Introduction
Controlled ovarian stimulation using a
gonadotrophin-releasing hormone (GnRH) antagonist is a potential
treatment option for patients with a low response to fertility
treatments, as it appears to be at least as effective as
GnRH-agonist treatment (long protocol) (1).
Controlled ovarian stimulation with a GnRH
antagonist offers a number of advantages compared with the long
agonist protocol, including a reduction in the overall duration of
the treatment, the absence of peri-menopausal symptoms as a result
of pituitary desensitization, no risk of inadvertent GnRH agonist
administration at the early stages of pregnancy, no risk of ovarian
cyst formation during the mild luteal phase and reduced doses of
gonadotropins (2).
Therefore, in low responders, ovarian stimulation
without pituitary suppression may induce asynchronous follicular
development with a limited degree of potential follicle
development. Furthermore, in patients that are predicted to be poor
responders, for example with elevated serum levels of basal
follicle-stimulating hormone (FSH) due to a decreased ovarian
reserve, antral follicle sizes during the early follicular phase
are often markedly heterogeneous. This phenomenon is associated
with the early exposure of FSH-sensitive follicles to gradient FSH
concentrations during the previous luteal phase.
A potential disadvantage of GnRH-antagonist
protocols is that stimulation is generally initiated on days 2 or 3
of the menstrual cycle, which increases the difficulty of planning
stimulation and laboratory activities. However, cycle scheduling
may be used to avoid having to retrieve oocytes at the weekend and
to distribute the workload equally throughout the week, thereby
reducing the disturbance of incubators and the associated negative
impact on embryonic development. Furthermore, it can reduce the
amount of unplanned laboratory work, which may adversely affect the
concentration and efficiency of laboratory staff (3).
Oral contraceptive pills (OCPs) and synthetic
progestogens have previously been used to schedule the ovarian
stimulation cycle (4–6). Estrogens primarily inhibit FSH
secretion, whereas progestogens primarily control luteinizing
hormone (LH) secretion. The mechanisms underlying this process have
not been well defined; however, it has been postulated that the
gestagen component of OCPs may exert a negative impact on
endometrial receptivity in the subsequent cycle. Alternatively, low
concentrations of LH following OCP pretreatment may impair oocyte
quality or endometrial receptivity if ovarian stimulation is
performed using recombinant FSH (rFSH) but no LH is administered
(4–6).
According to in vitro fertilization (IVF)
stimulation protocols, GnRH antagonists are administered to prevent
a premature pituitary LH surge, which exerts a detrimental effect
in patients that undergo IVF. Treatment with GnRH antagonists may
result in reduced gonadotropin levels. In order to facilitate
follicle growth, FSH is administered exogenously, whereas LH is not
typically administered. Although GnRH antagonists prevent surges in
LH levels, there is evidence that the administration of GnRH
analogs may result in an excessive reduction in endogenous LH
levels, particularly in older women (7,8). A
number of studies have been performed to investigate whether the
additional administration of recombinant LH (rLH) with rFSH is able
to improve the ovarian stimulation cycle outcome (9,10). Few
studies have analyzed this factor in the context of GnRH antagonist
treatment, and the results of these trials obtained in older women
(>35-years-old) are inconsistent (11).
The use of rLH supplementation during ovarian
stimulation, as a part of an assisted reproductive technique (ART),
has not been demonstrated to increase pregnancy success rate, and
previous studies have reported conflicting results in women aged
≥35 years. Previous results do not support the hypothesis that the
addition of rLH increases the rate of pregnancy in unselected
patients treated with an ART protocol consisting of rFSH with a
GnRH antagonist. There may be a potential benefit associated with
the use of rLH supplementation during ovarian stimulation in women
who have a poor response or are of an advanced age (12).
Therefore, it may be valuable to conduct a study
focused on women aged ≥40 years, with the aim of determining if
women may be able to benefit from rLH supplementation during
ovarian stimulation and identifying the cohort of women that would
benefit, in order to improve IVF outcome.
