Introduction
Computed tomography (CT)-guided transthoracic needle
biopsy is a well-established, effective and safe technique for the
diagnosis of focal lung lesions (1,2) and has
exhibited high diagnostic accuracy and specificity (3). No differences have been detected
between single-needle transthoracic needle biopsy and a coaxial
technique with regard to diagnostic accuracy (4). Conventional CT-guided transthoracic
needle biopsy, however, is a method for locating lesion-edges that
increases the dwell time of the needle within the lung while
scanning prior to biopsy (3,5–8). The
reported dwell times during conventional CT range between 29 and 41
min (4,6,9,10). Longer dwell times are believed to
result in greater needle motion during respiration, leading to the
widening of the pleural puncture site (2,11–13).
Pneumothorax and hemorrhage are the most frequent complications
associated with the procedure (11,14–17).
Other potential factors affecting the rate of complications include
lesion depth and size, presence of atelectasis and patient age
(13–16,18–22).
In order to reduce the needle dwell time in the
lungs and reduce complications, we have established an extrapleural
locating (EPL) method that maintains the needle tip outside the
visceral pleura prior to biopsy. Our previous pilot studies have
involved only small sample sizes (23,24);
however, the preliminary results of these studies indicated that
the EPL method was accurate and safe, and decreased the dwell time
compared with conventional techniques (4,6,9,10).
Furthermore, EPL was shown to improve the false-negative rate
(23,24). Factors that affected accuracy
included lesion depth and size, necrosis and the number of pleural
passes (9,22,25,26).
The aim of the present study was to assess the
efficacy of the low-dose, CT-guided EPL method and extrapleural
percutaneous lung biopsy on a larger cohort of patients. We
hypothesized that this novel technique would reduce complications
during low-dose, CT-guided automated cutting needle biopsy (ACNB),
compared with results from previously published studies. Thus, a
range of factors affecting accuracy and safety were evaluated
during ACNB using a new EPL method.
Subjects and methods
Subjects
The Ethics Committee of the Zhongnan Hospital of
Wuhan University (Wuhan, China) approved the study protocol and
provided permission to perform this study. Each patient provided
signed informed consent prior to participation.
This study was a retrospective analysis of 1,106
percutaneous CT-guided ACNBs with EPL performed on 1,065 patients
between March 2005 and May 2012. All procedures were performed by
the two radiologists that were experienced in performing CT-guided
lung biopsies. Percutaneous CT-guided ACNB with an EPL technique
was indicated in any patient with a lung lesion requiring biopsy.
Patients with pleural-based tumors, such as mesothelioma, or
pleural-based metastases were excluded, as this study reported only
the results of percutaneous lung biopsy.
EPL method
A CT scan of the chest using a conventional CT
Scanner (Somatom Sensation 16; Siemens Healthcare, Forchheim,
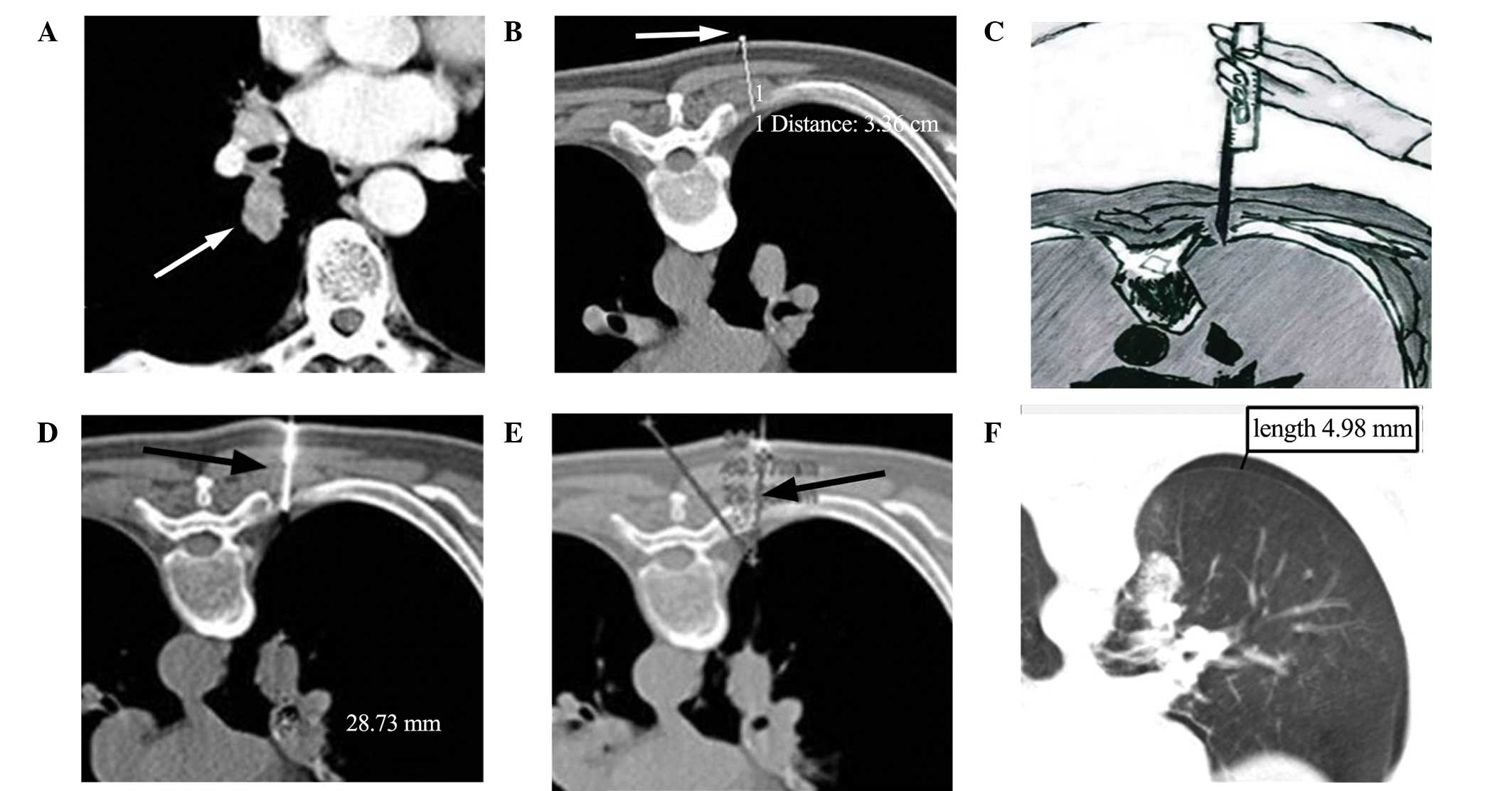
Germany) was initially performed to identify the lesion (Fig. 1A). Prior to the biopsy, patients with
deep intraparenchymal lesions or lesions adjacent to blood vessels
received 1 KU hemocoagulase (H20041730; Jinzhou Ahon
Pharmaceuticals Co., Ltd., Jinzhou, China) intramuscularly, as
previously described (27).
Prior to the procedure, the radiologist described
the biopsy process to the patient and positioned the patient in
supine, prone or lateral positions to minimize puncture depth, and
to avoid contact with bone and large blood vessels. After placing
the patient in the scanner, the patient was trained in the
breath-holding technique to ensure they were able to maintain the
required magnitude of breath-hold during the CT scans and
biopsy.
An initial localization scan with a low-dose
technique (Lung CARE Series, Siemens Sensation 16 CT scan: 20–50
mA; 120 kV; scan field, 30–60 mm) through the region of interest
was performed at a slice thickness of 5 mm and viewed on lung and
soft-tissue windows. Localization was performed following a review
of conventional CT images and by using laser positioning and metal
skin markers (Biopsy single series: 50 mA; 120 kV; 10-mm thickness)
to indicate the site of needle entry and direction of approach for
biopsy. During the procedure, the ribs, lung bullae, vessels,
fissures and low-density areas were avoided and the amount of
aerated lung tissue traversed was minimized.
After ensuring that the direction of needle approach
was perpendicular to the chest wall, the thickness of the thoracic
wall was measured from the skin marker to the pleural surface
(Fig. 1B) to determine the depth of
anesthesia required and the depth of needle insertion. Using an
aseptic technique with a 23-gauge needle, local anesthetic
(lidocaine 1%) was administered. The anesthetic needle was inserted
into the chest wall pleura as the locating needle. In patients with
a thin chest wall, in which the anesthetic needle was unable to be
fixed automatically, the operator directly applied a biopsy needle
(Bard® Max-Core® Core Needle Biopsy Instrument; Bard Biopsy
Systems, Tempe, AZ, USA) for positioning. Once the core gun had
been inserted into the chest wall, the radiology assistant, wearing
X-ray protective gear, fixed the core gun manually and the low-dose
CT scan, with a dose-length product (DLP) of 6 mGy/cm, was
performed for EPL positioning (Fig.
