Introduction
Osteoarthritis (OA) is a chronic joint disease,
which occurs most often among older people and affects the whole
joint structure. Although the details of the pathogenesis of OA are
unclear, pathological changes are mainly observed in cartilage,
subchondral bone (SB), the articular capsule and synovial membrane
(1). OA has traditionally been
considered a disease of cartilage; however, studies have
demonstrated that the SB plays an important role in the disease
(2–6), through the strong effect it has on
shock absorption and cartilage metabolism, for example (7). Furthermore, the alterations in the SB,
which are commonly involved in SB deterioration and sclerosis, may
occur during or following cartilage changes, and can contribute to
cartilage degradation in animal models (2,6,8). SB alterations are therefore closely
associated with the pathogenesis, initiation and progression of
OA.
In addition to SB alterations, cytokines are also
involved in the pathogenesis of OA. Catabolic cytokine tumor
necrosis factor-α (TNF-α) and interleukin-1 (IL-1), in particular,
can modulate cartilage degradation and synovial inflammation.
TNF-α, as one of the major inflammatory mediators during the
development of OA, has been found to be increased in osteoarthritic
SB during the inflammatory process associated with the pathology of
OA (9,10). Furthermore, TNF-α has been
demonstrated to injure osteoblasts (OBs) through the inhibition of
cell proliferation and differentiation, as well as through the
induction of apoptosis (11–14). These studies indicate that TNF-α may
also be involved in the SB alterations that occur during the
pathogenesis of OA.
Under pathological conditions, abnormal SB
remodeling is the main cause of SB alterations, and therefore,
regulating SB metabolism could slow down the progress of OA. OBs,
which form major parts of the SB framework, are responsible for
bone formation in the process of SB remodeling. Notably, SB OBs in
patients with OA exhibit abnormal metabolism (15,16),
suggesting that the regulation of the osteogenic ability of OBs
could contribute to SB metabolism.
Tougu Xiaotong capsule (TXC), which consists of
Radix Morindae Officinalis, Radix Paeoniae Alba, Rhizoma Ligusticum
Wallichii and Herba Sarcandrae Glabrae (17), has been used to treat OA in the
Second People's Hospital Affiliated to Fujian University of
Traditional Chinese Medicine (Fujian, China) for more than two
decades. In our previous studies we found that TXC was able to
reduce the rate and change the pattern of SB remodeling, leading to
a reduction in SB sclerosis (18,19);
however, the molecular mechanism of the effect of TXC on SB
remodeling remains unknown. A TNF-α-injured OB-like cell line was
therefore used to evaluate the protective effects of TXC on cell
proliferation and differentiation and investigate the underlying
mechanisms by which TXC affects SB remodeling in OA.
Materials and methods
Reagents
Eagle's minimum essential medium, α modification
(α-MEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS)
and 0.25% trypsin − 0.02% EDTA were HyClone products (GE
Healthcare, Logan, UT, USA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
from Sigma Group Inc. (Cream Ridge, NJ, USA) and rat TNF-α was from
PeproTech, Inc. (London, UK). Propidium iodide (PI), Hoechst 33258
and 4′,6-diamidino-2-phenylindole were purchased from MP
Biomedicals (Santa Ana, CA, USA), alizarin red S from Shanghai
Sangon Biological Engineering Technology & Services Co., Ltd.
(Shanghai, China)., dimethyl sulfoxide (DMSO) from Shanghai
Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and
tetracycline ointment from Beijing Solarbio Science &
Technology Co., Ltd. (Beijing, China). The alkaline phosphatase
(ALP) test kit and osteocalcin (OCN) ELISA test kit were obtained
from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China).
Cell culture
The OB-like cell line (UMR-106) was obtained from
the type culture collection of the Chinese Academy of Sciences
(Shanghai, China). Briefly, cells were cultured in α-MEM containing
10% FBS supplemented with 1% penicillin/streptomycin at 37°C in a
humidified atmosphere containing 5% CO2. Once the cells
had reached ~80% confluence, they were detached using 0.25% trypsin
− 0.02% EDTA, and diluted at 1:3.
TXC testing
TXC was obtained from the Second People's Hospital
Affiliated to Fujian University of Traditional Chinese Medicine
(Approval no. Ming Zhizi Z20100006). A 30 mg/ml stock solution of
TXC was prepared by dissolving TXC power in PBS and storing it at
−20°C. The working concentrations of TXC were diluted in the cell
culture medium.
Cell viability assay
Cells were seeded into 96-well plates at a density
of 1×104 cells/well in 0.1 ml medium, and then treated
with 30 ng/ml TNF-α as previously described (20,21) or
30 ng/ml TNF-α plus various concentrations of TXC (0.625, 1.25 and
2.5 mg/ml) for 24 h. Subsequently, 100 µl 0.5 mg/ml MTT solution
was added to each well and the plates were incubated at 37°C for 4
h; the purple-blue MTT formazan precipitate was then dissolved in
150 µl DMSO. Finally, the absorbance was measured at 570 nm using
an ELISA reader (ELX800; BioTek Instruments, Inc., Winooski, VT,
USA).
Observation and analysis of cell
death
A total of 1×105 cells were seeded into
35-mm-diameter Petri dishes in 1 ml medium, and treated with 30
ng/ml TNF-α or 30 ng/ml TNF-α plus 1.25 mg/ml TXC for 24 h; at the
end of the treatment, the live cells were stained by incubating
with 10 µg/ml Hoechst 33258 solution at 37°C for 30 min in the
dark, while the dead cells were stained by incubating with 10 µg/ml
PI at 37°C for another 10 min. The staining solution was then
discarded and, following supplementation with 0.1 mM PBS, the
stained cells were examined under a fluorescence microscope (EVOS™
FL; Life Technologies, Carlsbad, CA, USA) and further observed
using a laser scanning confocal microscope (LSCM; LSM 710; Zeiss,
Oberkochen, Germany). All images were captured at a magnification
of ×200 and the cellular mortality was analyzed using the ZEN 2009
Light Edition imaging analysis system (Zeiss).