In previous literature, the only study conducted in
women >35 years old that received a GnRH antagonist protocol,
postulated that the degree of hypothalamic-pituitary-ovarian
recovery following pretreatment with OCP administration, may be
used to identify patients that could benefit from rLH
supplementation (13). However, to
date no such perspective randomized study has been conducted to
investigate this topic.
Materials and methods
Presentation of the hypothesis
The currently proposed hypothesis is based on
rationale that, in older women that are predicted to respond poorly
to GnRH antagonist protocols, the extent to which serum levels of
endogenous gonadotrophins are returned to normal by action of the
hypothalamic-pituitary-ovarian axis following pretreatment with
OCPs may be useful for identifying whether women may benefit from
rLH supplementation, and if so, which ones.
In certain cases, older women who are predicted to
be poor responders exhibit an altered
hypothalamic-pituitary-ovarian regulatory mechanism due to the
dysregulation of E2 feedback. The aberrant responsiveness of the
hypothalamic-pituitary axis to estrogen feedback and the subsequent
generation of abnormal patterns of gonadotropin release (normal,
attenuated or increased) may accelerate spontaneous ovarian
follicular depletion and reduce the likelihood of a good ovarian
responsiveness during controlled ovarian hyperstimulation.
We hypothesize that patients with suppressed
activity of the hypothalamic-pituitary axis may benefit from rLH
supplementation, as GnRH antagonist administration during ovarian
stimulation has the potential to induce a marked reduction in LH
levels in such patients compared with that in patients with a
regular recovery of the activity of this axis following OCP.
Furthermore, we hypothesize that patients with
hyper-responsiveness of hypothalamic-pituitary-axis activity
following OCP may be affected by ‘low gonadotropin responsiveness’
comparable to that in patients with a mutation in the FSH receptor
(14). Thus, such cases may benefit
from the ability of rLH supplementation to potentiate exogenous FSH
activity in theca and granulosa cells.
Therefore, the primary aim of the currently proposed
study is to determine whether 1 month pretreatment with OCP is
useful for detecting the cohort of women in a pool of estimated
poor responders aged >40 years that may benefit from rLH
supplementation during the ovarian stimulation cycle according to
hypothalamic-pituitary-ovarian recovery after OCP administration. A
secondary aim of the proposed study is to detect the most effective
serum markers for assessing the cohort of patients that may benefit
from rLH administration during ovarian stimulation.
Testing the hypothesis
The proposed pilot study will include 120 women aged
between 40 and 50 years, that are predicted to be poor responders
according to the Bologna Criteria (15), with an ovarian biological age higher
than their chronological age (16–18).
Patients who have not previously undergone a first fresh non-donor
IVF cycle for primary infertility without rLH supplementation
during ovarian stimulation will be excluded.
All enrolled patients will be properly informed
regarding the aims of the study, and will be required to agree to
these aims and to the use of their data according to the Italian
Law for Privacy 675/96 prior to enrolment.
Patients with any of the following characteristics
will be excluded: History of smoking, deep endometriosis with
elevated CA125 serum value (19),
previous ART cycle in the preceding 3 months, BMI >30, karyotype
abnormalities, mutations of the cystic fibrosis gene, acquired or
inherited thrombophilia and immunological disorders, previous
chemotherapy and/or radiotherapy for neoplasia treatment and marked
qualitative and quantitative alteration in the semen used for
fertilization, according to World Health Organization guidelines
(20).
In cases of benign uterine lesions, such as
endometrial polyps, submucous myomas, intrauterine synechiae and/or
uterine septus, patients will be considered eligible for the study
if they have undergone hysteroscopic-adequate treatment at least 3
months previously (21–23).
