24 h (T2) and 48 h
(T3) postoperatively (20).
Preoperative assessment, including disease duration, co-morbid
diseases and medication at home was completed the day prior to
surgery. Venous blood (10 ml at each time-point) was drawn to
determine the concentrations of C-reactive protein (CRP), IL-1β and
IL-6 as representative systemic inflammatory mediators at T0, T1,
T2 and T3. Blood samples were drawn via an indwelling catheter
inserted into the forearm vein and put into heparin anticoagulant
tubes, transported to the hospital research laboratory within 30
min, and then centrifuged at 2,000 × g for 10 min at 4°C. Separated
plasma samples were stored at −80°C until assayed. Using
commercially available ELISA kits, plasma levels of the following
cytokines were measured using ELISA, 70-E-EK101B2 Human IL-1β ELISA
Kit, MultiSciences Biotech Co., Ltd, Hangzhou, China), IL-6 (using
ELISA, 70-E-EK1062 Human IL-6 ELISA Kit, MultiSciences Biotech Co.,
Ltd, Hangzhou, China), CRP (using Immunoturbidimetry, CRP Kit,
Meikang Biotech Co., Ltd, Ningbo, China).
Outcomes
The main outcome of the study was cognitive
function, which was defined according to the MMSE score. Compared
with baseline, postoperative POCD was considered according to the
criteria of MMSE score reductions of ≥1 ± standard deviation, as
described by Newman (21). Cognitive
impairment was defined and graded according to the MMSE score:
<27 indicates cognitive impairment; 21–27, mild cognitive
impairment; 9–21, moderate cognitive impairment; and <9, severe
cognitive impairment. On the basis of whether the patients had POCD
in the first day subsequent to surgery, patients were divided into
POCD and non-POCD groups, and a comparison was made between the
outcomes of these two groups.
Statistical analysis
Quantitative variables are presented as mean ±
standard deviation or median with interquartile range. Categorical
variables were analyzed using χ2 or Fisher's exact
tests. Continuous variables were tested with MannWhitney U tests or
Student's t-tests depending on the distribution of the data. Repeat
analysis of variance was used to compare the difference between the
different times in the two groups. P<0.05 was considered to
indicate a statistically significant difference and adjusted for
multiple comparisons when appropriate. The following software
packages were used to perform analysis: Excel 2000 (Microsoft,
Redmond, MA, USA) and SPSS version 10.0 (SPSS Inc., Chicago, IL,
USA).
Results
Comparison between DEX and control
groups
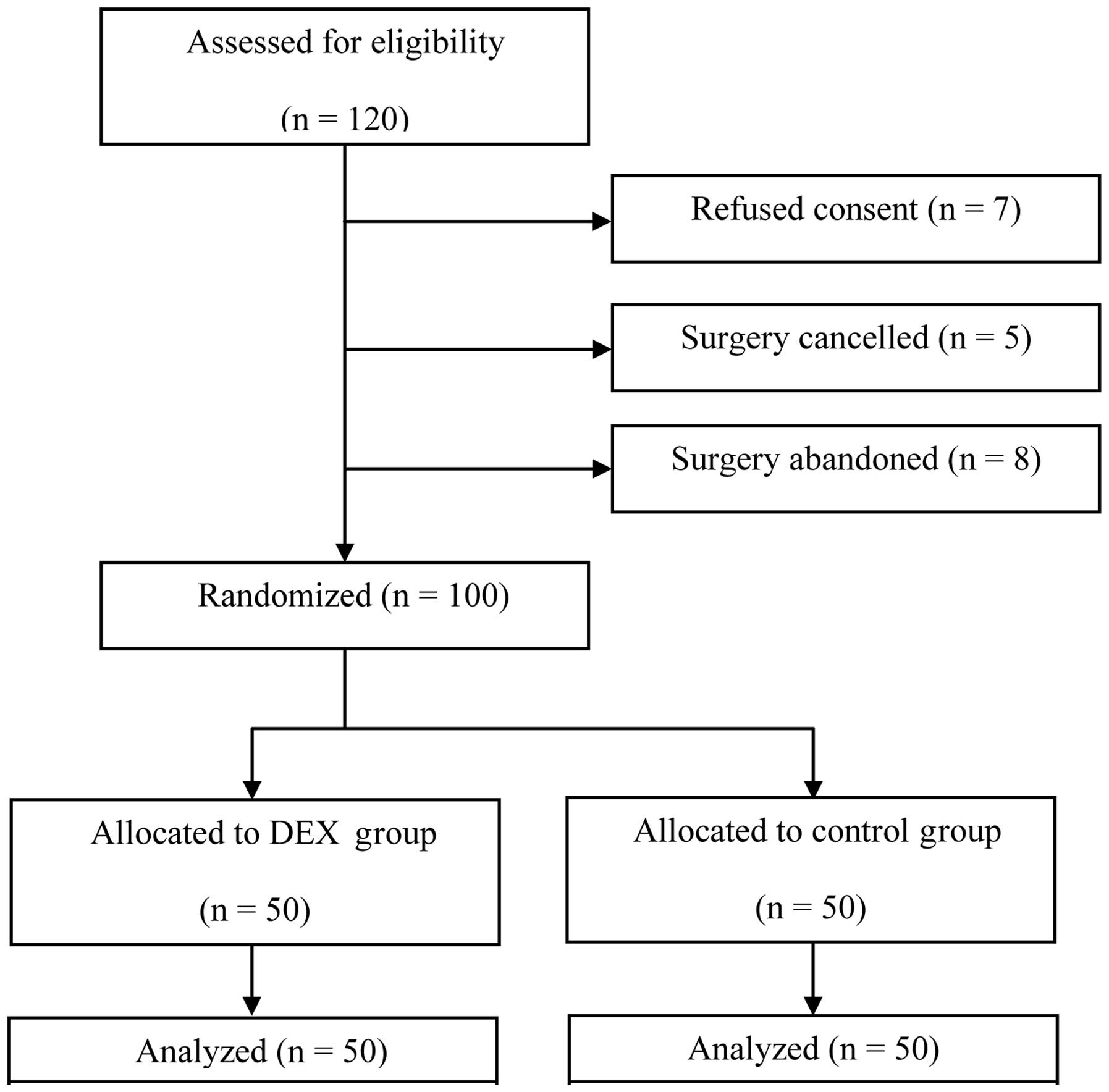