Cell ultrastructure evaluation
The cellular ultrastructure was observed using
transmission electronic microscope (TEM; H-7650; Hitachi, Ltd.,
Tokyo, Japan). Cells were seeded into 6-well plates at a density of
2×105 cells/well in 2 ml medium, and treated with 30
ng/ml TNF-α or 30 ng/ml TNF-α plus 1.25 mg/ml TXC for 24 h; the
cells were then scratched with 200 µl 2.5% glutaraldehyde in 1 ml
fresh medium, collected and adjusted to the concentration of
1×106 cells/ml. The cells were then pre-fixed in 2.5%
glutaraldehyde for 24 h at 4°C, rinsed with 0.1 mM PBS 3 times,
post-fixed in 1% osmic acid and 1.5% potassium hexacyanoferrate(II)
for 1.5 h and then rinsed with 0.1 mM PBS three more times. Next,
the cells were stained with uranyl acetate-saturated 70% ethanol
overnight at 4°C, dehydrated in ethanol and acetone solution of
ascending concentrations and embedded in epoxide resin 618.
Furthermore, the embedded blocks were ultrasectioned into 90-nm
sections and stained with uranyl acetate and lead citrate for 10
min, respectively. Finally, the stained ultrasections were observed
using TEM at 80 kV.
Assay of ALP activity and OCN
secretion
Cells were seeded into 24-well plates at a density
of 1×105 cells/well in 1 ml medium, and then treated
with 10 ng/ml TNF-α as previously described (22) or 10 ng/ml TNF-α plus 1.25 mg/ml TXC
for 14 days. The supernatant was collected every 2 days and stored
at −80°C until it was finally analyzed using the ALP assay kit with
colorimetric measurement using a semi-automatic biochemical
analyzer (BA-88A; Mindray, Shenzhen, China). The secretion of OCN
was evaluated using the ELISA kit, according to the manufacturer's
instructions; briefly, the OCN standard and samples were added to
the antibody-coated 96 wells, and incubated at 37°C for 1 h;
following repeated washes, the substrate was added to each well at
37°C for 15 min; finally the absorbance was measured at 450 nm
using the ELISA reader (BioTek Instruments, Inc.).
Optical morphology of mineralized
nodules
Two methods, alizarin red S and tetracycline
staining, were used to observe mineralized nodule formation. Cells
were seeded into 6-well plates at a density of 2×105
cells/well in 2 ml medium, or medium containing 50 µg/ml
tetracycline. After treating with TNF-α (10 ng/ml) or TNF-α plus
TXC (10 ng/ml + 1.25 mg/ml, respectively) for 14 days, the cells
were fixed with 4% paraformaldehyde at room temperature for 15 min,
rinsed with 0.1 mM PBS and stained with alizarin red S at 37°C for
30 min. The stained cells were observed using a phase contrast
microscope (Eclipse TS-100F; Nikon Corporation, Tokyo, Japan) and
the images were captured at a magnification of ×40. Tetracycline
staining was observed using an LSCM (Zeiss) and the images were
captured at a magnification of ×100.
Calcium content of mineralized
nodules
The calcium content was observed and analyzed using
a scanning electron microscope (SEM; Hitachi TM3000; Hitachi, Ltd.,
Tokyo, Japan). A total of 2×105 cells were seeded into
6-well plates, and treated with TNF-α (10 ng/ml) or TNF-α (10 ng/ml
+ 1.25 mg/ml, respectively) plus TXC for 14 days. The treated cells
were pre-fixed in 2.5% glutaraldehyde for 24 h at 4°C, rinsed with
0.1 mM PBS three times and washed with distilled water for 30 sec,
then immediately post-fixed in 1% osmic acid and 1.5% potassium
hexacyanoferrate(II) at 4°C for 1.5 h and rinsed with 0.1 mM PBS
three more times. Subsequently, the rinsed cells were washed with
distilled water for 30 sec, and then dehydrated in
tert-butyl alcohol solution of ascending concentrations.
They were then subjected to vacuum drying and were sputter-coated
with platinum using IB-5 ion sputtering equipment (Eiko, Co., Ltd.,
Hitachinaka, Japan). Finally, mineralized nodules were observed
using the SEM and the images were captured at a magnification of
×400. An energy dispersive spectroscopy (EDS; Quantax 70; Bruker,
Berlin, Germany) analysis was performed in order to further examine
the calcium content of mineralized nodules at a magnification of
×5,000.
Statistical analysis
GraphPad Prism® 6 for Windows (GraphPad Software,
Inc., La Jolla, CA, USA). was used for the statistical analysis.