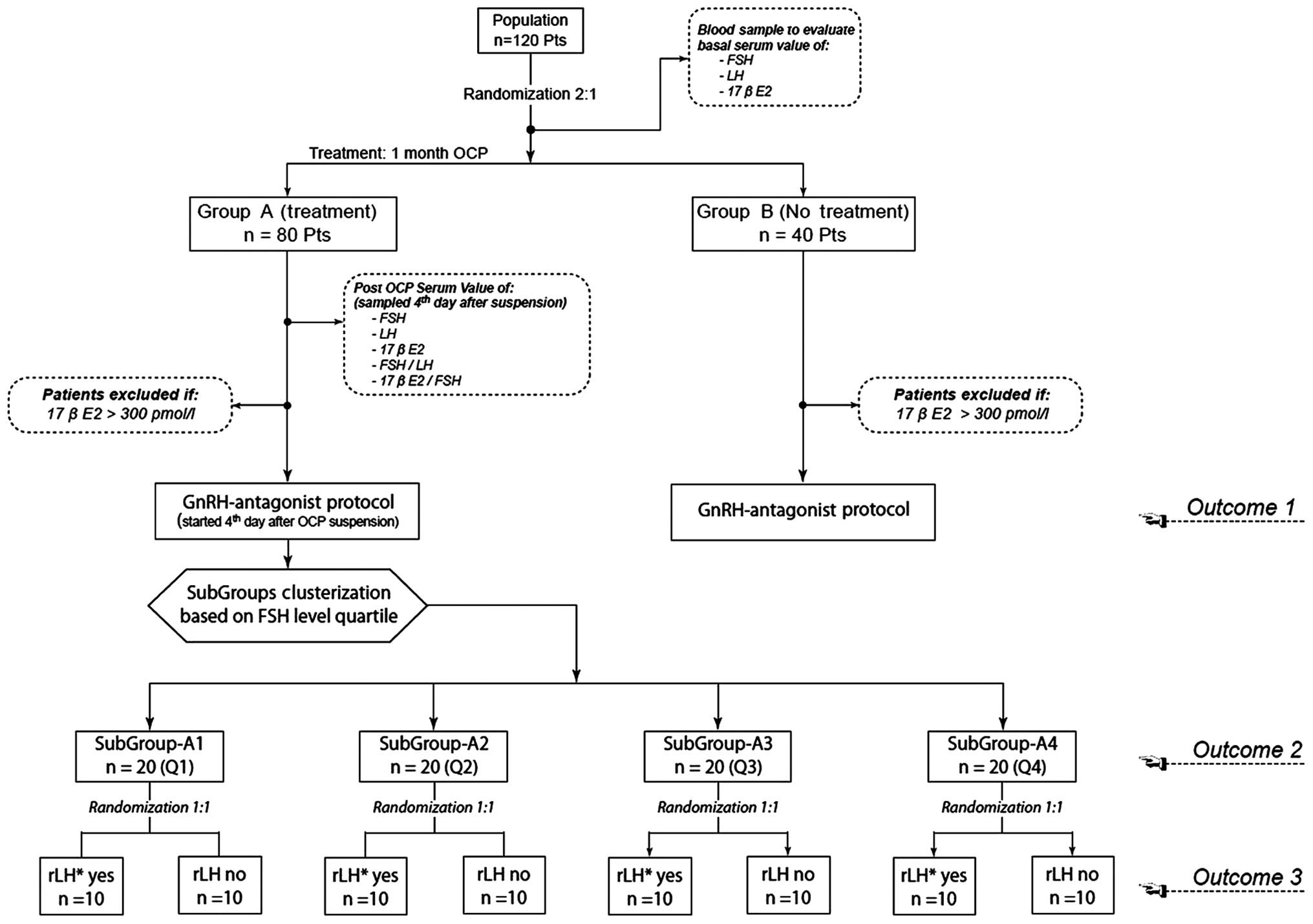
All women, accurately selected according to the
exclusion criteria, will be assigned at random (using 2:1
computerized randomization) to one of two study groups: Group A
(OCP-treated) or group B (control).