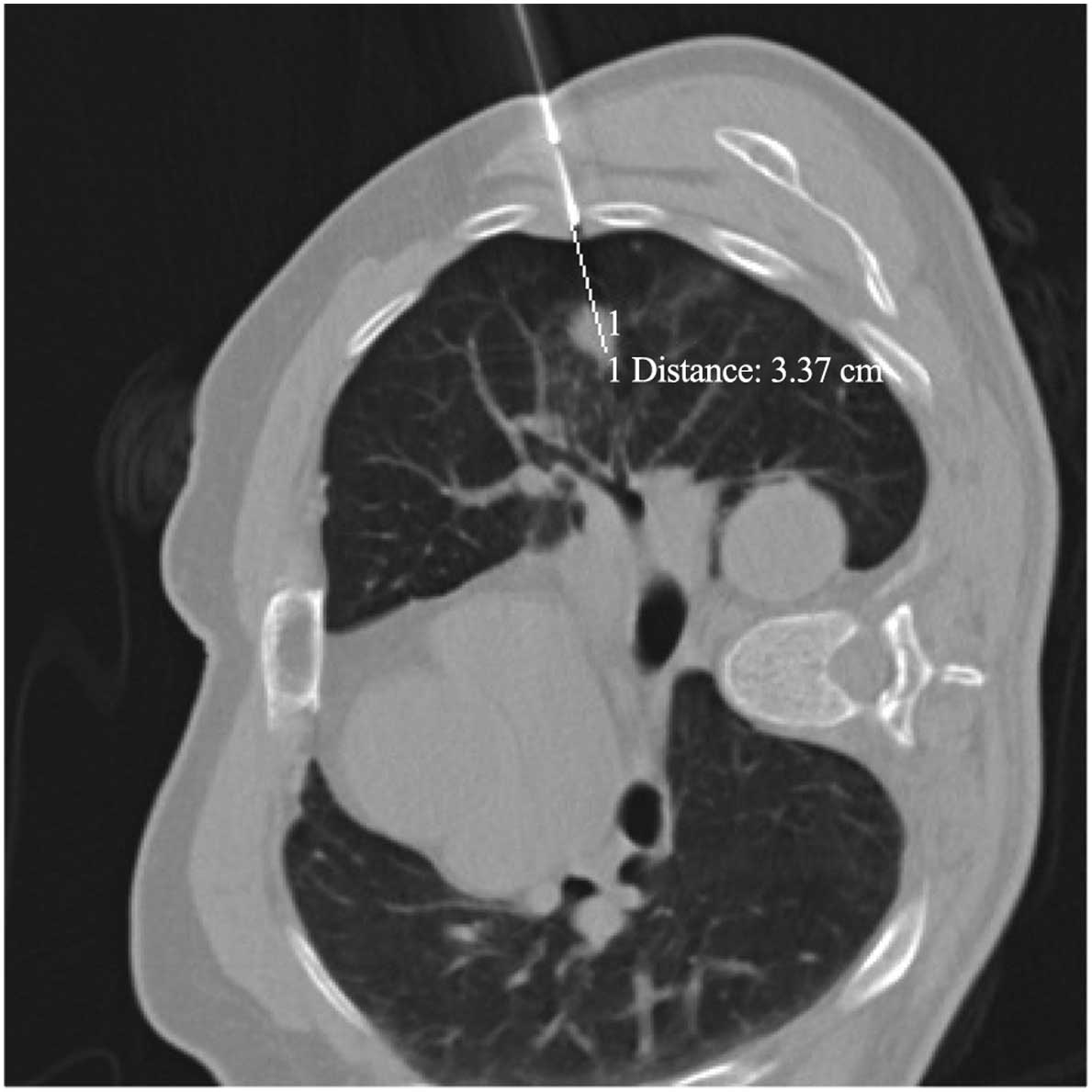
1C). The radiologist confirmed the puncture angle and distances
between the locating pin and lesion based on the CT data. The
position of the lesion in relation to the tip of the locating
needle, the precise distance to the margin of the lesion and the
needle-pleura angle (defined as the angle between the locating
needle and the line vertical to pleural surface) (Fig. 1D and E) were optimized using
sequential CT scanning (Biopsy single series: 50 mA; 120 kV; scan
field, 13.5 mm).
According to the data obtained from the repeat CT
scan, the trajectory of the locating needle was adjusted under the
chest wall pleura. The Bard Max-Core Core Needle was directly
advanced into the lung and fired to obtain a core of tissue
(typically 2-cm in length) from the lesion, based on data including
direction, angle and depth obtained using the locating needle,
following which the needle gun was withdrawn. The needle dwell time
within the lung was measured, as the time between the biopsy needle
puncturing the lung and its withdrawal from the lungs by the
radiologist. The extracted tissue was examined by the naked eye. If
soft tissue was evident in the sample, the biopsy was considered
successful and the specimens obtained were placed in formalin
solution using a saline-filled syringe. In general, to ensure that
the tissue block was sufficient for histological examination, the
biopsy was repeated 2–3 times, with slight adjustment of the
transthoracic puncture angle and depth used. If pneumothorax
occurred and caused the lesion to shift, or if adequate tissue was
not obtained during the procedure, a repeat localization CT scan
with EPL was performed. In order to avoid air embolism, the
procedure was terminated if the patient coughed or hemoptysis
ensued. Following completion of the biopsy, the biopsy area was
scanned using conventional CT guidance (Biopsy single series: 50
mA; 120 kV; thickness, 4.5 mm; scan field, 13.5 mm) to exclude
pneumothorax or hemorrhage (Fig.
1F). The biopsy gun should advance to the depth displayed in
Fig. 2.
Final diagnosis
The final diagnosis for each patient was based on
the results of the surgery, response to relevant therapy, or
clinical observations at month 24 of clinical follow-up. The biopsy
specimens were evaluated by an experienced pathologist and the
final diagnosis was confirmed by surgery. Histological findings
obtained by biopsy were compatible with the patient's clinical
disease manifestations. Atypical adenomatous hyperplasia was
defined as malignancy in this series, as previously described
(28).
Data collection
Data collected included details regarding patient
information (gender, age), nature of the lesion (location, type,
depth, size, presence of emphysema, contrast enhancement) and the
presence of atelectactic lung tissue adjacent to the lesion, which
could increase the difficulty of distinguishing between healthy and
tumor tissue. In addition, details of the procedure (position,
needle size, number of EPL attempts, needle-pleura angle),
complications (pneumothorax, hemorrhage, hemoptysis) and the
histological results (benign versus malignant) were collected.
Potential complications of the EPL method included
the presence of an immediate pneumothorax or hemorrhage on the
post-biopsy CT images, the presence of a pneumothorax on the chest
radiograph at 4 h post-biopsy and hemoptysis. Hemorrhage was also
graded using the following criteria: i) Mild, hemorrhage presenting
as haziness along the needle tracks or in adjacent air spaces on
the CT scan; ii) moderate, occurrence of fewer than five episodes
of hemoptysis estimated at <30 ml blood or minimal hemothorax;
and iii) severe, hemoptysis or hemothorax associated with
hemodynamic instability (13).
The presence of pneumothorax was assessed by a
low-dose CT technique immediately subsequent to biopsy while the
patient was on the CT scan table. Pneumothorax was graded using the
following criteria: i) Mild, lung surface retraction of ≤2 cm; ii)
moderate, lung surface retraction of between 2 and 4 cm; and iii)
severe, lung surface retraction of ≥4 cm (13). Patients that were clinically stable
remained under medical observation for 12 h prior to discharge
(29). A chest radiograph was
performed for all patients at 4 h after the procedure or sooner if
they exhibited symptoms before this time.