From March 2010 to July 2012, 120 elderly patients
were recruited in accordance with the inclusion criteria. However,
20 patients were subsequently excluded: 7 patients declined to give
consent; surgery was cancelled for 5 patients and surgery was
abandoned for another 8 patients. The remaining 100 patients were
included in randomization and data analysis (Fig. 1). No significant differences in
pre-test variables such as demographic and surgical details were
observed between the DEX and control groups (Table I).
 | Table I.Baseline characteristics of patients
and procedures in the DEX and control groups. |
Table I.
Baseline characteristics of patients
and procedures in the DEX and control groups.
| Characteristics | DEX group | Control group | P-value |
|---|
| Age, years |
69±5 |
70±6 | 0.556 |
| Gender n/N (%
female) | 22/50 (44) | 24/50 (48) | 0.841 |
| Body weight,
kg |
59±7 |
60±8 | 0.519 |
| Height, cm | 163±8 | 164±7 | 0.294 |
| BMI,
kg/m2 |
22±3 |
22±3 | 0.906 |
| Disease duration,
years | 3
(2–3.5) | 3
(2–3.6) | 0.561 |
| No. of co-morbid
diseases | 1 (1–2) | 1 (1–2) | 0.123 |
| No. of medication
at home | 2 (1–3) | 2 (1–3) | 0.587 |
| ASAa group, n/N (%) |
|
| 0.912 |
| Group
I | 21/50 (42) | 22/50 (44) |
|
| Group
II | 22/50 (44) | 20/50 (40) |
|
| Group
III | 7/50
(14) | 8/50
(16) |
|
| Surgical duration,
min | 59±8 | 60±10 | 0.734 |
| Anesthetic
duration, min | 77±9 | 78±11 | 0.524 |
| Amount of fluid
administered, ml |
|
|
|
|
Ringer's lactate solution |
92±15 |
90±15 | 0.176 |
| 6%
hydroxyethyl starch | 576±45 | 568±63 | 0.314 |
| Post-operative
diagnosis, n/N (%) |
|
| 0.953 |
| Chronic
cholecystitis | 23/50 (46) | 22/50 (44) |
|
| Gall
bladder stone | 19/50 (38) | 20/50 (40) |
|
| Gall
bladder polyps | 5/50
(10) | 6/50
(12) |
|
| Biliary
colic | 3/50 (6) | 2/50 (4) |
|
Table II illustrates
that the preoperative MMSE scores and the scores on day 2 after
surgery were similar in the two groups (P>0.05). However,
compared with the control group, the MMSE scores at 6 h and 1 day
after surgery were significantly higher in the DEX group
(P<0.001, P=0.012, respectively). Postoperatively 19 patients in
the control group and 9 patients in the DEX group developed mild
cognitive impairment (P=0.026). The number of patients with
moderate cognitive impairment in the DEX and control groups was 1
and 2 patients, respectively, with no significant difference
between the groups (P=0.558). None of the patients developed severe
cognitive impairment (Table
III).
 | Table II.MMSE scores in the DEX and control
groups. |
Table II.