All quantitative data are expressed as the mean ± standard
deviation. Statistical analysis among different groups was carried
out with the paired t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
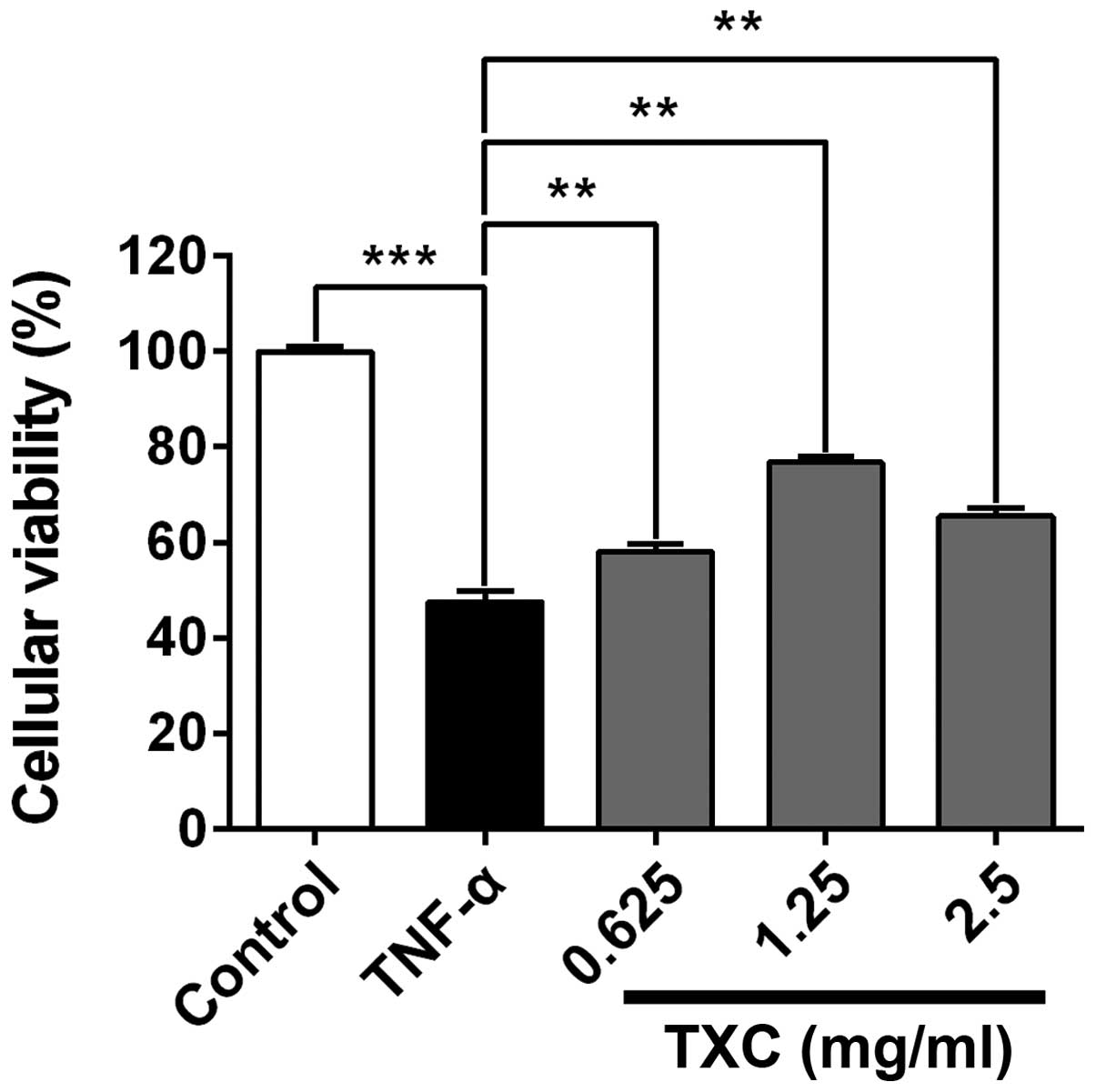
TXC promotes cell proliferation
Following treatment with TXC for 24 h, the viability
of the injured cells was determined using MTT assay. As shown in
Fig. 1, the cellular viability of
the TNF-α group was significantly lower compared with that of the
control group, indicating that TNF-α inhibited the viability of
OB-like cells. The cell viability of the TXC groups was promoted
when compared with that of the TNF-α group (P<0.01), suggesting
that TXC protected the OB-like cells from TNF-α-induced injury.
Notably, 1.25 mg/ml TXC had a markedly higher protective effect
than the other concentrations of TXC, further implying that the
protective role of TXC was not dose-dependent.
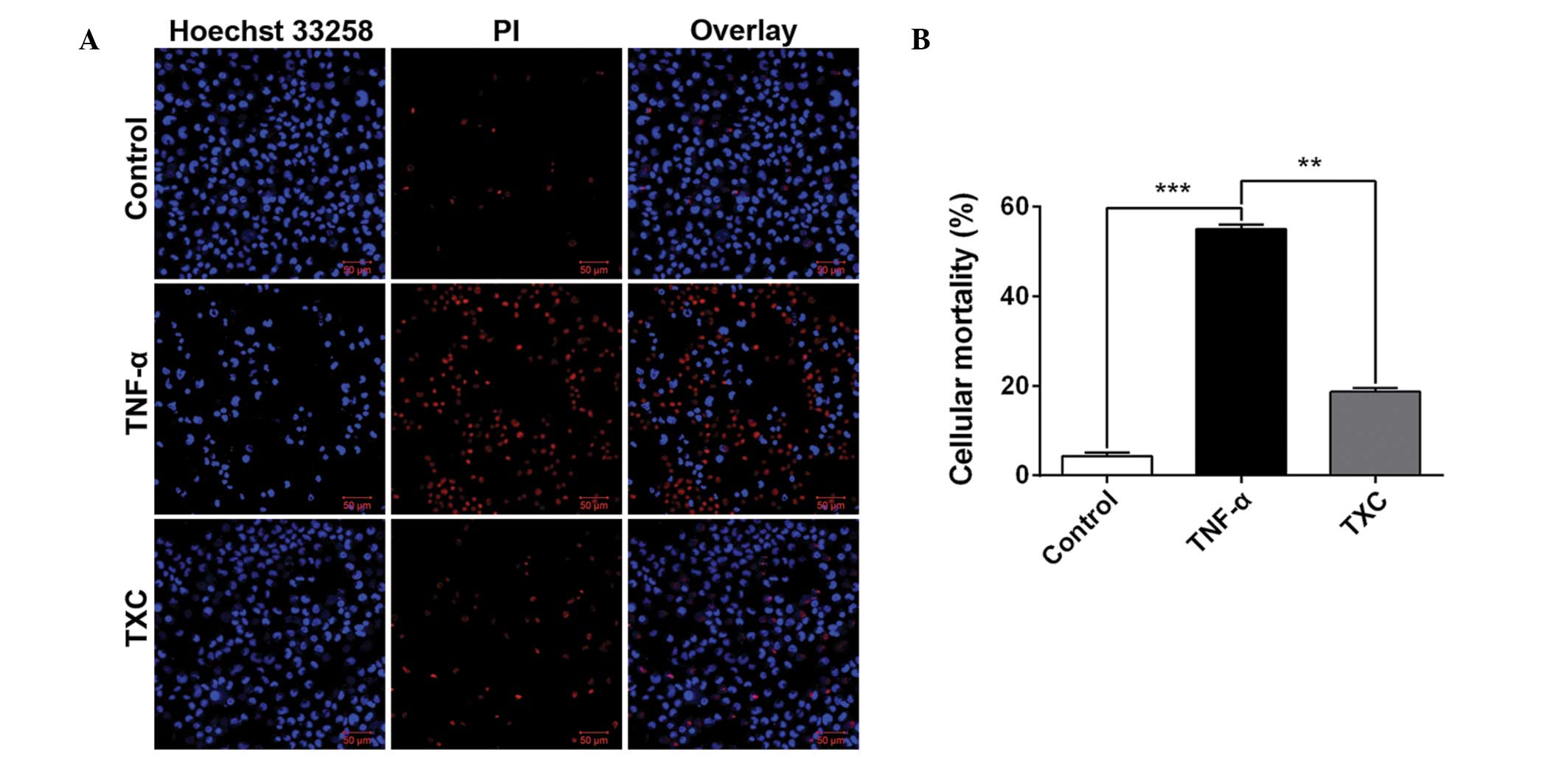
TXC reduces cell death
To further investigate the cell growth following TXC
treatment, cellular mortality was examined using fluorescence
microscopy and an LSCM. As shown in Fig.
2, the living cells (blue nuclei, stained with Hoechst 33258)
were significantly fewer and the mean intensity significantly lower
in the TNF-α group compared with those in the control group, but
the number of dead cells (red nuclei, stained by PI) and mean
intensity were significantly higher in the TNF-α group than in the
control group. This finding suggests that TNF-α is able to inhibit