Lesion size was measured along the needle path
maximum long-axis diameter on the mediastinal windows. Lesion depth
was measured from the point of pleural puncture to the nearest edge
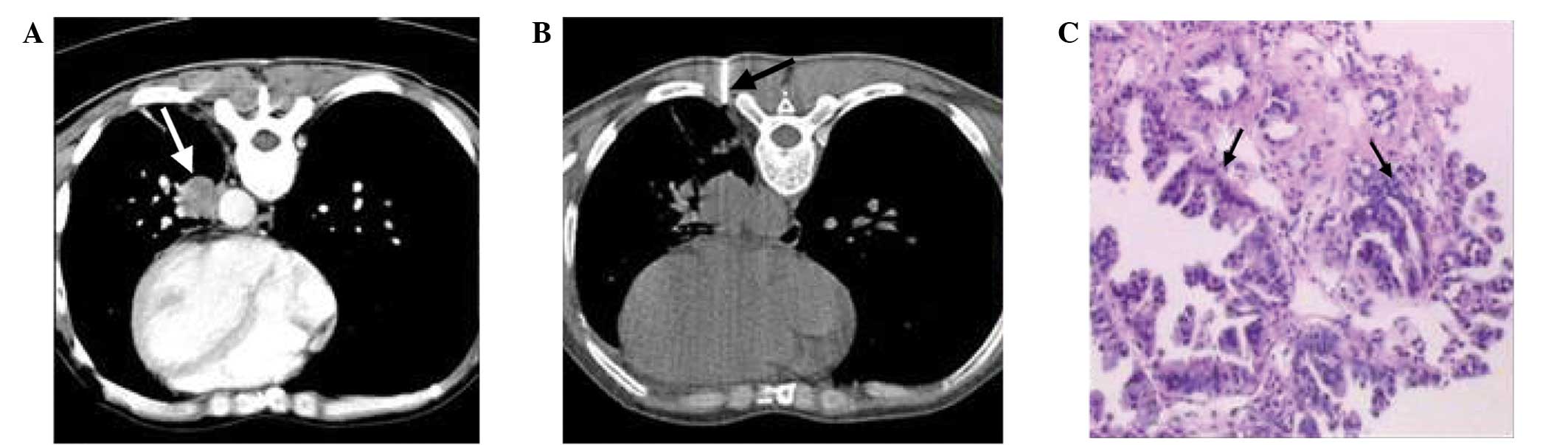
of the lung lesion along the needle path. Lesion type was divided
into central versus peripheral. The definitions of central and
peripheral lesions were based on imaging observation. Lesions that
originated in the segmental bronchi or above bronchial lesions were
graded as central lesions (Fig. 3),
whereas lesions that originated in the lower bronchi were graded as
peripheral lesions.
Emphysema was defined as ≤900 HU, in which ≤900 HU
was used as the threshold for determining emphysema (30). The emphysema status was classified as
follows: Group 1, no emphysemic tissue surrounding the lesion;
group 2, emphysemic tissue surrounding the lesion, but distant from
the needle tract; and group 3, prominent emphysemic tissue in the
needle tract.
The Somatom Sensation 16 CT biopsy scanner was able
to record the radiation dose administered throughout the entire
scanning process. Total effective dose (ED) for all procedures was
obtained directly from the CT station. Theoretically, ED (mSv) =
DLP × k, where k=0.014 (mSv/mGy/cm) (31). Procedural duration was defined as the
time between the first and last CT slice performed during the
procedure.
Statistical analysis
Distributions of quantitative data were compared
using the Student's t-test or the Mann-Whitney U-test, for
analyzing the factors with or without a normal distribution,
respectively. Distribution of qualitative data was compared using a
χ2 test or a bilateral Fisher's exact test. Categorical
variables are presented as counts and percentages, with
χ2 tests for group comparisons. Logistic regression
models were performed to detect the risk factors for diagnostic
accuracy and complications. Continuous variables were stratified
into groups for logistic regression analyses of complications.
Using a forward procedure, only variables considered to be
significant on univariate analysis (P<0.05) were subsequently
introduced to multivariate logistic regression modeling to assess
their contribution to diagnostic accuracy and the risk of
pneumothorax, hemorrhage and hemoptysis. A two-sided P<0.05 was
considered to indicate a statistically significant difference. SAS
statistics software, version 9.2 (SAS Institute Inc., Cary, NC,
USA) was used for all analyses.
Results
General data
A total of 1,106 percutaneous ACNBs were performed
in 1,065 patients (733 men and 332 women) with a mean age of 59±12
years, including 290 outpatients and 775 inpatients. Among these,
198 cases underwent the procedure using a biopsy needle as a
locating needle for EPL. The patients were primarily inpatients
requiring pathological evaluation of their lesions. Among the 1,106
biopsies performed, 794 lesions were malignant and 312 were benign
(Table I).
 | Table I.Characteristics of malignant (n=794)
and benign (n=312) lesions. |
Table I.
Characteristics of malignant (n=794)
and benign (n=312) lesions.
| Characteristic | Total, n (%) | Malignant, n
(%) | Benign, n (%) | P-value |
|---|
| Gender |
|
|
| 0.017a |
|
Male | 757
(68.44) | 560 (70.53) | 197 (63.14) |
|
|
Female | 349
(31.56) | 234 (29.47) | 115 (36.86) |
|
| Age, years |
|
|
|
<0.001a |
|
≤50 | 236
(21.34) | 113 (14.23) | 123 (39.42) |
|
|
51–60 | 331
(29.93) | 238 (29.97) | 93
(29.81) |
|
|
61–70 | 330
(29.84) | 264 (33.25) | 66
(21.15) |
|
|
>70 | 209 (18.9) | 179 (22.54) | 30 (9.62) |
|
| Lesion location,
lobe |
|
|
| 0.012a |
| Right
upper | 333
(30.11) | 251 (31.61) | 82
(26.28) |
|
| Right
middle | 46
(4.16) | 24 (3.02) | 22 (7.05) |
|
| Right
lower | 242
(21.88) | 169 (21.28) | 73 (23.4) |
|
| Left
upper | 273
(24.68) | 203 (25.57) | 70
(22.44) |
|
| Left
lower | 212
(19.17) | 147 (18.51) | 65
(20.83) |
|
| Lesion type |
|
|
|
<0.001a |
|
Central | 265
(23.96) | 247 (31.11) | 18 (5.77) |
|
|
Peripheral | 841
(76.04) | 547 (68.89) | 294 (94.23) |
|
| Lesion depth,
mm |
|
|
|
<0.001a |
| 0 | 521
(47.11) | 348 (43.83) | 173
(55.45) |
|
|
1–10 | 195
(17.63) | 117 (14.74) | 78 (25) |
|
|
11–20 | 216
(19.53) | 175 (22.04) |
41 (13.14) |
|
|
21–30 | 108
(9.76) | 94
(11.84) | 14
(4.49) |
|
|
>30 |
66 (5.97) | 60 (7.56) |
6 (1.92) |
|
| Lesion size,
mm |
|
|
|
<0.001a |
|
≤10 |
57 (5.15) | 22 (2.77) | 35
(11.22) |
|
|
11–20 | 213
(19.26) | 132 (16.62) | 81
(25.96) |
|
|
21–30 | 273
(24.68) | 171 (21.54) | 102 (32.69) |
|
|
31–40 | 227
(20.52) | 174 (21.91) | 53
(16.99) |
|
|
>40 | 336
(30.38) | 295 (37.15) | 41
(13.14) |
|
| Emphysema |
|
|
| 0.063 |
|
0b | 423 (38.25) | 287 (36.15) | 136 (43.59) |
|
|
1c | 450 (40.69) | 337 (42.44) | 113 (36.22) |
|
|
2d | 233 (21.07) | 170 (21.41) | 63
(20.19) |
|
| Contrast
enhancement |
|
|
| 0.087 |
|
Yes | 336
(30.38) | 253 (31.86) | 83
(26.60) |
|
| No | 770
(69.62) | 541 (68.14) | 229 (73.40) |
|
| Atelectasis |
|
|
| 0.005a |
|
Yes | 112
(10.13) | 93
(11.71) | 19 (6.09) |
|
| No | 994
(89.87) | 701 (88.29) | 293 (93.91) |
|
| Position |
|
|
| 0.665 |
|
Lateral | 240 (21.7) | 176 (22.17) | 64
(20.51) |
|
|
Prone | 586
(52.98) | 414 (52.14) | 172 (55.13) |
|
|
Supine | 280
(25.32) | 204 (25.69) | 76
(24.36) |
|
| Needle size, G |
|
|
| 0.008a |
| 16 | 105 (9.49) | 87
(10.96) | 18 (5.77) |
|
| 18 | 1,001 (90.51) | 707 (89.04) | 294 (94.23) |
|
| Number of
attempts |
|
|
| 0.034a |
| ≤2 | 396 (35.8) | 277 (34.89) | 119 (38.14) |
|
| 3 | 581
(52.53) | 412 (51.89) | 169 (54.17) |
|
| ≥4 | 129
(11.66) | 105 (13.22) | 24 (7.69) |
|
| Needle-pleura
angle, degrees |
|
|
| 0.071 |
|
≤15 | 923
(83.45) | 672 (84.63) | 251 (80.45) |
|
|
16–30 | 110 (9.95) | 78 (9.82) | 32
(10.26) |
|
|
>30 | 73
(6.60) | 44 (5.54) | 29 (9.29) |
|
| Pneumothorax |
|
|
| 0.783 |
|
Yes | 207
(18.72) | 147 (18.51) | 60
(19.23) |
|
| No | 899
(81.28) | 647 (81.49) | 252 (80.77) |
|
| Hemorrhage |
|
|
| 0.003a |
|
Yes | 251
(22.69) | 199 (25.06) | 52
(16.67) |
|
| No | 855
(77.31) | 595 (74.94) | 260 (83.33) |
|
| Hemoptysis |
|
|
| 0.276 |
|
Yes | 58
(5.24) | 38 (4.79) | 20 (6.41) |
|
| No | 1,048 (94.76) | 756 (95.21) | 292 (93.59) |
|
| Diagnosis |
|
|
|
<0.001a |
|
Positive | 730
(66.00) | 730 (91.94) | 0 (0.00) |
|
|
Negative | 376
(34.00) | 64 (8.06) | 312 (100.00) |
|
| Specific
diagnosis |
|
|
| 0.245 |
|
Yes | 925
(83.63) | 671 (84.51) | 254 (81.41) |
|
| No | 181
(16.37) | 123 (15.49) | 58
(18.59) |
|
| False negative |
|
|
|
|
|
Yes | 64
(8.06) | 64 (8.06) |
|
|
| No | 730
(91.94) | 730 (91.94) |
|
|
The overall diagnostic accuracy was 94.2%. A
specific diagnosis was achieved in 81.4% of the benign and 84.5% of
the malignant lesions (Table I). The
overall specific diagnostic rate was 83.6%.