MMSE scores in the DEX and control
groups.
| Time of test | DEX group | Control group | P-value |
|---|
| T0 | 28.4±1.3 | 28.3±1.4 |
0.710 |
| T1 | 27.3±2.3 | 24.3±2.3 | <0.001 |
| T2 | 27.9±1.7 | 26.7±1.9 |
0.012 |
| T3 | 28.0±1.6 | 28.2±1.3 |
0.604 |
 | Table III.Degree of cognitive impairment 24 h
after surgery. |
Table III.
Degree of cognitive impairment 24 h
after surgery.
|
| Rate of impairment,
n/N (%) |
|
|---|
|
|
|
|
|---|
| Degree of
impairment | DEX group | Control group | P-value |
| Mild | 9/50 (18) | 19/50 (38) | 0.026 |
| Moderate | 1/50 (2) | 2/50 (4) | 0.558 |
| Severe | 0 | 0 | – |
Hemodynamic data
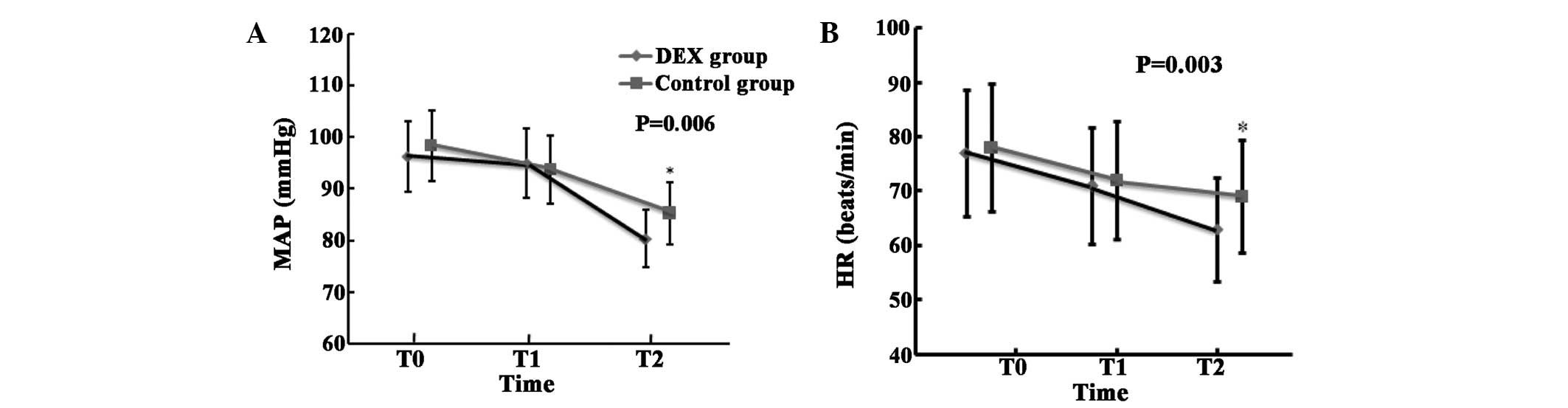
Dexmedetomidine administration resulted in
significant reductions in HR and MAP at the end of peritoneal
closure (T2, the definition is different from that in the rest of
the paper, which is 1 day after surgery) (P=0.006 for MAP; P=0.003
for HR). There were no significant differences in MAP and HR
observed between the DEX and control groups at the time-points of
T0 (prior to induction of anesthesia) and T1 (10 min following
infusion of study drug, this definition is different from that in
the rest of the paper, which is 6 h after surgery; Fig. 2).