OB-like cell growth; however, the larger number of living cells and
their higher mean intensity, as well as the smaller number of dead
cells and their corresponding mean intensity that were observed in
the TXC group when compared with the TNF-α group, clearly
demonstrated that TXC prevented the OB-like cells from undergoing
TNF-α injuries.
TXC improves cellular
ultrastructure
To reveal the protective effects of TXC on cellular
ultrastructure, TEM was used to observe the ultrathin sections of
OB-like cells following treatment with TNF-α and TXC for 24 h. As
shown in Fig. 3, the cells of the
control group were adherent with a long-fusiform shape, and the
nucleus and chromatin were normal and had generally regular
contours. By contrast, the TNF-α-injured cells exhibited typical
apoptotic characteristics, such as round shape, nuclear chromatin
condensation, mitochondrial pyknosis and expansion of the
endoplasmic reticulum. By contrast, the nucleus and other
organelles of the TXC-treated cells appeared clearly improved
following treatment, which indicated that TXC attenuated the
TNF-α-induced injuries of the OB-like cells.
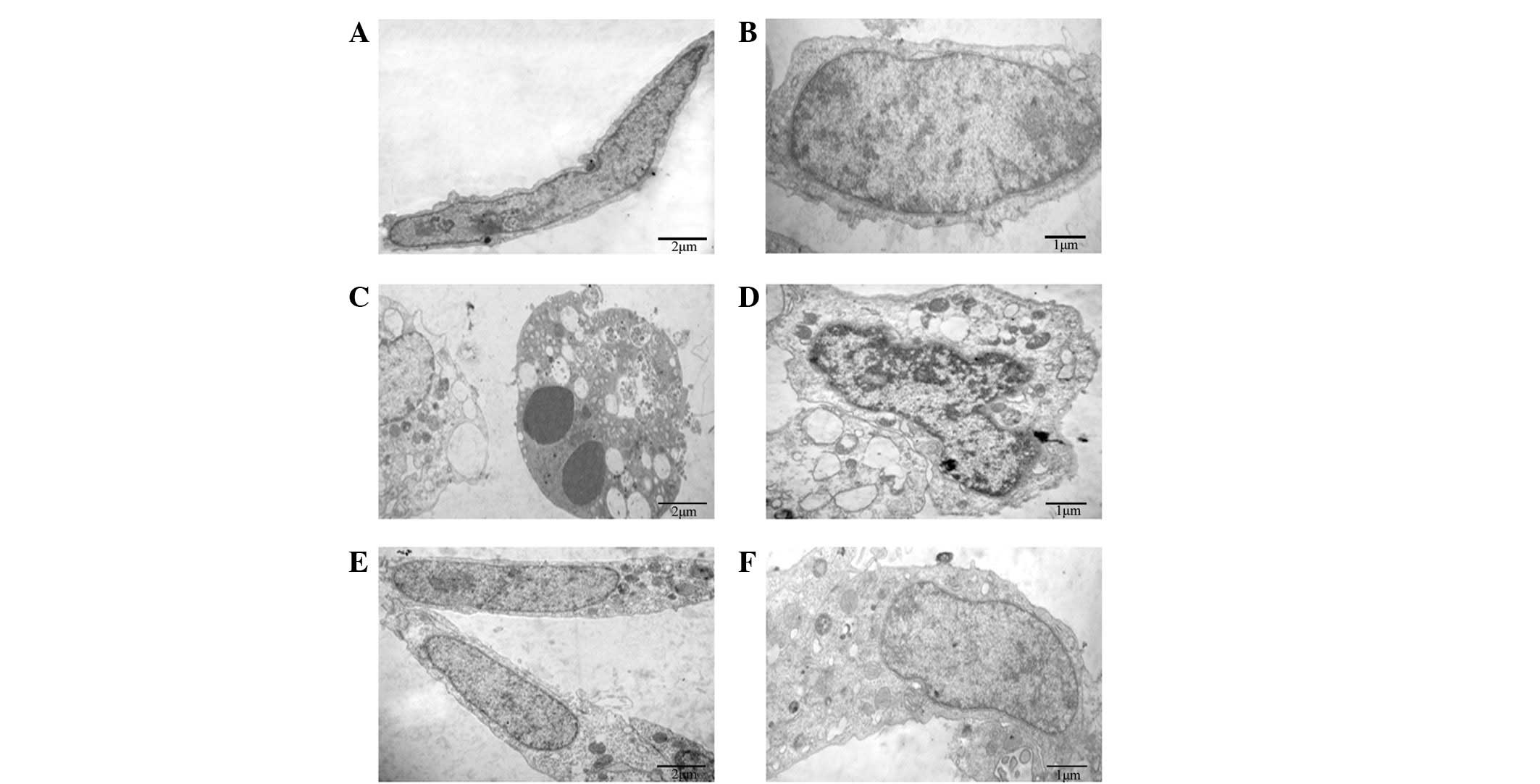 | Figure 3.Effect of TXC on the cell
ultrastructure of TNF-α-injured OB-like cells. (A and B) The normal
cells mainly presented a long fusiform shape and the nucleus and
chromatin were normal with generally regular contours. (C and D)
TNF-α-injured OB-like cells showed typical apoptotic