Risk factors affecting accuracy
The risk factors affecting accuracy are presented in
Table II. Results of the univariate
logistic regression analysis indicated that younger age, lesion
type and depth, atelectasis and hemoptysis significantly affected
accuracy (P<0.05; Table II).
Factors that were significant on the univariate analyses were
included in the multivariate model.
 | Table II.Univariate and multivariate analysis
of risk factors affecting accuracy (false-negative rate). |
Table II.
Univariate and multivariate analysis
of risk factors affecting accuracy (false-negative rate).
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Risk
factor/reference | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Gender/female | 1.54
(0.83–2.85) | 0.167 |
|
|
| Age, years | 0.97
(0.95–0.99) | 0.009a | 0.97
(0.95–1.00) | 0.027 |
| Lesion
location/right upper lobe |
|
|
|
|
| Right
middle lobe | 0.86
(0.19–3.88) | 0.844 |
|
|
| Right
lower lobe | 0.72
(0.35–1.49) | 0.379 |
|
|
| Left
upper lobe | 0.81
(0.42–1.57) | 0.531 |
|
|
| Left
lower lobe | 0.69
(0.32–1.49) | 0.344 |
|
|
| Lesion
type/central | 0.34
(0.2–0.57) |
<0.001a | 0.72
(0.37–1.39) | 0.321 |
| Lesion depth,
mm | 1.03
(1.01–1.05) | 0.007a | 1.03
(1.01–1.05) | 0.008a |
| Lesion size,
mm | 1.00
(0.99–1.02) | 0.894 |
|
|
|
Emphysema/0b | 1.06
(0.61–1.82) | 0.847 |
|
|
|
1c | 0.65
(0.37–1.15) | 0.142 |
|
|
|
2d | 0.68
(0.34–1.36) | 0.273 |
|
|
| Contrast
enhancement/no | 1.05
(0.61–1.81) | 0.865 |
|
|
| Atelectasis/no | 5.75
(3.27–10.1) |
<0.001a | 6.53
(3.12–13.67) |
<0.001a |
|
Position/lateral |
|
|
|
|
|
Prone | 0.87
(0.46–1.65) | 0.668 |
|
|
|
Supine | 1.04
(0.51–2.13) | 0.917 |
|
|
| Needle size/16
G | 2.64
(0.81–8.61) | 0.107 |
|
|
| Number of
attempts/≤2 |
|
|
|
|
| 3 | 0.68
(0.38–1.21) | 0.196 |
|
|
| ≥4 | 1.76
(0.88–3.50) | 0.109 |
|
|
| Needle-pleura
angle/≤15° |
|
|
|
|
|
16–30° | 0.95 (0.4–2.3) | 0.916 |
|
|
|
>30° | 1.14
(0.4–3.32) | 0.804 |
|
|
| Hemostatic/no | 0.89
(0.31–2.54) | 0.824 |
|
|
|
Pneumothorax/no | 1.52
(0.84–2.77) | 0.166 |
|
|
| Hemorrhage/no | 1.29
(0.74–2.26) | 0.374 |
|
|
| Hemoptysis/no | 4.64
(2.14–10.06) |
<0.001a | 5.06
(2.19–11.70) |
<0.001a |
Results of the multivariate analysis suggested that
the significant risk factors affecting accuracy included younger
age, atelectasis, hemoptysis and lesion depth (P<0.03; Table II). In the 794 cases of malignant
lesions, there were 64 false-negative results, including 15
false-negative cases in patients ≤50 years old (15/113, 13.27%), 19
false-negative cases in patients 51–60 years old (19/238, 8.00%),
22 false-negative cases in patients 61–70 years old (22/264, 8.33%)
and 8 false-negative cases in patients aged >70 years (8/179,
4.47%). Among the 64 cases of malignant lesions, atelectasis was
present in 24 cases (24/93, 25.81%), while there were 40
false-negative cases with no atelectasis (40/701, 5.71%);
hemoptysis appeared in 10 cases (10/38, 26.32%), while there was no
hemoptysis in 54 cases (54/756, 7.14%). Combining the malignant and
benign lesions, hemorrhage occurred in 28 subjects (28/251, 11.16%)
that had been administered hemocoagulase and 5 of the 58 subjects
(8.62%) with hemoptysis had received hemocoagulase.
Lesion size had no effect on the false-negative
results. Among the 64 false-negative cases, there were 17 cases
with a lesion size measuring ≤20 mm, 11 measuring 21–30 mm, 9
measuring 31–40 mm, 12 measuring 41–50 mm and 15 measuring 51–88
mm.
The results of the multivariate logistic regression
model showed that the risk of malignant lesions receiving a
false-negative diagnosis decreased for every year increase in
patient age [odds ratio (OR), 0.97; P=0.027] and increased with
every millimeter increase in lesion depth (OR, 1.03; P=0.008)
(Table II). The false-negative
rates were 6.9% for a lesion depth of 0 mm, 2.56% for a lesion
depth of 1–10 mm, 10.8% for 11–20 mm, 11.70% for 21–30 mm and
11.67% for a lesion depth >30 mm from the pleural surface.
Additional significant risk factors affecting accuracy
(false-negative diagnosis) included the presence of hemoptysis (OR,
5.06) and atelectasis (OR, 6.53) (Table
II).
False-negative results occurred in 64 malignant
cases (8.06%) (Table I). Among the
64 false-negative cases, a final diagnosis was confirmed by
clinical manifestation of disease in 26 cases (40.6%), by
definitive histology obtained at surgery in 18 cases (28%), by a
secondary biopsy in 15 cases (23.4%) and by bronchofibroscope in 3
cases (4.0%). One case (2%) was confirmed by the detection of
cancer cells in the hydrothorax, and one case was confirmed via
thoracoscopy (2%) (Table III).
 | Table III.Distribution of diagnostic methods
used to confirm the false-negative results. |
Table III.
Distribution of diagnostic methods
used to confirm the false-negative results.
| Confirmation
method | False-negative
rate, n (%) |
|---|
| Clinical
manifestations of disease | 26 (40.6) |
| Surgery | 18 (28.0) |
| Secondary
biopsy | 15 (23.4) |
|
Bronchofibroscope | 3 (4.0) |
| Thoracoscope | 1 (2.0) |
| Cancer cell in
hydrothorax | 1 (2.0) |
| Total | 64 (100) |
Complications of the procedure: Risk
factors affecting pneumothorax
The incidence and distribution of pneumothorax,
hemorrhage and hemoptysis are presented in Table IV. Among the 1,106 lesions biopsied,
207 (18.71%) biopsies caused pneumothorax, 251 biopsies were
associated with hemorrhage and 58 biopsies were associated with
hemoptysis. The risk factors affecting complication rates are
presented in Table V.
 | Table IV.Complications. |
Table IV.