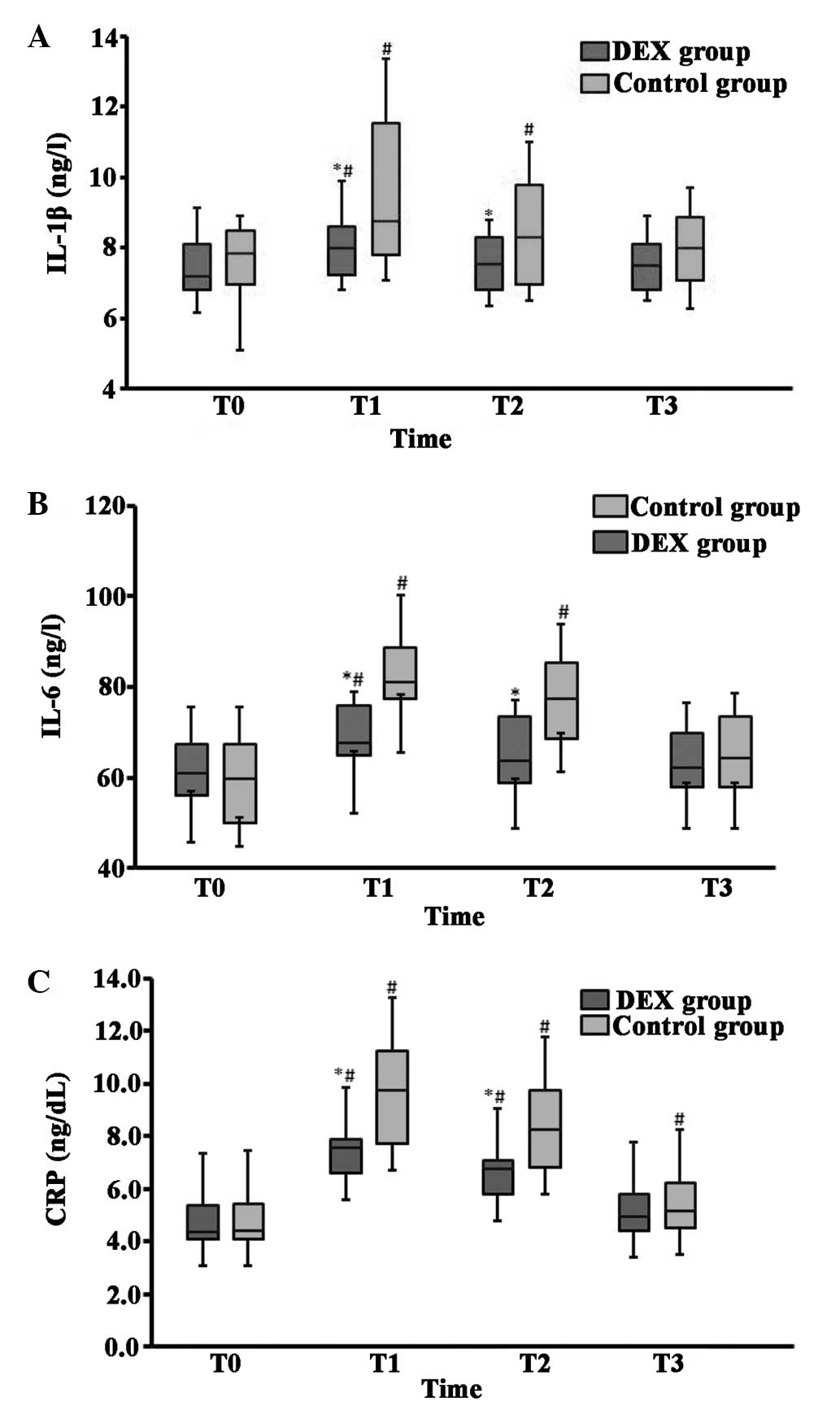
The baseline plasma values of the cytokines IL-1β,
IL-6 and CRP were comparable in the DEX and control groups;
however, the concentrations of IL-1β, IL-6 and CRP were increased
significantly 6 h after surgery and 1 day after surgery in the
control group compared with those in the DEX group (Fig. 3). When compared with baseline values,
the levels of IL-1β, IL-6 and CRP were markedly increased at the
6-h time-point for patients in the DEX group, while they were
increased at 6 h and 1 day following surgery for patients in the
control group. Two days postoperatively, IL-1β and IL-6 levels were
not significantly different from baseline levels in the control
group, while CRP levels remained marginally elevated (Fig. 3C). At 2 days after surgery the three
mediators returned to baseline levels for patients in the DEX
group.
Comparison between patients with and
without POCD
The patients were divided into two groups according
to whether or not they developed POCD. The characteristics of the
patients with POCD and without POCD are presented in Table IV. Those who developed POCD had a
longer disease duration (mean, 3.5 vs. 2.5 years, P=0.009) and more
co-morbid diseases (P=0.017). Other characteristics, including MMSE
scores, age, weight, height and ASA score were similar in patients
with or without POCD. Plasma levels of IL-1β, IL-6, and CRP on day
1 after surgery were significantly higher in patients with POCD
(P<0.05), while surgical duration, anesthesia duration and the
amount of fluid infusion were similar between the patients with and
without POCD. Hemodynamic data were also comparable in the patients
with and without POCD (data not shown).
 | Table IV.Comparison between the patients who
developed POCD one day after surgery and the patients without
POCD. |
Table IV.