characteristics, and the nuclear chromatin and mitochondria, as
well as the endoplasmic reticulum presented an abnormal
ultrastructure. (E and F) TXC-treated OB-like cells also mainly
presented a long fusiform shape, however, many of their organelles,
including the nucleus, mitochondria and endoplasmic reticulum,
appeared clearly improved following treatment. TXC, Tougu Xiaotong
capsule; TNF-α, tumor necrosis factor-α; OB, osteoblast. |
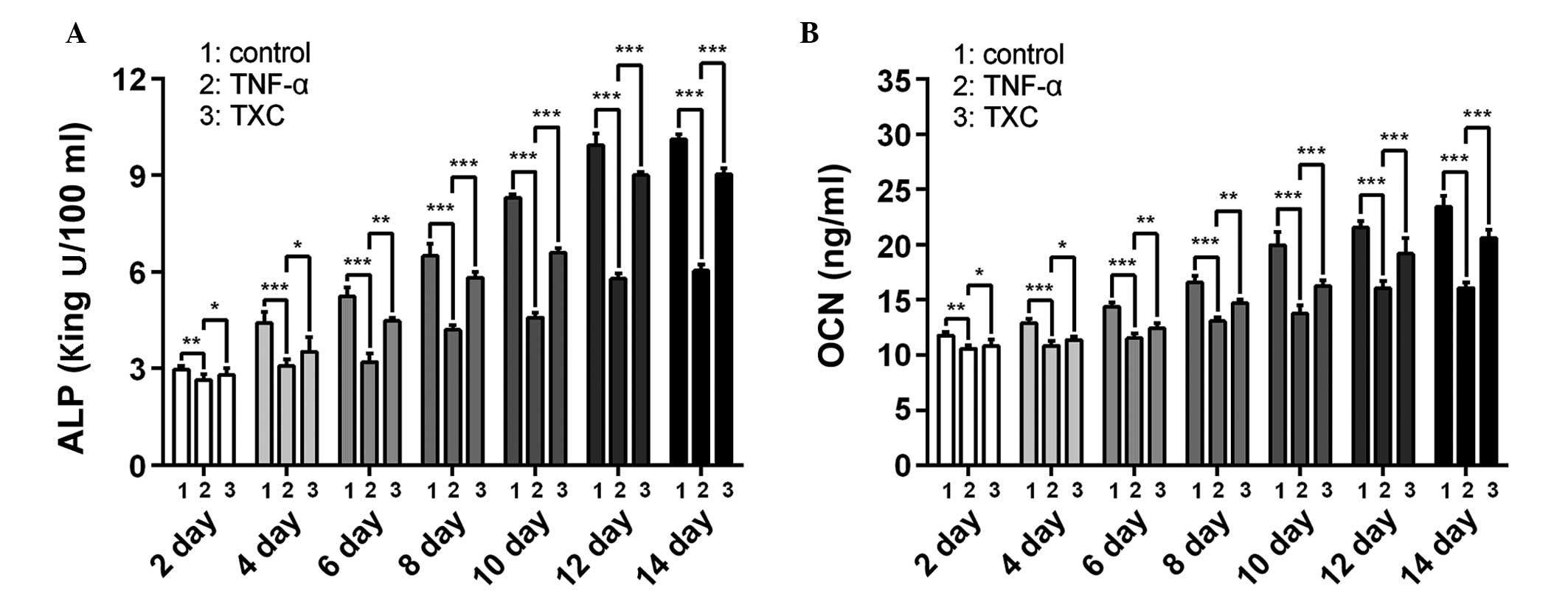
TXC promotes ALP activity and OCN
secretion
ALP is an early index and OCN a late-stage marker of
OB differentiation; therefore, to further evaluate the effects of
TXC on the progress of OB-like cell differentiation, the ALP
activity and OCN secretion were determined once every 2 days,
following the initial treatment for 14 days. As shown in Fig 4, ALP and OCN were significantly
decreased in the TNF-α group compared with the control group
between days 2 and 14 of differentiation, suggesting that TNF-α
inhibited the differentiation of the OB-like cells. By contrast,
the TXC group showed a significant increase in ALP activity and OCN
secretion between days 2 and 14 of differentiation compared with
those in the TNF-α group, suggesting that TXC promoted
differentiation of the TNF-α-treated OB-like cells, partly through
the upregulation of ALP activity and OCN secretion. Furthermore,
ALP activity and OCN secretion were not decreased as the treatment
time was extended, which indicates that the cell injuries did not
persist for a prolonged period of time.