Complications.
|
| Pneumothorax, n
(%) | Hemorrhage, n
(%) | Hemoptysis, n
(%) |
|---|
|
|
|
|
|
|---|
| Parameter | No | Yes | P-value | No | Yes | P-value | No | Yes | P-value |
|---|
| Total, n | 899 | 207 | − | 855 | 251 | − | 1,048 | 58 | − |
| Gender |
|
|
0.060 |
|
|
0.033 |
|
|
0.005 |
|
Male | 604 (79.79) | 153 (20.21) |
| 599 (79.13) | 158 (20.87) |
| 727 (96.04) | 30 (3.96) |
|
|
Female | 295 (84.53) | 54 (15.47) |
| 256 (73.35) | 93 (26.65) |
| 321 (91.98) | 28 (8.02) |
|
| Age, years |
|
|
0.274 |
|
|
0.003 |
|
|
0.265 |
|
≤50 | 195 (82.63) | 41 (17.37) |
| 189 (80.08) | 47 (19.92) |
| 228 (96.61) | 8
(3.39) |
|
|
51–60 | 266 (80.36) | 65 (19.64) |
| 244 (73.72) | 87 (26.28) |
| 308 (93.05) | 23 (6.95) |
|
|
61–70 | 260 (78.79) | 70 (21.21) |
| 243 (73.64) | 87 (26.36) |
| 312 (94.55) | 18 (5.45) |
|
|
>70 | 178 (85.17) | 31 (14.83) |
| 179 (85.65) | 30 (14.35) |
| 200 (95.69) | 9
(4.31) |
|
| Lesion location,
lobe |
|
|
0.004 |
|
|
0.021 |
|
|
0.147 |
| Right
upper | 287 (86.19) | 46 (13.81) |
| 238 (71.47) | 95 (28.53) |
| 311 (93.39) | 22 (6.61) |
|
| Right
middle | 31
(67.39) | 15 (32.61) |
| 38
(82.61) | 8 (17.39) |
| 43
(93.48) | 3
(6.52) |
|
| Right
lower | 184 (76.03) | 58 (23.97) |
| 185 (76.45) | 57 (23.55) |
| 225 (92.98) | 17 (7.02) |
|
| Left
upper | 223 (81.68) | 50 (18.32) |
| 221 (80.95) | 52 (19.05) |
| 263 (96.34) | 10 (3.66) |
|
| Left
lower | 174 (82.08) | 38 (17.92) |
| 173 (81.60) | 39 (18.40) |
| 206 (97.17) | 6
(2.83) |
|
| Lesion type |
|
|
0.121 |
|
|
0.414 |
|
|
0.974 |
|
Central | 224 (84.53) | 41 (15.47) |
| 200 (75.47) | 65 (24.53) |
| 251 (94.72) | 14 (5.28) |
|
|
Peripheral | 675 (80.26) | 166 (19.74) |
| 655 (77.88) | 186 (22.12) |
| 797 (94.77) | 44 (5.23) |
|
| Lesion depth,
mm |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
| 0 | 497 (95.39) | 24 (4.61) |
| 490 (94.05) | 31 (5.95) |
| 503 (96.55) | 18 (3.45) |
|
|
1–10 | 144 (73.85) | 51 (26.15) |
| 174 (89.23) | 21 (10.77) |
| 191 (97.95) | 4
(2.05) |
|
|
11–20 | 149 (68.98) | 67 (31.02) |
| 128 (59.26) | 88 (40.74) |
| 202 (93.52) | 14 (6.48) |
|
|
21–30 | 72
(66.67) | 36 (33.33) |
| 42
(38.89) | 66 (61.11) |
| 95
(87.96) | 13 (12.04) |
|
|
>30 | 37
(56.06) | 29 (43.94) |
| 21
(31.82) | 45 (68.18) |
| 57
(86.36) | 9
(13.64) |
|
| Lesion size,
mm |
|
| <0.001 |
|
|
0.001 |
|
|
0.052 |
|
≤10 | 38
(66.67) | 19 (33.33) |
| 44
(77.19) | 13 (22.81) |
| 55
(96.49) | 2 (3.51) |
|
|
11–20 | 150 (70.42) | 63 (29.58) |
| 156 (73.24) | 57 (26.76) |
| 199 (93.43) | 14 (6.57) |
|
|
21–30 | 213 (78.02) | 60 (21.98) |
| 194 (71.06) | 79 (28.94) |
| 253 (92.67) | 20 (7.33) |
|
|
31–40 | 192 (84.58) | 35 (15.42) |
| 175 (77.09) | 52 (22.91) |
| 213 (93.83) | 14 (6.17) |
|
|
>40 | 306 (91.07) | 30 (8.93) |
| 286 (85.12) | 50 (14.88) |
| 328 (97.62) | 8
(2.38) |
|
| Emphysema |
|
| <0.001 |
|
| <0.001 |
|
|
0.030 |
|
0a | 406 (95.98) | 17 (4.02) |
| 309 (73.05) | 114 (26.95) |
| 392 (92.67) | 31 (7.33) |
|
|
1b | 436 (96.89) | 14 (3.11) |
| 398 (88.44) | 52 (11.56) |
| 435 (96.67) | 15 (3.33) |
|
|
2c | 57
(24.46) | 176 (75.54) |
| 148 (63.52) | 85 (36.48) |
| 221 (94.85) | 12 (5.15) |
|
| Atelectasis |
|
|
0.001 |
|
|
0.001 |
|
|
0.696 |
|
Yes | 104 (92.86) | 8 (7.14) |
| 100 (89.29) | 12 (10.71) |
| 107 (95.54) | 5
(4.46) |
|
| No | 795 (79.98) | 199 (20.02) |
| 755 (75.96) | 239 (24.04) |
| 941 (94.67) | 53 (5.33) |
|
| Position |
|
| <0.001 |
|
|
0.422 |
|
|
0.691 |
|
Lateral | 156 (65.00) | 84 (35.00) |
| 193 (80.42) | 47 (19.58) |
| 230 (95.83) | 10 (4.17) |
|
|
Prone | 497 (84.81) | 89 (15.19) |
| 449 (76.62) | 137 (23.38) |
| 554 (94.54) | 32 (5.46) |
|
|
Supine | 246 (87.86) | 34 (12.14) |
| 213 (76.07) | 67 (23.93) |
| 264 (94.29) | 16 (5.71) |
|
| Needle size, G |
|
| <0.001 |
|
| <0.001 |
|
|
0.488 |
| 16 | 102 (97.14) | 3 (2.86) |
| 98 (93.33) | 7 (6.67) |
| 101 (96.19) | 4
(3.81) |
|
| 18 | 797 (79.62) | 204 (20.38) |
| 757 (75.62) | 244 (24.38) |
| 947 (94.61) | 54 (5.39) |
|
| Number of
attempts |
|
| <0.001 |
|
| <0.001 |
|
| <0.001 |
| ≤2 | 296 (74.75) | 100 (25.25) |
| 262 (66.16) | 134 (33.84) |
| 360 (90.91) | 36 (9.09) |
|
| 3 | 496 (85.37) | 85 (14.63) |
| 485 (83.48) | 96 (16.52) |
| 563 (96.90) | 18 (3.10) |
|
| ≥4 | 107 (82.95) | 22 (17.05) |
| 108 (83.72) | 21 (16.28) |
| 125 (96.90) | 4
(3.10) |
|
| Needle-pleura
angle, degrees |
|
|
0.632 |
|
|
0.900 |
|
|
0.064 |
|
≤15 | 746 (80.82) | 177 (19.18) |
| 714 (77.36) | 209 (22.64) |
| 877 (95.02) | 46 (4.98) |
|
|
16–30 | 91
(82.73) | 19 (17.27) |
| 86
(78.18) | 24 (21.82) |
| 106 (96.36) | 4
(3.64) |
|
|
>30 | 62
(84.93) | 11 (15.07) |
| 55
(75.34) | 18 (24.66) |
| 65
(89.04) |
8 (10.96) |
|
| Hemostatic |
|
| <0.001 |
|
| <0.001 |
|
|
0.381 |
|
Yes | 40
(60.61) | 26 (39.39) |
| 38
(57.58) | 28 (42.42) |
| 61
(92.42) | 5
(7.58) |
|
| No | 859 (82.60) | 181 (17.40) |
| 817 (78.56) | 223 (21.44) |
| 987 (94.90) | 53 (5.10) |
|
 | Table V.Statistical analysis of risk factors
for complications (pneumothorax, hemorrhage and hemoptysis). |
Table V.
Statistical analysis of risk factors
for complications (pneumothorax, hemorrhage and hemoptysis).
| Risk
factor/reference | OR (95% CI) | P-value |
|---|
| Pneumothorax |
|
|
| Lesion size/≤10
mm |
|
|
|
11–20 | 1.25
(0.46–3.37) | 0.662 |
|
21–30 | 0.64
(0.23–1.73) | 0.377 |
|
31–40 | 0.48
(0.17–1.35) | 0.162 |
|
>40 | 0.32
(0.11–0.95) | 0.040a |
|
Emphysema/0b |
|
|
|
1c | 1.00
(0.47–2.12) | 0.999 |
|
2d |
72.11
(37.35–139.22) |
<0.001a |
| Hemorrhage |
|
|
| Age/≤50 years |
|
|
|
51–60 | 1.68
(1.03–2.75) | 0.038a |
|
61–70 | 1.75
(1.05–2.90) | 0.031a |
|
>70 | 0.99
(0.53–1.82) | 0.961 |
| Lesion
location/right upper lobe |
|
|
| Right
middle lobe | 0.39
(0.15–1.00) | 0.050 |
| Right
lower lobe | 0.54
(0.33–0.88) | 0.014a |
| Left
upper lobe | 0.61
(0.38–0.99) | 0.044 |
| Left
lower lobe | 0.48
(0.29–0.80) | 0.005a |
| Lesion depth/0
mm |
|
|
|
1–10 | 1.62
(0.88–2.98) | 0.122 |
|
11–20 | 9.56
(5.83–15.68) |
<0.001a |
|
21–30 | 24.49
(13.36–44.87) |
<0.001a |
|
>30 | 31.51
(15.22–65.24) |
<0.001a |
|
Emphysema/0b |
|
|
|
1c | 0.55
(0.35–0.87) | 0.010a |
|
2d | 0.51
(0.33–0.80) | 0.004a |
| Hemoptysis |
|
|
| Gender/female | 0.55
(0.31–0.96) | 0.036a |
| Lesion depth/0
mm |
|
|
|
1–10 | 0.50
(0.16–1.53) | 0.226 |
|
11–20 | 1.70
(0.79–3.66) | 0.174 |
|
21–30 | 3.10
(1.35–7.12) | 0.008a |
|
>30 | 3.85
(1.49–10.00) | 0.006a |
| Number of
attempts/≤2 |
|
|
| 3 | 0.40
(0.21–0.73) | 0.003a |
| ≥4 | 0.42
(0.14–1.25) | 0.118 |
Table IV shows that
lesion location, depth and size, as well as emphysema, atelectasis,
position, needle size, number of attempts and hemostasis, had
significant effects on the incidence of pneumothorax. The 207 cases
of pneumothorax included 192 mild, 11 moderate and 4 severe
pneumothoraces that required chest tube drainage.