Comparison between the patients who
developed POCD one day after surgery and the patients without
POCD.
| Characteristic | No POCD | POCD | P-value |
|---|
| N | 72 | 28 | – |
| Gender n/N (%
female) | 33/72 (46) | 13/28 (46) | 0.830 |
| Age, years | 69±5 | 69±6 | 0.770 |
| ASA group, n/N
(%) |
|
| 0.208 |
| Group
I | 33/72 (46) | 10/28 (36) | – |
| Group
II | 31/72 (43) | 11/28 (39) | – |
| Group
III | 8/72 (11) | 7/28 (25) | – |
| Weight, kg | 62±7 | 61±8 | 0.342 |
| Height, cm | 164±7 | 163±6 | 0.667 |
| BMI,
kg/m2 | 23.3±3.1 | 22.8±3.7 | 0.538 |
| Disease duration,
years | 2.5 (2–3.5) | 3.5 (2.5–4) | 0.009 |
| No. of co-morbid
diseases | 1 (1–2) | 2 (1–2) | 0.017 |
| No. of medication
at home | 2 (1–3) | 2 (1–3) | 0.544 |
| Surgical duration,
min | 61±9 | 60±10 | 0.699 |
| Anesthesia
duration, min | 78±10 | 78±9 | 0.866 |
| 6% hydroxyethyl
starch, ml | 87±17 | 91±12 | 0.227 |
| Ringer's lactate,
ml | 555±65 | 568±62 | 0.364 |
| IL-1β, ng/l | 7.8±1.6 | 8.9±1.9 | 0.013 |
| IL-6, ng/l | 69.0±11.6 | 77.7±16.5 | 0.015 |
| CRP, mg/dl | 7.1±1.1 | 8.1±1.8 | 0.010 |
| Baseline MMSE | 28.4±1.6 | 28.2±1.6 | 0.626 |
Discussion
Previous studies have shown that patients >60
years of age may have a higher risk of developing POCD. Moller
et al (6) reported that POCD
was observed in 25.8% of elderly patients 1 week after surgery and
9.9% of patients 3 months after surgery (22). Anesthesia may also be one of the
causes of POCD. Unlike general anesthesia, local anesthesia does
not have the potential advantage of reducing the incidence of POCD
(23). General anesthetics,
particularly inhaled agents are likely to be associated with POCD
(24–27). The present study revealed that 31% of
the elderly patients undergoing selective LC developed POCD 24 h
after surgery. The study also demonstrated that the incidence of
POCD was markedly reduced by intravenously administered
dexmedetomidine (20 vs. 42% in the control group; P<0.01), which
is consistent with a previous study (28). Although this current and previous
research (28) revealed that DEX may
provide neuroprotection in elderly patients following surgery, the
mechanisms associated with this neuroprotective effect are not
clear. It is well-known that anesthesia and surgical trauma,
combined with primary disease requiring surgery and commodities
constitute sources of stress to patients, which is also considered
to be responsible for POCD (6,8). The
interaction of the neuroendocrine and sympathetic nervous systems
promotes the release of inflammatory factors, particularly the
nonspecific inflammatory reaction of the nervous system, likely
promoting the development of POCD in elderly surgical patients
(9–15). It has been demonstrated that the
pathological processes of central nervous system disease are
associated with inflammatory and immunological responses (29,30). In
addition to surgery, anesthetic techniques and the anesthetics
themselves could trigger the release of stress hormones and
cytokines. Factors such as IL-1β, IL-6 and tumor necrosis factor-α
(TNF-α), a cytokine with a wide bioactivity, can cross the blood
brain barrier, promote brain cell permeability, and cause an
inflammatory reaction in the central nervous system, thereby
affecting the functioning of synaptic connections, resulting in
cognitive function damage. Prolonged elevation of levels of IL-1β
in the brain, specifically in the hippocampus, may be responsible
for learning and memory impairments as such an elevation has been
shown to damage hippocampal long-term potentiation (31). TNF-α and IL-1β can stimulate actin in
the brain and cause actin regeneration, which plays a key role in
the process of nerve degeneration (32). Clinical studies have shown that the
levels of CRP, TNF-α, IL-6 and IL-1 increase markedly
postoperatively in elderly hip-fracture patients (33,34). The
levels of cytokines, particularly IL-6, have been observed to
increase significantly in patients with impaired mental status
(33,34). Compared with the major traumatic
surgeries such as open thoracotomy and laparotomy or orthopedic
surgery, LC is considered to be a minor surgery. Recently Kang
et al (35) reported that LC
also induced the release of inflammatory mediators. Thus, for
elderly patients, the inhibition of the release of inflammatory
factors associated with the LC procedure is important for the
reduction of postoperative complications, including POCD.