TXC promotes mineralized nodule
formation and calcium secretion
Mineralized nodules, which contain large amounts of
calcium salt, are a marker of mature OB differentiation. In order
to explore the protective effect of TXC on OB mineralization,
specific staining (alizarin red S and tetracycline staining) was
performed and the morphological features of the mineralized nodules
were observed under an SEM. As shown in Fig. 5A, mineralized nodule formation was
inhibited in the TNF-α group, indicating that TNF-α inhibited the
differentiation of the mature OB-like cells, whereas there was a
clear increase in the number and width of mineralized nodules in
the TXC group when compared with those in the TNF-α group,
suggesting that TXC promoted the differentiation of mature OB-like
cells. To further determine the effect of TXC on mineralized
nodules, EDS analysis was used to quantify the calcium content. As
shown in Fig. 5B, the calcium
content (mass and atomic percentage) was significantly decreased in
the TNF-α group compared with that in the control group, while the
calcium content of the TXC group was significantly increased
compared with that in the TNF-α group. This demonstrates the
protective effect of TXC against TNF-α-injured OB-like cell
mineralization.
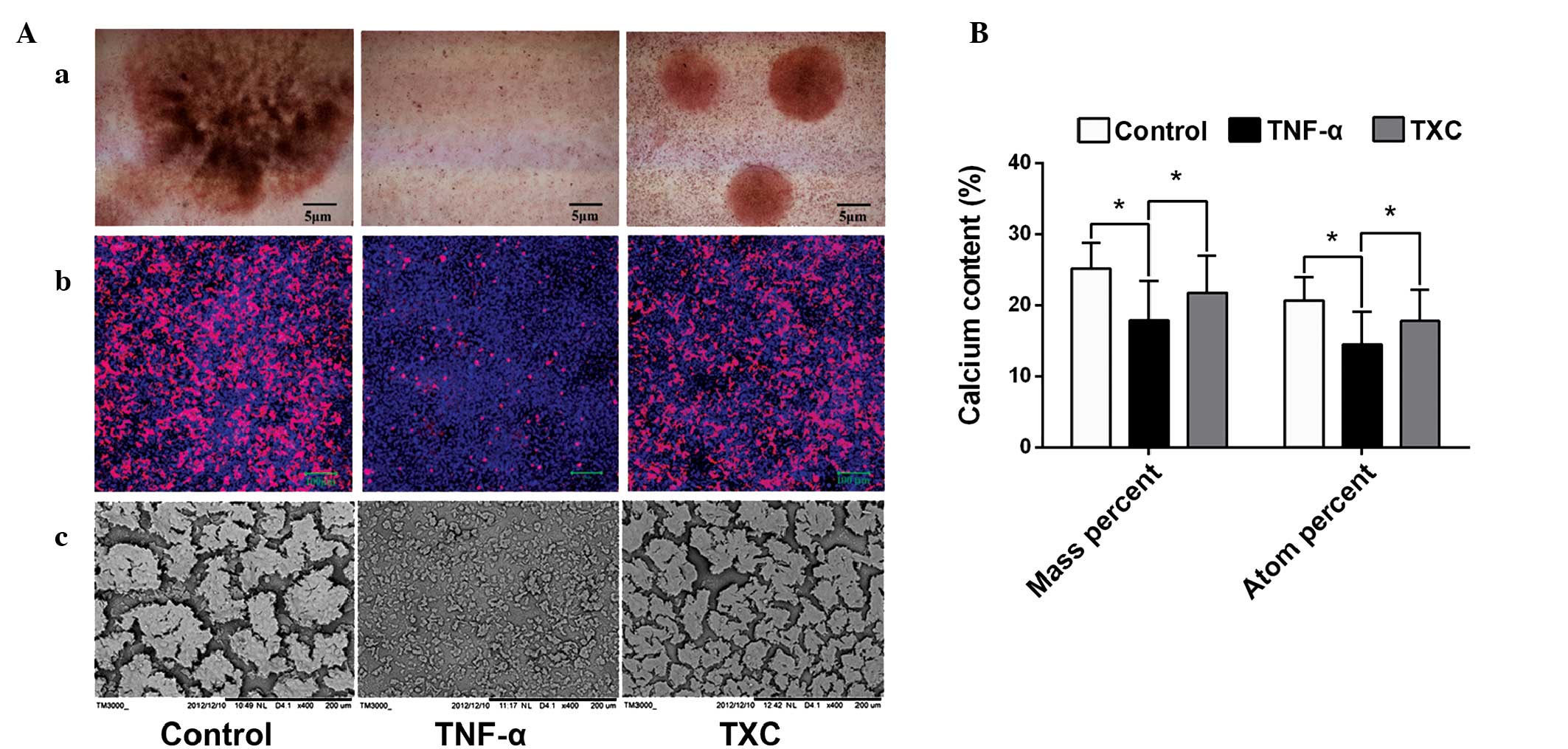 | Figure 5.Observation and quantification of
mineralized nodules in TNF-α-injured OB-like cells. Compared with
the TNF-α group, TXC treatment significantly promoted the secretion
of cell mineralized nodules and increased the calcium content,
which was observed using a phase contrast microscope, LSCM and SEM,
and measured by EDS. (Aa) Alizarin red S staining was observed
using phase contrast microscopy and (Ab) tetracycline staining was
observed using an LSCM. (Ac) The morphological changes of
mineralized nodules were also observed using an SEM. (B) EDS
analysis was used for the quantification of the calcium content.