A higher frequency of pneumothorax was observed in
patients with CT evidence of emphysema (75.54%) in the needle tract
(Table IV). The pneumothorax rate
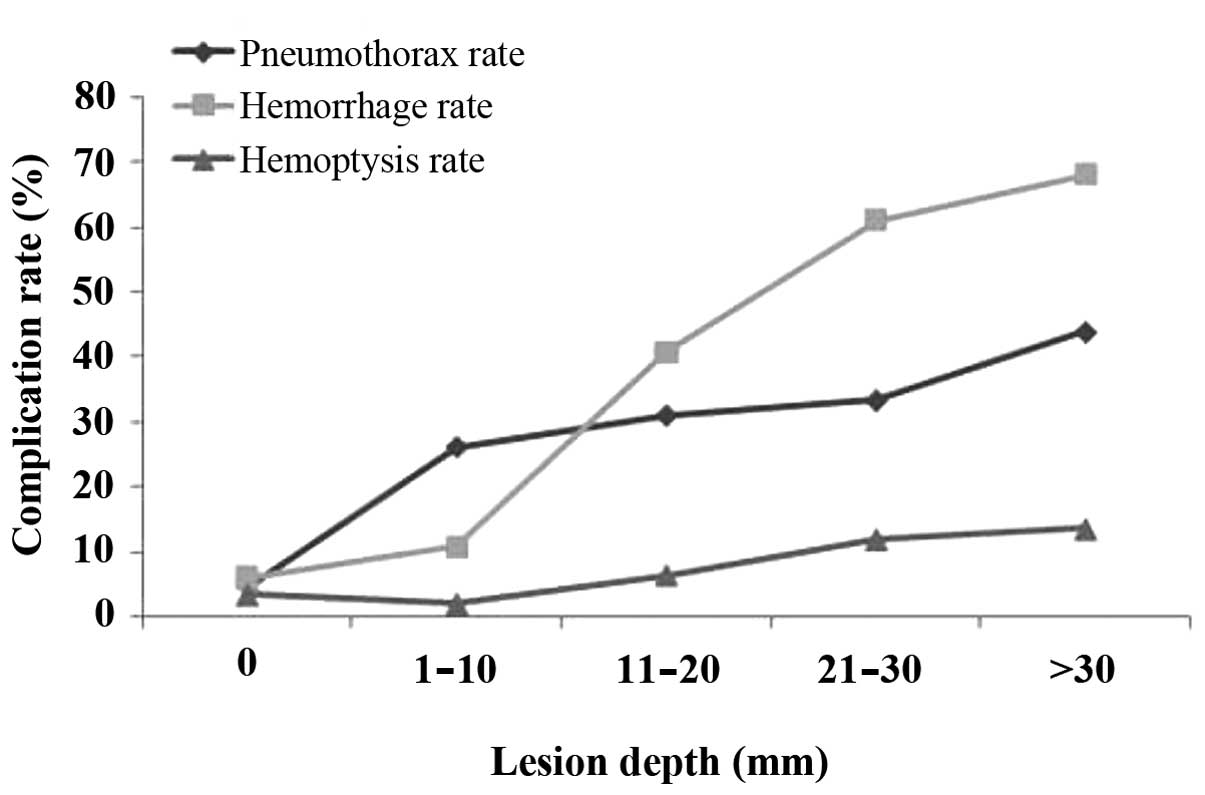
increased with increasing lesion depth. The pneumothorax rate was
4.61, 26.15, 31.02, 33.33 and 43.94% for the five groups of lesion
depth (0, 1–10, 11–20, 21–30 and ﹥30 mm, respectively) (Fig. 4).
Increasing lesion size >10 mm was inversely
correlated with the incidence of pneumothorax. In the five groups
of lesion size (≤10, 11–20, 21–30, 31–40 and ﹥40 mm), the
pneumothorax rate was 33.33, 29.58, 21.98, 15.42 and 8.93%,
respectively (Table IV).
From multivariate logistic regression analysis, only
lesion size and emphysema appeared to significantly affect the
incidence of pneumothorax (P<0.05; Table V). The odds of pneumothorax were
significantly reduced in subjects with a lesion size of >40 mm
(OR, 0.32; P=0.04) compared with those in subjects with a lesion
size of <10 mm and were significantly higher in subjects with
prominent emphysemic tissue in the needle tract (OR, 72.11;
P<0.001) (Table V) compared with
those in subjects with no emphysemic tissue surrounding the
lesions. As shown in Fig. 4, the
pneumothorax rates increased with increasing lesion depth
(P<0.001), as did the rates of hemorrhage and hemoptysis
(P<0.001).
Complications of the procedure: Risk
factors affecting hemorrhage
A total of 251 biopsies (22.7%) caused perifocal and
needle track hemorrhages, including 193 mild, 50 moderate and 8
severe, of which 52 were hemopneumothoraces. Gender, age,
emphysema, atelectasis, needle size, number of attempts and
hemostasis, as well as lesion location, depth and size, appeared to
have a significant effect on the incidence of hemorrhage (Table IV). Lesions >10 mm in depth were
associated with an increased incidence of hemorrhage (Table IV). Lesions in the right upper lobe
were more prone to hemorrhage compared with those in other
locations (28.53%, Table IV). The
hemorrhage rate in the group that had emphysemic tissue in the
needle tract (36.48%) was markedly increased compared with the
bleeding rate from the needle pathway without emphysema (26.95%)
(Table IV). Based on multivariate
logistic regression analysis, age, lesion location and depth and
emphysema all had a significant effect on the incidence of
hemorrhage (P<0.05). The odds of hemorrhage were significantly
higher in patients in the 61–70-year-old group (OR, 1.75; P=0.031)
compared with those in patients aged ≤50 years and were
significantly lower in those with lesions located in lobes other
than the right upper lobe (all P<0.05). The odds of hemorrhage
were significantly higher in subjects with lesion depths of 11–20,
21–30 and >30 mm (ORs of 9.56, 24.49 and 31.51, respectively;
P<0.001) compared with those in subjects with a lesion depth of
0 mm. Multivariate analysis, following adjustment for confounding
factors, showed that the odds of hemorrhage were significantly
reduced in lesions with some or prominent emphysemic tissue
compared with those in lesions without surrounding emphysemic
tissue (ORs of 0.55 and 0.51, respectively; P=0.010 and 0.004,
respectively; Table V).
Complications of the procedure: Risk
factors affecting hemoptysis
The hemoptysis rate was 5.2% (Table IV). Gender, lesion depth, emphysema
and number of attempts appeared to have a significant effect on the
incidence of hemoptysis (Table IV).
Based on multivariate logistic regression analysis, gender, lesion
depth and number of biopsy attempts had a significant effect on the
incidence of hemoptysis (P<0.05). The odds of hemoptysis were
significantly lower in males compared with those in females (OR,
0.55; P=0.036); were significantly higher in subjects with lesion
depths of 21–30 and >30 mm compared with those in patients with
a lesion depth of 0 mm (ORs of 3.1 and 3.85, respectively; P=0.008
and 0.006, respectively); and were significantly lower in subjects
with 3 biopsy attempts compared with those in subjects with ≤2
attempts (OR, 0.4; P=0.003; Table
V).
Total ED and needle dwell time
The total mean ED was 0.54±0.14 mSv (range,
0.21–0.95 mSv). The mean duration of the procedure was 16±2 min and
the mean time spent by the needle in the parenchyma (mean needle
dwell time) was 5±3 sec (range, 3–8 sec) per biopsy. A total of 396
patients (35.8%) each received 2 biopsy attempts and 581 patients
(52.53%) each received 3 attempts (Table
I).