These results indicate that dexmedetomidine can
decrease the incidence of POCD in elderly patients; however, the
following caveats should be considered. The present study found
that dexmedetomidine administration decreased the incidence of mild
cognitive impairment 24 h after surgery, which was consistent with
the findings in Chen et al (28); however, the difference from the
controls did not reach statistical significance in the previous
study (28). The differences in the
characteristics, including older age and more severe pre-operative
morbidity in the current study. The study by Kang et al
(35) showed that the levels of
pro-inflammatory cytokines remained almost the same during and 1 h
after the surgery for patients with a mean age of <45 years, ASA
I or II undergoing LC surgery in the control group. The present
data revealed that the levels of IL-1β, IL-6 and CRP were higher 6
h postoperatively in the control group than in the DEX group. The
possible contributing factors include the condition, age and other
concomitant diseases of the patients. Further study is necessary to
confirm these.
The aim of the present study was to investigate the
effects of dexmedetomidine on the incidence of POCD through the
suppression of harmful inflammatory mediators. It was found that
the levels of IL-1β, IL-6 and CRP were attenuated by the
administration of dexmedetomidine to elderly patients undergoing
LC, although at 6 h following surgery, the plasma levels of IL-1β
and IL-6 also were significantly increased compared with baseline
data in patients in the DEX group. The concentration of CRP was
also higher than baseline 1 day following the procedure in the DEX
group. When a comparison was made between the DEX and control
groups, the baseline values were comparable, and the concentrations
of IL-1β, IL-6 and CRP were significantly lower in the DEX group at
6 h and 1 day following surgery. These findings indicated that
dexmedetomidine administration during surgery can reduce the
inflammatory reaction associated with surgery and anesthetics
peri-operatively. When the patients were separated into two groups
according to whether they developed POCD by day 1 postoperatively,
it was found that the levels of IL-1β, IL-6 and CRP were
significantly higher in patients with POCD. Disease duration and
the number of co-morbid diseases were contributing factors to POCD
development. Additionally, it was found that MMSE scores were
significantly lower in the control group 6 h and 1 day following
surgery. Such changes in inflammatory factors were consistent with
the lower MMSE scores, suggesting that DEX reduced the incidence of
POCD through the suppression of the inflammatory response. To the
best of our knowledge, the present study is the first to
investigate the association between the development of POCD and
pro-inflammatory cytokines. These findings are consistent with the
hypothesis that dexmedetomidine can decrease systemic inflammatory
factors and, therefore, reduce the incidence of POCD.
The results of the current study with elderly
patients undergoing minor surgery are consistent with those of
previous studies, indicating that dexmedetomidine decreases
cytokine secretion (36,37). The detailed mechanism of the
anti-inflammatory reaction of dexmedetomidine during surgery
remains unclear. The suppression of the production of cytokines may
be associated with pre-synaptic α2-adrenoceptor-related mechanisms.
The binding of dexmedetomidine to α2-adrenoceptors present in
presynaptic membranes is expected to cause
lipopolysaccharide-induced apoptosis of lymphocytes and
monocytes/macrophages, thus reducing the amount of inflammatory
cytokines (36,37). Additionally, subsequent to
dexmedetomidine combining with the post-synaptic α2-adrenoceptors,
the majority of which are thought to be post-synaptic in the spinal
cord, the concentration of norepinephrine increases, leading to
negative immunoregulatory effects through suppression of the
production of proinflammatory cytokines such as IL-6 and TNF-α
(36,38). Surgery-associated tissue damage
stimulates the inflammatory cascade, including nociceptive and
inflammatory responses, accompanied by elevation of cytokines
(39). Dexmedetomidine decreases the
projection of ascending nociceptive neurons in the dorsal horn of
the spinal cord, and then produces an analgesic effect. It is
likely that dexmedetomidine reduces cytokine levels by inhibiting
nociception. α2-adrenoceptor agonists are also considered to
stimulate the neural microenvironment and change the phenotype of
proinflammatory cytokines, reducing the expression of inflammatory
cytokines (40). In the present
study, dexmedetomidine administration decreased the HR and MAP at
the end of the surgical procedure, and this may have been induced
by a central sympatholytic effect mediated by α2-adrenoceptor
activation and the consequent activation of cholinergic activity,
reducing the production of cytokines through the cholinergic
anti-inflammatory pathway (38).
Therefore, it appears that dexmedetomidine may exert its
anti-inflammatory effect through a variety of mechanisms.
Limitations of the present study included the fact
that the plasma concentrations of anti-inflammatory cytokines were
not detected. Westhoff et al (12) stressed the role of decreased levels
of anti-inflammatory cytokines in the occurrence of delirium, an
acute impairment in attention and cognition. A future study will
explore the effect of dexmedetomidine treatment on the balance of
pro-inflammatory and anti-inflammatory cytokines.