Group A patients will be pre-treated with OCPs
containing 2 mg dienogest and 0.03 mg ethinyl estradiol (Effiprev®;
Effik Italia SpA, Milan, Italy) for 21 days during the menstrual
cycle prior to the scheduled IVF/intra-cytoplasmic sperm
injection.
For all patients, serum values of FSH, LH and E2
will be determined on day 3 of the cycle preceding OCP, considered
as the basal values, and at the beginning of stimulation (4 days
after the final OCP for group A patients and 4 days after the
initiation of menses for group B patients).
Single absolute numbers and Δ-variation from
baseline (expressed as a percentage) will be considered for all
markers, the FSH/LH ratio and the E2/FSH ratio in the blood
samples.
Δ-variation from the baseline FSH and LH values will
be used to categorize patients into one of four quartiles (Q1, Q2,
Q3 and Q4), in a similar manner to the study proposed by Schmitz
et al (24).
Patients will be classified based on the following
criteria: Q1, patients with negative baseline variation >25%;
Q2, patients with negative baseline variation between 5 and 25%;
Q3, patients with positive baseline variation between 5 and 25%;
and Q4, patients with positive baseline variation >25%.
According to their obtained FSH quartile, patients
will be admitted to the subgroup A1 in cases of Q1, to subgroup A2
in cases of Q2, to subgroup A3 in cases of Q3 and to subgroup A4 in
cases of Q4.
Patients admitted to each of the subgroups A1-4
(n=20 per subgroup) will be secondly randomized to determine
whether they will receive rLH supplementation (ratio, 1:1) during
ovarian stimulation, starting from day 4 of stimulation. A detailed
flow chart of the proposed study protocol is shown in Fig. 1.
Patients in groups A and B will be treated with
GnRH-antagonist (short protocol) stimulation according to our Units
Protocol (25,26). All stimulation cycles will be
performed using 350 IU rFSH (Gonal F; Merck Serono S.A., Geneva,
Switzerland) daily for the first 5 days. Subsequent adjustments to
the treatment will be decided by the clinicians according to
ovarian response and various biochemical and ultrasound features.
In addition, 0.25 mg GnRH antagonist (Ganirelix; MSD Italia S.R.L.,
Rome, Italy) will be administered daily, starting from the
ultrasonographic detection of at least one follicle of >14 mm
diameter and continued until human chorionic gonadotropin (hCG)
administration.
Starting at day 6 of stimulation, all patients will
undergo serum sampling for the measurement of hormone levels (17β
estradiol, progesterone and LH) and pelvic ultrasound.
After an adequate number of follicles (≥3 follicles
with a diameter of >18 mm) have been detected, 250 µg
recombinant hCG (r-hCG) (Ovitrelle; Merck Serono S.A.) will be
administered to induce ovulation.
Oocyte retrieval will be conducted at 36 h after hCG
administration. Oocytes will be fertilized according to standard
IVF techniques.
Between 1 and 3 embryos will be transferred on days
2 to 3 following oocyte retrieval. The number of embryos
transferred will be determined on the basis of the age of the
patient and embryo quality.
Furthermore, 400 mg vaginal progesterone (Progeffik;
Effik Italia SpA) will be administered daily as a luteal support
until day 14 after oocyte retrieval, and the treatment will be
discontinued in the event of a negative β-hCG serum test.
Pregnancy will be confirmed by the detection of
increased β-hCG concentrations at week 2 after embryo transfer (ET)
and with sonographic observation of an intrauterine gestational sac
at weeks 3–4 after ET. Continuing pregnancy will be confirmed by
the detection of an embryo heart beat with transvaginal
sonography.