Discussion
Low-dose, CT-guided ACNB of pulmonary lesions with
EPL has high diagnostic accuracy and improved safety compared with
conventional CT-guided biopsy. The sensitivity, specificity,
positive predictive value and negative predictive value of the EPL
method were 91.9, 100, 100 and 82.9%, respectively, and showed
improvements compared with the values obtained in previous studies
(13,18–24). The
overall diagnostic accuracy of EPL in the present study was 94.2%.
Results of multivariate analysis demonstrated that significant risk
factors affecting accuracy included younger age, atelectasis,
hemoptysis and lesion depth (all P<0.03). Younger age (≤50 years
old) has not been investigated in previously published studies as a
risk factor affecting accuracy and is, therefore, a novel finding.
Among the 1,106 lesions biopsied, 207 resulted in pneumothorax, 251
in hemorrhage and 58 in hemoptysis. Lesion size and emphysema were
demonstrated to exert a significant influence on the incidence of
pneumothorax. Age, lesion location and depth and emphysema
significantly affected the incidence of hemorrhage. In addition,
gender, lesion depth and number of attempts had a significant
effect on the incidence of hemoptysis.
With regard to the risk factors affecting accuracy,
the results of multivariate analysis showed that significant risk
factors affecting accuracy included younger age, atelectasis,
hemoptysis and lesion depth (all P<0.03). Younger age (≤50 years
old) has not previously been reported in the literature as a risk
factor affecting accuracy and is, therefore, a novel finding. To
the best of our knowledge, this is the first study to show a
correlation between younger age and diagnostic accuracy. Other
studies have investigated the effect of age on accuracy using
various lung biopsy techniques (26,32,33). All
three of these studies, however, failed to find any correlation
between accuracy and age (26,32,33). The
reason for the discrepancy between previously published results and
the present findings may be associated with the high false-negative
rate in the younger patients in the malignant group. This high
false-negative rate may have been due to the increased inflammation
and necrosis in the tumors of younger patients, which increased the
difficulty of confirming a malignant diagnosis. Among the 15
false-negative cases in the malignant group of patients aged ≤50
years, 4 cases exhibited obvious necrosis and 3 cases exhibited a
combination of inflammation and necrosis. The final diagnoses
included 8 cases of lung cancer, 5 cases of metastatic tumor, one
malignant lesion and one case of malignant fibrous histiocytoma
(data not shown).
The effect of atelectasis on accuracy may have been
due to difficulty in distinguishing lesions from atelectasis, as
the majority of lesions complicated with atelectasis were of a
central type and exhibited necrosis. In addition, enhancement on CT
scan appeared to exert no effect on diagnostic accuracy in the
present study. The effect of hemoptysis on accuracy may have been
due to the fact that the procedure was terminated due to the risk
of air embolism if the patients developed serious cough or
hemoptysis (25). Finally, it
appeared that diagnostic accuracy decreased in proportion to
increasing lesion depth >10 mm; this finding was expected, as
deeper lesions are more difficult to biopsy.
The EPL method exhibits an acceptable complication
rate. Among the 1,106 lesions biopsied, 207 biopsies (18.71%) led
to pneumothorax. The EPL method appears to reduce damage to the
visceral pleura, which may partly explain the reduced pneumothorax
rate in the present study compared with rates reported using other
techniques (13–15,19–21).
Furthermore, the pneumothorax rate was relatively low despite
multiple needle passes, which may have been due to the breath-hold
training undergone by the patients at the beginning of the
procedure.
As reported in previous studies, deeper lesions were
associated with a higher rate of pneumothorax (10,14,34). The
pneumothorax rates in the present study increased with increasing
lesion depth (P<0.001). Furthermore, the pneumothorax rate in
the present study was elevated in patients with emphysemic tissue
surrounding the lesions, which increased the possibility of
pulmonary vascular tearing, while it remained relatively low in the
group without emphysema, younger patients or lesions situated in
superficial parts.
For conventional CT-guided ACNB, the incidence of
pulmonary hemorrhage reported in the literature varies between 4
and 42%, and the hemoptysis rate varies between 2 and 25% (13,14,18,19,22,24,28). In
the present study, 251 biopsies (22.69%) were associated with
hemorrhage and 58 biopsies (5.24%) were associated with hemoptysis
(Table IV). Few reports have
described an association between lesion location and hemorrhage
(35,36). Analysis of the present data showed
that right upper lobe lesions had a higher rate of hemorrhage;
however, this finding may have been due to random error.
Furthermore, the rates of hemorrhage and hemoptysis increased
significantly with increasing lesion depth (P<0.001). The risk
of hemorrhage was significantly lower in lesions with some or
prominent emphysemic tissue compared with that in lesions with no
surrounding emphysemic tissue (OR, 0.55 and 0.51, respectively;
Table V). The risk of hemoptysis was
also significantly lower in subjects with 3 biopsy attempts
compared with that in patients that underwent ≤2 attempts, which
may have been due to the fact that fewer deep-lesion biopsy
attempts were made than superficial-lesion biopsy attempts
(Table VI). Biopsy duration can
increase if the surgeon consideres more tissue biopsy specimens are
required, which can result in a relatively high rate of bleeding.
In addition, the administration of hemostatic drugs can reduce the
rate of hemoptysis.
 | Table VI.Number of biopsy attempts required
for each lesion depth range. |
Table VI.
Number of biopsy attempts required
for each lesion depth range.
|
| Number of biopsy
attempts, n (%) |
|
|---|
|
|
|
|
|---|
| Lesion depth,
mm | ≤2 | 3 | ≥4 | P-value |
|---|
| 0 | 9
(50.00) | 9
(50.00) | 0 (0.00) | 0.003 |
| 1–10 | 2
(50.00) | 2
(50.00) | 0 (0.00) |
|
| 11–20 | 5
(35.71) | 5
(35.71) | 4
(28.57) |
|
| 21–30 | 12 (92.31) | 1 (7.69) | 0 (0.00) |
|
| >30 | 8
(88.89) | 1
(11.11) | 0 (0.00) |
|
The present study contained several limitations,
including its retrospective nature and the fact that the study was
conducted at a single hospital and included primarily inpatients.
In addition, hemocoagulase was used in patients with a high risk of
bleeding prior to surgery, as it has been demonstrated to reduce
bleeding from our clinical observations, particularly the incidence
of hemoptysis. The injection of hemocoagulase was not randomized or
controlled and was used only in certain cases. Thus, the
inconsistent application of hemocoagulase may have affected the
assessment of hemorrhage and hemoptysis (i.e., without the benefit
of a randomized control, it may have affected the statistical
results). As this study was retrospective, this limitation could
not be altered.
In conclusion, the present EPL method for performing
CT-guided ACNB is a novel technique that has been described in one
previous study in the literature (24). This EPL method has been demonstrated
to be a safe, fast and accurate diagnostic method with reduced
dwell time compared with conventional techniques. The EPL method
maintains the needle tip outside the visceral pleural prior to
biopsy. Consequently, the time spent by the needle within the
parenchyma (dwell time) is significantly decreased compared with
that associated with conventional methods (2,19,22,27).
In addition, the entire procedure, on average, has a duration of
16±2 min, and requires a lower dose of radiation compared with
those employed in previous studies (37,38). The
results of the multivariate analysis indicated that significant
risk factors affecting accuracy included younger age, atelectasis,
hemoptysis and lesion depth (P<0.03). To the best of our
knowledge, younger age (≤50 years old) has not been previously
reported in the literature as a risk factor affecting accuracy and
is therefore a novel observation.
Acknowledgements
The present study was supported by the Key
Foundation of Hubei Natural Science Funds (no. 2012FFB04414).
Glossary
Abbreviations
Abbreviations:
|
ACNB
|
automated cutting needle lung
biopsy
|
|
EPL
|
extrapleural locating
|
|
CT
|
computed tomography
|
|
OR
|
odds ratio
|
|
ED
|
effective dose
|
|
DLP
|
dose-length product
|
References
|
1
|
Wallace MJ, Krishnamurthy S, Broemeling
LD, Gupta S, Ahrar K, Morello FA Jr and Hicks ME: CT-guided
percutaneous fine-needle aspiration biopsy of small (≤1 cm)
pulmonary lesions. Radiology. 225:823–828. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laurent F, Latrabe V, Vergier B, Montaudon
M, Vernejoux JM and Dubrez J: CT-guided transthoracic needle biopsy
of pulmonary nodules smaller than 20 mm: Results with an automated
20-gauge coaxial cutting needle. Clin Radiol. 55:281–287. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guimaraes MD, de Andrade MQ, da Fonte AC,
Chojniak R and Gross JL: CT-guided cutting needle biopsy of lung
lesions - an effective procedure for adequate material and specific
diagnose. Eur J Radiol. 80:e488–e490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu RH, Tzeng WS, Lee WJ, Chang SC, Chen
CH, Fung JL, Wang YJ and Mak CW: CT-guided transthoracic cutting
needle biopsy of intrathoracic lesions, comparison between coaxial
and single needle technique. Eur J Radiol. 81:e712–e716. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hiraki T, Mimura H, Gobara H, Iguchi T,
Fujiwara H, Sakurai J, Matsui Y, Inoue D, Toyooka S, Sano Y and
Kanazawa S: CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions
performed with 20-gauge coaxial cutting needles: Diagnostic yield
and risk factors for diagnostic failure. Chest. 136:1612–1617.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geraghty PR, Kee ST, McFarlane G, Razavi
MK, Sze DY and Dake MD: CT-guided transthoracic needle aspiration
biopsy of pulmonary nodules: Needle size and pneumothorax rate.