In conclusion, treatment with dexmedetomidine during
LC inhibits the secretion of pro-inflammatory cytokines, which may
be beneficial for postoperative cognitive function in elderly
patients. The present findings support the hypothesis that
dexdetomidine reduces the incidence of POCD by downregulating the
inflammatory response. Dexmedetomidine can be safely applied during
anesthesia in a straightforward surgery such as LC in elderly
patients. It may be considered as a method to prevent POCD in
elderly patients.
Acknowledgements
The authors would like to thank Dr. Xue-Jun Dong,
the physician of Shaoxing People's Hospital, for assistance in the
measurement of cytokines. This project was supported by Zhejiang
Medical Association, (grant no. 2012ZYC-A72) and Wenling Science
and Technology bureau (grant no. w112114).
References
|
1
|
Tang JX: The characteristics and operation
for elderly patients with inguinal hernia. Lin Chuang Er Bi Yan Hou
Tou Jing Wai Ke Za Zhi. 16:224–226. 2008.(In Chinese).
|
|
2
|
Monk TG, Weldon BC, Garvan CW, Dede DE,
van der Aa MT, Heilman KM and Gravenstein JS: Predictors of
cognitive dysfunction after major noncardiac surgery.
Anesthesiology. 108:18–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinmetz J, Christensen KB, Lund T, Lohse
N and Rasmussen LS: ISPOCD Group: Long-term consequences of
postoperative cognitive dysfunction. Anesthesiology. 110:548–555.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cibelli M, Fidalgo AR, Terrando N, Ma D,
Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS
and Maze M: Role of interleukin-1beta in postoperative cognitive
dysfunction. Ann Neurol. 68:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sauër AM, Kalkman C and van Dijk D:
Postoperative cognitive decline. J Anesth. 23:256–259. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moller JT, Cluitmans P, Rasmussen LS, Houx
P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD,
et al: Long-term postoperative cognitive dysfunction in the elderly
ISPOCD1 study. ISPOCD investigators. International Study of
Post-Operative Cognitive Dysfunction. Lancet. 351:857–861. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McDonagh DL, Mathew JP, White WD,
Philips-Bute B, Laskowitz DP, Podgoreanu MV and Newman MF:
Neurologic Outcome Research Group: Cognitive function after major
noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of
brain injury. Anesthesiology. 112:852–859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rasmussen LS: Postoperative cognitive
dysfunction: Incidence and prevention. Best Pract Res Clin
Anaesthesiol. 20:315–330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wan Y, Xu J, Ma D, Zeng Y, Cibelli M and
Maze M: Postoperative impairment of cognitive function in rats: A
possible role for cytokine-mediated inflammation in the
hippocampus. Anesthesiology. 106:436–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li YC, Xi CH, An YF, Dong WH and Zhou M:
Perioperative inflammatory response and protein S-100β
concentrations - relationship with post-operative cognitive
dysfunction in elderly patients. Acta Anaesthesiol Scand.
56:595–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji MH, Yuan HM, Zhang GF, Li XM, Dong L,
Li WY, Zhou ZQ and Yang JJ: Changes in plasma and cerebrospinal
fluid biomarkers in aged patients with early postoperative
cognitive dysfunction following total hip-replacement surgery. J
Anesth. 27:236–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Westhoff D, Witlox J, Koenderman L,
Kalisvaart KJ, de Jonghe JF, van Stijn MF, Houdijk AP, Hoogland IC,
Maclullich AM, van Westerloo DJ, et al: Preoperative cerebrospinal
fluid cytokine levels and the risk of postoperative delirium in
elderly hip fracture patients. J Neuroinflammation. 10:1222013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie G, Zhang W, Chang Y and Chu Q:
Relationship between perioperative inflammatory response and
postoperative cognitive dysfunction in the elderly. Med Hypotheses.
73:402–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fidalgo AR, Cibelli M, White JP, Nagy I,
Maze M and Ma D: Systemic inflammation enhances surgery-induced
cognitive dysfunction in mice. Neurosci Lett. 498:63–66. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng L, Xu L and Ouyang W: Role of
peripheral inflammatory markers in postoperative cognitive
dysfunction (POCD): A meta-analysis. PLoS One. 8:e796242013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantz J, Josserand J and Hamada S:
Dexmedetomidine: New insights. Eur J Anaesthesiol. 28:3–6. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su F and Hammer GB: Dexmedetomidine:
Pediatric pharmacology, clinical uses and safety. Expert Opin Drug
Saf. 10:55–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shukry M and Miller JA: Update on
dexmedetomidine: Use in nonintubated patients requiring sedation
for surgical procedures. Ther Clin Risk Manag. 6:111–121. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Monk TG and Price CC: Postoperative
cognitive disorders. Curr Opin Crit Care. 17:376–381. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Folstein MF, Folstein SE and McHugh PR:
Mini-mental state. A practical method for grading the cognitive
state of patients for the clinician J Psychiatr Res. 12:189–198.