The primary aim of the proposed study is to compare
the following parameters between groups A and B: Number of IVF
cycles is this correct that are cancelled prior to ovarian
stimulation (due to a basal E2 value of >300 pmol/l) and during
ovarian stimulation (due to <3 follicles with a diameter of
>14 mm); total dose of rFSH administered during ovarian
stimulation (IUs); length of stimulation (days); total number of
follicle and number of follicles >14 mm in diameter; total
number of retrieved oocytes and number of mature oocytes; number
and quality of obtained embryos, endometrial thickness at oocyte
retrieval; pregnancy rate; and continuing pregnancy rate.
A secondary aim of the study is to compare the
following parameters among the four A subgroups: Total dose of rFSH
administered during ovarian stimulation (IUs), length of
stimulation (days), total number of follicle and number of
follicles >14 mm in diameter, total number of retrieved oocytes
and number of mature oocytes, number and quality of obtained
embryos, endometrial thickness at oocyte retrieval, pregnancy rate
and continuing pregnancy rate in order to detect which, if any, FSH
quartile will be a predictor of an improved IVF outcome.
Finally, a third intended outcome is to detect
whether rLH supplementation improves the IVF outcomes in each
subgroup-A patient, determine in which of the interquartile
subgroups A1-4 the rLH supplementation produces the most improved
results and identify whether variations in other serum markers
after OCP have a greater predictive capability than the FSH serum
value in the detection of patients that may benefit from rLH
supplementation. Detailed steps of the proposed study protocol are
summarized in Fig. 1.
Statistical analysis will be performed using SPSS
software for Windows, version 19.0 (IBM SPSS, Armonk, USA),
applying parametric and non-parametric tests when appropriate. The
Kolmogorov-Smirnov test will be used to assess the normality of
distribution. Continuous variables will be expressed as absolute
numbers, the mean ± standard deviation, and will be analyzed using
Students t-test or analysis of variance when appropriate.
Categorical variables will be expressed as percentages and analyzed
using the χ2 test or Fisher's exact test. P<0.05 will
be considered to indicate a statistically significant
difference.
Discussion
If the current hypothesis is confirmed, it should
facilitate the identification of women that could benefit from rLH
supplementation during ovarian stimulation, among the cohort of
predicted poor responders. Consequently, previous inconclusive
results concerning rLH supplementation may be clarified.
If patients supplemented with rLH (according to
abnormal recovery of the activity of the hypothalamic-pituitary
axis following OCP treatment) display improvements in terms of
ovarian response during IVF and subsequently pregnancy rate, the
OCP test pre-IVF may be considered to be a useful tool for
improving the success of ARTs in poor responders, reducing cost and
patient stress.
It is reasonable to expect that the number of
patients that consent to the 1-month OCP study will be high, as the
proposed study involves a low-expense, well-tolerated intervention,
with no obvious contraindications.
If the predictive efficacy of the OCP-test is
confirmed, its use in patients prior to GnRH antagonist protocols
to detect patients requiring rLH supplementation during ovarian
stimulation may be extended to a large scale population.
Glossary
Abbreviations
Abbreviations:
|
ART
|
assisted reproductive technique
|
|
E2
|
17β estradiol
|
|
ET
|
embryo transfer
|
|
GnRH
|
gonadotropin-releasing hormone
|
|
r-hCG
|
recombinant human chorionic
gonadotropin
|
|
IVF
|
in vitro fertilization
|
|
OCP
|
oral contraceptive pill
|
|
r-FSH
|
recombinant follicular-stimulating
hormone
|
|
rLH
|
recombinant luteinizing hormone
|
References
|
1
|
Kim CH, Jeon GH, Cheon YP, Jeon I, Kim SH,
Chae HD and Kang BM: Comparison of GnRH antagonist protocol with or
without oral contraceptive pill pretreatment and GnRH agonist
low-dose long protocol in low responders undergoing
IVF/intracytoplasmic sperm injection. Fertil Steril. 92:1758–1760.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Devroey P, Aboulghar M, Garcia-Velasco J,
Griesinger G, Humaidan P, Kolibianakis E, Ledger W, Tomás C and
Fauser BC: Improving the patient's experience of IVF/ICSI: A
proposal for an ovarian stimulation protocol with GnRH antagonist
co-treatment. Hum Reprod. 24:764–774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Inany HG, Youssef MA, Aboulghar M,
Broekmans F, Sterrenburg M, Smit J and Abou-Setta AM:
Gonadotrophin-releasing hormone antagonists for assisted
reproductive technology. Cochrane Database Syst Rev.