For all groups, n=10. *P<0.05. TXC, Tougu Xiaotong capsule;
TNF-α, tumor necrosis factor-α; OB, osteoblast; LSCM, laser
scanning confocal microscope; SEM, scanning electron microscope;
EDS, energy dispersive spectroscopy. |
Discussion
TXC is a clinically effective drug in the treatment
of OA, which is able to alleviate osteoarthritic symptoms such as
pain, swelling and limited joint mobility (17,23,24).
Previous studies have reported that the mechanisms underlying TXC
treatment include the upregulation of Bcl-2 and downregulation of
p53 and caspase-3 and −9, as well as the regulation of Atg12/LC3
conjugation (17,25); however, the mechanisms through which
TXC regulates osteoarthritic SB remodeling remain to be elucidated.
The present study found that TXC was able to prevent TNF-α-induced
injuries of OB-like cells and promote cell proliferation and
differentiation. This suggests that TXC may be able to protect bone
osteogenesis from inflammation, which could be a molecular
mechanism through which TXC regulates SB remodeling.
TNF-α, as one of the key inflammatory factors, is
generally considered to be responsible for the degeneration of
articular cartilage and synovial membrane inflammation. TNF-α has
been observed to affect the SB during the inflammatory process
(10). Data have also shown that
TNF-α can inhibit OB proliferation and differentiation and induce
cellular apoptosis in vitro (13–16,20–22); therefore, TNF-α
could directly or indirectly inhibit OB metabolism, leading to
osteoarthritic SB alterations. The present study confirmed that
TNF-α inhibited the viability of OB-like cells, promoted cellular
mortality and apoptosis and inhibited cell differentiation by the
downregulation of ALP activity, OCN secretion and nodule
mineralization, in addition to reducing the calcium content of
mineralized nodules, a finding that was consistent with a previous
study (14). However, it was also
found that ALP and OCN were not decreased as the treatment time was
extended, which may have been due to the fact that the dose of
TNF-α was not able to entirely inhibit cellular activities during
the differentiation process.
The UMR-106 cell line is a clonal derivative of a
transplantable rat osteosarcoma that is considered to be an OB-like
cell line and has been used to study bone formation (26–28). In
the present study, the protective effects of TXC against injuries
in an OB-like cell line were investigated. Using MTT assay and LSCM
analysis, it was found that TXC promoted the viability of the
OB-like cells and reduced cellular mortality. In addition, the
cellular nucleus and other organelles were better developed in the
TXC-treated cells than in the untreated ones, which suggests that
TXC promoted cell proliferation of the TNF-α-injured OB-like cells.
The results also demonstrated that TXC significantly upregulated
the cellular activity of ALP, the secretion of OCN and the
mineralization of nodules in TNF-α-injured OB-like cells.
Furthermore, the SEM observation and EDS analysis further confirmed
that TXC promoted calcium secretion in TNF-α-exposed OB-like cells.
These findings indicate that TXC may be able to promote the
differentiation and mineralization of OBs.
In conclusion, TXC protected an OB-like cell line
from TNF-α-induced injuries by promoting cell proliferation and
differentiation. In agreement with our previous study (18,19), it
was found that TXC has the potential to regulate
inflammation-induced alterations of SB. Further studies are
required in order to investigate the mechanisms underlying the
protective effects of TXC on OB injuries.
Acknowledgements
This study was supported by the Key Project of
Fujian Province Department of Science and Technology (Grant no.
2012Y4006), the Natural Science Foundation of Fujian Province
(Grant no. 2014J01356), the National Natural Science Foundation of
China (Grant no. 81202836) and the Chen Keji Development Fund for
Integrative Medicine (Grant no. CKJ2011004).
References
|
1
|
Glasson SS, Askew R, Sheppard B, Carito B,
Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, et al:
Deletion of active ADAMTS5 prevents cartilage degradation in a
murine model of osteoarthritis. Nature. 434:644–648. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayami T, Pickarski M, Zhuo Y, Wesolowski
GA, Rodan GA and le T Duong: Characterization of articular
cartilage and subchondral bone changes in the rat anterior cruciate
ligament transection and meniscectomized models of osteoarthritis.
Bone. 38:234–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tat SK, Pelletier JP, Mineau F, Caron J
and Martel-Pelletier J: Strontium ranelate inhibits key factors
affecting bone remodeling in human osteoarthritic subchondral bone
osteoblasts. Bone. 49:559–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuroki K, Cook CR and Cook JL: Subchondral
bone changes in three different canine models of osteoarthritis.
Osteoarthritis Cartilage. 19:1142–1149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madry H, van Dijk CN and Mueller-Gerbl M:
The basic science of the subchondral bone. Knee Surg Sports
Traumatol Arthrosc. 18:419–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castañeda S, Roman-Blas JA, Largo R and
Herrero-Beaumont G: Subchondral bone as a key target for
osteoarthritis treatment. Biochem Pharmacol. 83:315–323. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imhof H, Sulzbacher I, Grampp S, Czerny C,
Youssefzadeh S and Kainberger F: Subchondral bone and cartilage
disease: A rediscovered functional unit. Invest Radiol. 35:581–588.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muraoka T, Hagino H, Okano T, Enokida M
and Teshima R: Role of subchondral bone in osteoarthritis
development: A comparative study of two strains of guinea pigs with
and without spontaneously occurring osteoarthritis. Arthritis
Rheum. 56:3366–3374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stannus O, Jones G, Cicuttini F,
Parameswaran V, Quinn S, Burgess J and Ding C: Circulating levels
of IL-6 and TNF-α are associated with knee radiographic
osteoarthritis and knee cartilage loss in older adults.
Osteoarthritis Cartilage. 18:1441–1447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hulejová H, Baresová V, Klézl Z, Polanská
M, Adam M and Senolt L: Increased level of cytokines and matrix
metalloproteinases in osteoarthritic subchondral bone. Cytokine.