1975.PubMed/NCBI
|
|
21
|
Newman SP: Analysis and interpretation of
neuropsychologic tests in cardiac surgery. Ann Thorac Surg.
59:1351–1355. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bedford PD: Adverse cerebral effects of
anaesthesia on old people. Lancet. 269:259–263. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rasmussen LS: Postoperative cognitive
dysfunction: Incidence and prevention. Best Pract Res Clin
Anaesthesiol. 20:315–330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zurek AA, Bridgwater EM and Orser BA:
Inhibition of α5 γ-aminobutyric acid type A receptors restores
recognition memory after general anesthesia. Anesth Analg.
114:845–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Callaway JK, Jones NC and Royse CF:
Isoflurane induces cognitive deficits in the Morris water maze task
in rats. Eur J Anaesthesiol. 29:239–245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Le Freche H, Brouillette J,
Fernandez-Gomez FJ, Patin P, Caillierez R, Zommer N, Sergeant N,
Buée-Scherrer V, Lebuffe G, Blum D and Buée L: Tau phosphorylation
and sevoflurane anesthesia: An association to postoperative
cognitive impairment. Anesthesiology. 116:779–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kavanagh T and Buggy DJ: Can anaesthetic
technique effect postoperative outcome? Curr Opin Anaesthesiol.
25:185–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen J, Yan J and Han X: Dexmedetomidine
may benefit cognitive function after laparoscopic cholecystectomy
in elderly patients. Exp Ther Med. 5:489–494. 2013.PubMed/NCBI
|
|
29
|
Emsley HC and Hopkins SJ: Acute ischaemic
stroke and infection: Recent and emerging concepts. Lancet Neurol.
7:341–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Emsley HC, Smith CJ, Gavin CM, Georgiou
RF, Vail A, Barberan EM, Hallenbeck JM, del Zoppo GJ, Rothwell NJ,
Tyrrell PJ and Hopkins SJ: An early and sustained peripheral
inflammatory response in acute ischaemic stroke: Relationships with
infection and atherosclerosis. J Neuroimmunol. 139:93–101. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barrientos RM, Frank MG, Hein AM, Higgins
EA, Watkins LR, Rudy JW and Maier SF: Time course of hippocampal
IL-1 beta and memory consolidation impairments in aging rats
following peripheral infection. Brain Behav Immun. 23:46–54. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salminen A, Ojala J, Kauppinen A,
Kaarniranta K and Suuronen T: Inflammation in Alzheimer's disease:
Amyloid-beta oligomers trigger innate immunity defence via pattern
recognition receptors. Prog Neurobiol. 87:181–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barrientos RM, Higgins EA, Biedenkapp JC,
Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW and Maier SF:
Peripheral infection and aging interact to impair hippocampal
memory consolidation. Neurobiol Aging. 27:723–732. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beloosesky Y, Hendel D, Weiss A,
Hershkovitz A, Grinblat J, Pirotsky A and Barak V: Cytokines and
C-reactive protein production in hip-fracture-operated elderly
patients. J Gerontol A Biol Sci Med Sci. 62:420–426. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang SH, Kim YS, Hong TH, Chae MS, Cho ML,
Her YM and Lee J: Effects of dexmedetomidine on inflammatory
responses in patients undergoing laparoscopic cholecystectomy. Acta
Anaesthesiol Scand. 57:480–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maes M, Lin A, Kenis G, Egyed B and
Bosmans E: The effects of noradrenaline and alpha-2 adrenoceptor
agents on the production of monocytic products. Psychiatry Res.
96:245–253. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Szelényi J, Kiss JP and Vizi ES:
Differential involvement of sympathetic nervous system and immune
system in the modulation of TNF-alpha production by alpha-2- and
beta-adrenoceptors in mice. J Neuroimmunol. 103:34–40. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Klimscha W, Tong C and Eisenach JC:
Intrathecal alpha 2-adrenergic agonists stimulate acetylcholine and
norepinephrine release from the spinal cord dorsal horn in sheep.
An in vivo microdialysis study. Anesthesiology. 87:110–116. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shavit Y, Fridel K and Beilin B:
Postoperative pain management and proinflammatory cytokines: Animal
and human studies. J Neuroimmune Pharmacol. 1:443–451. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Devor M, Jänig W and Michaelis M:
Modulation of activity in dorsal root ganglion neurons by
sympathetic activation in nerve-injured rats. J Neurophysiol.
71:38–47. 1994.PubMed/NCBI
|