5:CD0017502011.PubMed/NCBI
|
|
4
|
Griesinger G, Kolibianakis EM, Venetis C,
Diedrich K and Tarlatzis B: Oral contraceptive pretreatment
significantly reduces ongoing pregnancy likelihood in
gonadotropin-releasing hormone antagonist cycles: An updated
meta-analysis. Fertil Steril. 94:2382–2384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kolibianakis EM, Papanikolau EG, Camus M,
Tournaye H, Van Steirteghem AC and Devroey P: Effect of oral
contraceptive pill pretreatment on ongoing pregnancy rates in
patients stimulated with GnRH antagonists and recombinant FSH for
IVF. A randomized controlled trial. Hum Reprod. 21:352–357. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rombauts L, Healy D and Norman RJ:
Comparative randomized trial to assess the impact of oral
contraceptive pretreatment on follicular growth and hormone
profiles in GnRH antagonist-treated patients. Hum Reprod.
21:95–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gizzo S, Andrisani A, Noventa M, Manfè S,
Oliva A, Gangemi M, Nardelli GB and Ambrosini G: Recombinant LH
supplementation during IVF cycles with a GnRH-antagonist in
estimated poor responders: A cross-matched pilot investigation of
the optimal daily dose and timing. Mol Med Rep. 12:4219–4229.
2015.PubMed/NCBI
|
|
8
|
Alviggi C, Clarizia R, Mollo A, Ranieri A
and De Placido G: Who needs LH in ovarian stimulation? Reprod
Biomed Online. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gizzo S, Quaranta M, Andrisani A, Bordin
L, Vitagliano A, Esposito F, Venturella R, Zicchina C, Gangemi M
and Noventa M: Serum stem cell factor assay in elderly poor
responder patients undergoing IVF: A new biomarker to customize
follicle aspiration cycle by cycle. Reprod Sci. July 7–2015.((Epub
ahead of print))
|
|
10
|
Hill MJ, Levens ED, Levy G, Ryan ME,
Csokmay JM, DeCherney AH and Whitcomb BW: The use of recombinant
luteinizing hormone in patients undergoing assisted reproductive
techniques with advanced reproductive age: a systematic review and
meta-analysis. Fertil Steril. February 24–2012.((Epub ahead of
print)). View Article : Google Scholar
|
|
11
|
König TE, van der Houwen LE, Overbeek A,
Hendriks ML, Beutler-Beemsterboer SN, Kuchenbecker WK, Renckens CN,
Bernardus RE, Schats R, Homburg R, et al: Recombinant LH
supplementation to a standard GnRH antagonist protocol in women of
35 years or older undergoing IVF/ICSI: A randomized controlled
multicentre study. Hum Reprod. 28:2804–2812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
König TE, van der Houwen LE and Lambalk
CB: Recombinant LH supplementation in women of 35 years and older
undergoing IVF? Fertil Steril. 98:e10–e11. 2012. View Article : Google Scholar
|
|
13
|
Schmitz C, Bocca S, Beydoun H, Stadtmauer
L and Oehninger S: Does the degree of
hypothalamic-pituitary-ovarian recovery after oral contraceptive
pills affect outcomes of IVF/ICSI cycles receiving GnRH-antagonist
adjuvant therapy in women over 35 years of age? J Assist Reprod
Genet. 29:877–882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Desai SS, Roy BS and Mahale SD: Mutations
and polymorphisms in FSH receptor: functional implications in human
reproduction. Reproduction. 146:R235–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferraretti AP, La Marca A, Fauser BC,
Tarlatzis B, Nargund G and Gianaroli L: ESHRE Working Group On Poor
Ovarian Response Definition: ESHRE consensus on the definition of
‘poor response’ to ovarian stimulation for in vitro
fertilization: The Bologna criteria. Hum Reprod. 26:1616–1624.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patrelli TS, Gizzo S, Sianesi N, Levati L,
Pezzuto A, Ferrari B and Modena A Bacchi: Anti-Müllerian hormone
serum values and ovarian reserve: Can it predict a decrease in
fertility after ovarian stimulation by ART cycles? PLoS One.