38:151–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tat S Kwan, Pelletier JP, Lajeunesse D,
Fahmi H, Lavigne M and Martel-Pelletier J: The differential
expression of osteoprotegerin (OPG) and receptor activator of
nuclear factor kappaB ligand (RANKL) in human osteoarthritic
subchondral bone osteoblasts is an indicator of the metabolic state
of these disease cells. Clin Exp Rheumatol. 26:295–304.
2008.PubMed/NCBI
|
|
12
|
Tat SK, Pelletier JP, Vergés J, Lajeunesse
D, Montell E, Fahmi H, Lavigne M and Martel-Pelletier J:
Chondroitin and glucosamine sulfate in combination decrease the
pro-resorptive properties of human osteoarthritis subchondral bone
osteoblasts: A basic science study. Arthritis Res Ther. 9:R1172007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xue LW, Zhang J, Wang XX, Bu T and Liu M:
Effects of tumor necrosis factor-α on the growth of rat
osteoblasts. Hua Xi Kou Qiang Yi Xue Za Zhi. 27:378–380. 2009.(In
Chinese). PubMed/NCBI
|
|
14
|
Tsukasaki M, Yamada A, Suzuki D, Aizawa R,
Miyazono A, Miyamoto Y, Suzawa T, Takami M, Yoshimura K, Morimura
N, et al: Expression of POEM, a positive regulator of osteoblast
differentiation, is suppressed by TNF-α. Biochem Biophys Res
Commun. 410:766–770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cunningham CC, Mills E, Mielke LA,
O'Farrell LK, Lavelle E, Mori A, McCarthy GM, Mills KH and Dunne A:
Osteoarthritis-associated basic calcium phosphate crystals induce
pro-inflammatory cytokines and damage-associated molecules via
activation of Syk and PI3 kinase. Clin Immunol. 144:228–236. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang W, Shang WL, Wang HD, Wu WW and Hou
SX: Sirt1 overexpression protects murine osteoblasts against
TNF-alpha-induced injury in vitro by suppressing the NF-kappaB
signaling pathway. Acta Pharmacol Sin. 33:668–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li XH, Wu MX, Ye HZ, Chen WL, Lin JM,
Zheng LP and Liu XX: Experimental study on the suppression of
sodium nitroprussiate-induced chondrocyte apoptosis by Tougu
Xiaotong Capsule-containing serum. Chin J Integr Med. 17:436–443.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang YM, Chen WL, Liu XX, Huang MY, Lun
RH, Li M, Xiao CY and Wu GW: Histochemical study of osteoarthritis
treated by Tougu Xiaotong Granule. Zhongguo Zhong Yi Gu Shang Ke.
19:1–3. 2011.(In Chinese).
|
|
19
|
Li M, Guo YE, Liu XX, Wei SS, Chen WL, Lin
JM, Huang YM, Huang MY and Wu ZL: Molecular mechanism of Tougu
Xiaotong capsules for subchondral bone remodeling in knee
osteoarthritis. Zhongguo Zu Zhi Gong Cheng Yan Jiu. 16:2669–2673.
2012.(In Chinese).
|
|
20
|
Minamitani C, Tokuda H, Adachi S,
Matsushima-Nishiwaki R, Yamauchi J, Kato K, Natsume H, Mizutani J,
Kozawa O and Otsuka T: p70 S6 kinase limits tumor necrosis
factor-alpha-induced interleukin-6 synthesis in osteoblast-like
cells. Mol Cell Endocrinol. 315:195–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chae HJ, Chae SW, Kang JS, Bang BG, Cho
SB, Park RK, So HS, Kim YK, Kim HM and Kim HR: Dexamethasone
suppresses tumor necrosis factor-alpha-induced apoptosis in
osteoblasts: Possible role for ceramide. Endocrinology.
141:2904–2913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mukai T, Otsuka F, Otani H, Yamashita M,
Takasugi K, Inagaki K, Yamamura M and Makino H: TNF-alpha inhibits
BMP-induced osteoblast differentiation through activating SAPK/JNK
signaling. Biochem Biophys Res Commun. 356:1004–1010. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin MN and Liu XX: Tougu Xiaotong
Decoction for treating 30 cases of osteoarthritis of the knee.
Fujian Zhong Yi Yao. 36:15–16. 2005.(In Chinese).
|
|
24
|
Zheng CS, Ye HZ, Xu XJ and Liu XX:
Computational pharmacology study of tougu xiaotong granule in
preventing and treating knee osteoarthritis. Chin J Integr Med.
15:371–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Liu F, Liang W, Ye H, Li H, Yu F,
Chen J, Chen W, Lin R, Zheng C, et al: Tougu Xiaotong capsule
promotes chondrocyte autophagy by regulating the Atg12/LC3
conjugation systems. Int J Mol Med. 34:545–552. 2014.PubMed/NCBI
|
|
26
|
Cruz A Dela, Mattocks M, Sugamori KS,
Grynpas MD and Mitchell J: Reduced trabecular bone mass and
strength in mice overexpressing Gα11 protein in cells of
theosteoblast lineage. Bone. 59:211–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pon-On W, Charoenphandhu N,
Teerapornpuntakit J, Thongbunchoo J, Krishnamra N and Tang IM: In
vitro study of vancomycin release and osteoblast-like cell growth
on structured calcium phosphate-collagen. Mater Sci Eng C Mater
Biol Appl. 33:1423–1431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joung YH, Lim EJ, Darvin P, Chung SC, Jang
JW, Do Park K, Lee HK, Kim HS, Park T and Yang YM: MSM enhances GH
signaling via the Jak2/STAT5b pathway in osteoblast-like cells and
osteoblast differentiation through the activation of STAT5b in
MSCs. PLoS One. 7:e474772012. View Article : Google Scholar : PubMed/NCBI
|