Radiology. 229:475–481. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta S, Krishnamurthy S, Broemeling LD,
Morello FA Jr, Wallace MJ, Ahrar K, Madoff DC, Murthy R and Hicks
ME: Small (</=2-cm) subpleural pulmonary lesions: Short-versus
long-needle-path CT-guided Biopsy - comparison of diagnostic yields
and complications. Radiology. 234:631–637. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haramati LB and Aviram G: What constitutes
effective management of pneumothorax after CT-guided needle biopsy
of the lung? Chest. 121:1013–1015. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klein JS, Salomon G and Stewart EA:
Transthoracic needle biopsy with a coaxially placed 20-gauge
automated cutting needle: Results in 122 patients. Radiology.
198:715–720. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saji H, Nakamura H, Tsuchida T, Tsoboi M,
Kawate N, Konaka C and Kato H: The incidence and the risk of
pneumothorax and chest tube placement after percutaneous CT-guided
lung biopsy: The angle of the needle trajectory is a novel
predictor. Chest. 121:1521–1526. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beşir FH, Altin R, Kart L, Akkoyunlu M,
Ozdemir H, Ornek T and Gündoğdu S: The results of computed
tomography guided tru-cut transthoracic biopsy: Complications and
related risk factors. Wien Klin Wochenschr. 123:79–82. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan MF, Straub R, Moghaddam SR, Maataoui
A, Gurung J, Wagner TO, Ackermann H, Thalhammer A, Vogl TJ and
Jacobi V: Variables affecting the risk of pneumothorax and
intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur
Radiol. 18:1356–1363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW,
Cheung YC and Chou AS: Risk factors of pneumothorax and bleeding:
Multivariate analysis of 660 CT-guided coaxial cutting needle lung
biopsies. Chest. 126:748–754. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ko JP, Shepard JO, Drucker EA, Aquino SL,
Sharma A, Sabloff B, Halpern E and McLoud TC: Factors influencing
pneumothorax rate at lung biopsy: Are dwell time and angle of
pleural puncture contributing factors? Radiology. 218:491–496.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yildirim E, Kirbas I, Harman A, Ozyer U,
Tore HG, Aytekin C and Boyvat F: CT-guided cutting needle lung
biopsy using modified coaxial technique: factors effecting risk of
complications. Eur J Radiol. 70:57–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Charig MJ and Phillips AJ: CT-guided
cutting needle biopsy of lung lesions-safety and efficacy of an
out-patient service. Clin Radiol. 55:964–969. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Topal U and Berkman YM: Effect of needle
tract bleeding on occurrence of pneumothorax after transthoracic
needle biopsy. Eur J Radiol. 53:495–499. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaura H, Inaba Y, Arai Y, Matsueda K and
Hatooka S: Massive intrathoracic haemorrhage after CT-guided lung
biopsy. Br J Radiol. 73:1105–1107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Laurent F, Michel P, Latrabe V, de Lara M
Tunon and Marthan R: Pneumothoraces and chest tube placement after
CT-guided transthoracic lung biopsy using a coaxial technique:
Incidence and risk factors. AJR Am J Roentgenol. 172:1049–1053.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Topal U and Ediz B: Transthoracic needle
biopsy: Factors effecting risk of pneumothorax. Eur J Radiol.
48:263–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chakrabarti B, Earis JE, Pandey R, Jones
Y, Slaven K, Amin S, McCann C, Jones PL, Thwaite E, Curtis JM and
Warburton CJ: Risk assessment of pneumothorax and pulmonary
haemorrhage complicating percutaneous co-axial cutting needle lung
biopsy. Respir Med. 103:449–455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsukada H, Satou T, Iwashima A and Souma
T: Diagnostic accuracy of CT-guided automated needle biopsy of lung
nodules. AJR Am J Roentgenol. 175:239–243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei YH, Liao MY and Xu LY: Diagnostic
value of CT-guided extrapleural locating transthoracic automated
cutting needle biopsy of lung lesions. Zhong Hua Zhong Liu Za Zhi.
33:473–475. 2011.(In Chinese).
|
|
24
|
Liao MY, Zhou YF, Tian ZX, Luo R, Qu YJ
and Xu LY: The factor analysis of the incidence of complication in
CT-guided lung automated cutting needle biopsy with extrapleural
locating method. Zhong Hua Yi Xue Za Zhi. 90:1747–1751. 2010.(In
Chinese).
|
|
25
|
Burbank F, Kaye K, Belville J, Ekuan J and
Blumenfeld M: Image-guided automated core biopsies of the breast,
chest, abdomen and pelvis. Radiology. 191:165–171. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Montaudon M, Latrabe V, Pariente A,
Corneloup O, Begueret H and Laurent F: Factors influencing accuracy
of CT-guided percutaneous biopsies of pulmonary lesions. Eur
Radiol. 14:1234–1240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Funk C, Gmür J, Herold R and Straub PW:
Reptilase-R - a new reagent in blood coagulation. Br J Haematol.
21:43–52. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kinoshita F, Kato T, Sugiura K, Nishimura
M, Kinoshita T, Hashimoto M, Kaminoh T and Ogawa T: CT-guided
transthoracic needle biopsy using a puncture site-down positioning
technique. AJR Am J Roentgenol. 187:926–932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dennie CJ, Matzinger FR, Marriner JR and
Maziak DE: Transthoracic needle biopsy of the lung: Results of
early discharge in 506 outpatients. Radiology. 219:247–251. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watanuki Y, Suzuki S, Nishikawa M,
Miyashita A and Okubo T: Correlation of quantitative CT with
selective alveolobronchogram and pulmonary function tests in
emphysema. Chest. 106:806–813. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McCullough C, Cody D, Edyvean S, Geise R,
Gould B, Keat N, Huda W, Judy P, Kalender W, McNitt-Gray M, et al:
The measurement, reporting, and management of radiation dose in CT.
Report of the AAPM Task Group 23 of the Diagnostic Imaging Council
CT CommitteeAmerican Association of Physicists in Medicine. College
Park, MD, USA: pp. 1–8. 2008
|
|
32
|
Choi JW, Park CM, Goo JM, Park YK, Sung W,
Lee HJ, Lee SM, Ko JY and Shim MS: C-arm cone-beam CT-guided
percutaneous transthoracic needle biopsy of small (≤20 mm) lung
nodules: Diagnostic accuracy and complications in 161 patients. AJR
Am J Roentgenol. 199:W322–W330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heyer CM, Reichelt S, Peters SA, Walther
JW, Müller KM and Nicolas V: Computed tomography-navigated
transthoracic core biopsy of pulmonary lesions: Which factors
affect diagnostic yield and complication rates? Acad Radiol.
15:1017–1026. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yeow KM, See LC, Lui KW, Lin MC, Tsao TC,
Ng KF and Liu HP: Risk factors for pneumothorax and bleeding after
CT-guided percutaneous coaxial cutting needle biopsy of lung
lesions. J Vasc Interv Radiol. 12:1305–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nour-Eldin NE, Alsubhi M, Naguib NN,
Lehnert T, Emam A, Beeres M, Bodelle B, Koitka K, Vogl TJ and
Jacobi V: Risk factor analysis of pulmonary hemorrhage complicating
CT-guided lung biopsy in coaxial and non-coaxial core biopsy
techniques in 650 patients. Eur J Radiol. 83:1945–1952. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rizzo S, Preda L, Raimondi S, Meroni S,
Belmonte M, Monfardini L, Veronesi G and Bellomi M: Risk factors
for complications of CT-guided lung biopsies. Radiol Med.
116:548–563. 2011.(In English and Italian). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smith JC, Jin DH, Watkins GE, Miller TR,
Karst JG and Oyoyo UE: Ultra-low-dose protocol for CT-guided lung
biopsies. J Vasc Interv Radiol. 22:431–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heyer CM, Lemburg SP, Kagel T, Mueller KM,
Nuesslein TG, Rieger CH and Nicolas V: Evaluation of chronic
infectious interstitial pulmonary disease in children by low-dose
CT-guided transthoracic lung biopsy. Eur Radiol. 15:1289–1295.
2005. View Article : Google Scholar : PubMed/NCBI
|