7:e445712012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gizzo S, Andrisani A, Esposito F, Oliva A,
Zicchina C, Capuzzo D, Gangemi M and Nardelli GB: Ovarian reserve
test: An impartial means to resolve the mismatch between
chronological and biological age in the assessment of female
reproductive chances. Reprod Sci. 21:632–639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Litta P, D'Agostino G, Conte L, Saccardi
C, Cela V, Angioni S and Plebani M: Anti-Müllerian hormone trend
after laparoscopic surgery in women with ovarian endometrioma.
Gynecol Endocrinol. 29:452–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patrelli TS, Berretta R, Gizzo S, Pezzuto
A, Franchi L, Lukanovic A, Nardelli GB and Modena AB: CA 125 serum
values in surgically treated endometriosis patients and its
relationships with anatomic sites of endometriosis and pregnancy
rate. Fertil Steril. 95:393–396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
World Health Organization: WHO Laboratory
Manual for the Examination of Human Semen and Sperm-Cervical Mucus
Interaction. Cambridge University Press; Cambridge, UK: 1999
|
|
21
|
Litta P, Cosmi E, Saccardi C, Esposito C,
Rui R and Ambrosini G: Outpatient operative polypectomy using a 5
mm-hysteroscope without anaesthesia and/or analgesia: Advantages
and limits. Eur J Obstet Gynecol Reprod Biol. 139:210–214. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saccardi C, Conte L, Fabris A, De Marchi
F, Borghero A, Gizzo S and Litta P: Hysteroscopic enucleation in
toto of submucous type 2 myomas: Long term follow up in women
affected by menorrhagia. J Minim Invasive Gynecol. 21:426–430.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Litta P, Spiller E, Saccardi C, Ambrosini
G, Caserta D and Cosmi E: Resectoscope or Versapoint for
hysteroscopic metroplasty. Int J Gynaecol Obstet. 101:39–42. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmitz C, Bocca S, Beydoun H, Stadtmauer
L and Oehninger S: Does the degree of
hypothalamic-pituitary-ovarian recovery after oral contraceptive
pills affect outcomes of IVF/ICSI cycles receiving GnRH-antagonist
adjuvant therapy in women over 35 years of age? J Assist Reprod
Genet. 29:877–882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gizzo S, Andrisani A, Noventa M, Quaranta
M, Esposito F, Armanini D, Gangemi M, Nardelli GB, Litta P,
D'Antona D and Ambrosini G: Menstrual cycle length: a surrogate
measure of reproductive health capable of improving the accuracy of
biochemical/sonographical ovarian reserve test in estimating the
reproductive chances of women referred to ART. Reprod Biol
Endocrinol. April 10–2015.((Epub ahead of print)). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gizzo S, Andrisani A, Esposito F, Noventa
M, Di Gangi S, Angioni S, Litta P, Gangemi M and Nardelli GB: Which
luteal phase support is better for each IVF stimulation protocol to
achieve the highest pregnancy rate? A superiority randomized
clinical trial. Gynecol Endocrinol. September 30–2014.((Epub ahead
of print)). PubMed/NCBI
|