Introduction
Since the initial separation and naming of bone
morphogenetic protein (BMP) by Urist and Strates in 1971 (1), there have been significant advances in
the development of recombinant human BMP (rhBMP) (2–4). Studies
have demonstrated that rhBMP-2 exhibits good osteoinductive
activity, inducing the transformation of undifferentiated
mesenchymal cells and osteoblasts into chondrocytes and
skeletogenous cells and contributing to the formation of cartilage
and bone (5–7). Since the bioactivity of rhBMP-2 is
easily lost due to diffusion and degradation in the body fluid, it
is necessary to develop a suitable carrier that enables the slow
release of rhBMP-2 to achieve the strongest efficacy (6,8).
With the development of materials science for
controlling and targeting release, it is currently possible to
increase the efficacy of drugs with a short half-life, such as
rhBMP-2 (2,9). Bioabsorbable polymerized materials,
which exhibit the same strength as human compact bone and can be
made into any shape, are commonly and effectively used as a carrier
for BMP in order to achieve good osteoinductive activity (2,10);
therefore, it may be possible to develop a novel absorbable,
bioactive compound plate containing rhBMP-2, which is beneficial
for bone growth and fracture healing, to replace the implant
currently used in clinical practice. This may be an effective way
to treat fracture or nonunion at a non-weight-bearing site. At
present, poly-D,L-lactic acid (PDLLA) is one of the most commonly
used bioabsorbable polymerized materials for drug carriers
(11). In the present study, a
super-high molecular weight PDLLA plate exhibiting the sustained
release of rhBMP-2 (PDLLA-rhBMP-2) was designed, and its effects on
the treatment of fracture, defect recovery and fixation and
degradation were evaluated, in order to provide a foundation for
the future study of bone frame tissue engineering and for the
clinical application of such designs.
Materials and methods
Materials
The rhBMP-2, dissolved in glycine buffer, was
provided by the Huadong Gene Technology Research Center (Hangzhou,
China). The rhBMP-2 had a molecular weight of 26 kDa, an
isoelectric point of 6.56 and alkaline phosphatase activity of
>100,000 U/mg. Each plate contained 0.05 mg rhBMP-2 with
super-high molecular weight PDLLA. The plate was then borated using
a mold, and a porous compound surface was formed using laminating
technology. Having been vacuumed, refrigerated and dried, the plate
was sterilized using Co-60. The PDLLA-rhBMP-2 exhibited the
following specifications (which were identical to those of the
normal super-high molecular weight PDLLA plate): Length, 40 mm;
width, 9 mm; thickness, 3mm; tensile strength, >50 MPa;
tri-point bending strength, >90 MPa; raw material intrinsic
viscosity, 1.6 dl/g; bioabsorbable internal fixing screw diameter,
2 mm. The PDLLA-rhBMP-2 and normal super-high molecular weight
PDLLA plate were made by Sichuan Dikang Sci & Tech
Pharmaceutical Industry Co., Ltd. (Chengdu, China). Other materials
included an Agfa computed radiography instrument (Agfa Healthcare,
Bonn, Germany), a light microscope (Leica Microsystems GmbH,
Wetzlar, Germany) and a high-resolution, color image analysis
system (Motic® Images Advanced 3.0; Department of Human Anatomy and
Histoembryology, Shanghai Fudan University, Shanghai, China).
Animal models and grouping
A total of 32 healthy New Zealand rabbits (3.0±0.5
kg) were purchased from the Experimental Animal Center of the
Shanghai No. 6 Municipal People's Hospital (Shanghai, China).
Ketamine (0.1 g/rabbit) and 2.5% sodium pentobarbital (30 mg/kg)
were used to anesthetize the rabbits. Following disinfection and
the placement of surgical towels, the dorsal aspect of the ulnar
was cut with a straight incision. The extensors and flexors of the
forearm were then bluntly dissected, and parts of the periosteum
were cut open and removed to expose the ulnar stem. The middle of
the ulna was cut with a scroll saw, and the broken ends were
planished with a dentistry bodkin, resulting in a 2.5-mm defect in
the periosteum of the ulna. The right side, which was used as the
experimental side, was fixed internally with the PDLLA-rhBMP-2 and
the left side, which was the control side, was fixed with the
normal PDLLA plate. Each broken end of the fractured bone was fixed
using two bioabsorbable screws. Finally, the incision was rinsed
with gentamicin sulfate and sutured layer-by-layer. The animals
were placed back in the cage and fed with normal forage. Every
other day, 80,000 units gentamicin sulfate was hypodermically
injected for three times in total. The animals were examined at 2,
4, 8 and 12 weeks after surgery.
Radiographic examination
The animals were anesthetized and an AP projection
of the ulna and radius was captured with the following conditions:
40 KV, 50 mA, 0.2 sec and 60-cm distance. The callus density, which
was the density of the identical area of bone defect on the same
graph was 100, and the identical area of ground color on the graph
was 0. The relative magnitude of the callus density of the region
was measured as the bone defect. The averaged callus density of the
bone defect represented the mean of the visible region.
Visual analysis
At 2, 4, 8 and 12 weeks after surgery, 8 animals
were sacrificed by i.v. pentobarbital sodium (60 mg/kg),
respectively, and the ulna and radius in the region of the bone
defect were cut. The degradation of the implant, compatibility
between the bone and implant, reaction of the surrounding soft
tissue, material degradation and fragmentation, ossification and
inflammatory reactions were assessed using the methods described
below.
Histopathological analysis
The broken ends of the fractured ulna (PDLLA-rhBMP-2
or control PDLLA plate) were isolated, fixed using 10% formaldehyde
solution, decalcified, embedded in paraffin and cut into 5-µm
laterigrade and longitudinal serial sections. The sections were
subsequently stained using hematoxylin and eosin and observed under
an optical microscope. Images were captured for the analysis of the
percentage of the target area (newly formed bone region).
Statistical analysis
Data are presented as the mean ± standard deviation,
and one-way analysis of variance was performed using the SPSS 13.0
statistical software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Radiographic examination
The results of the radiographic examination showed
that the ulna fractures were fixed stably with the two bioactive
plates. No movement was observed, and good reduction was
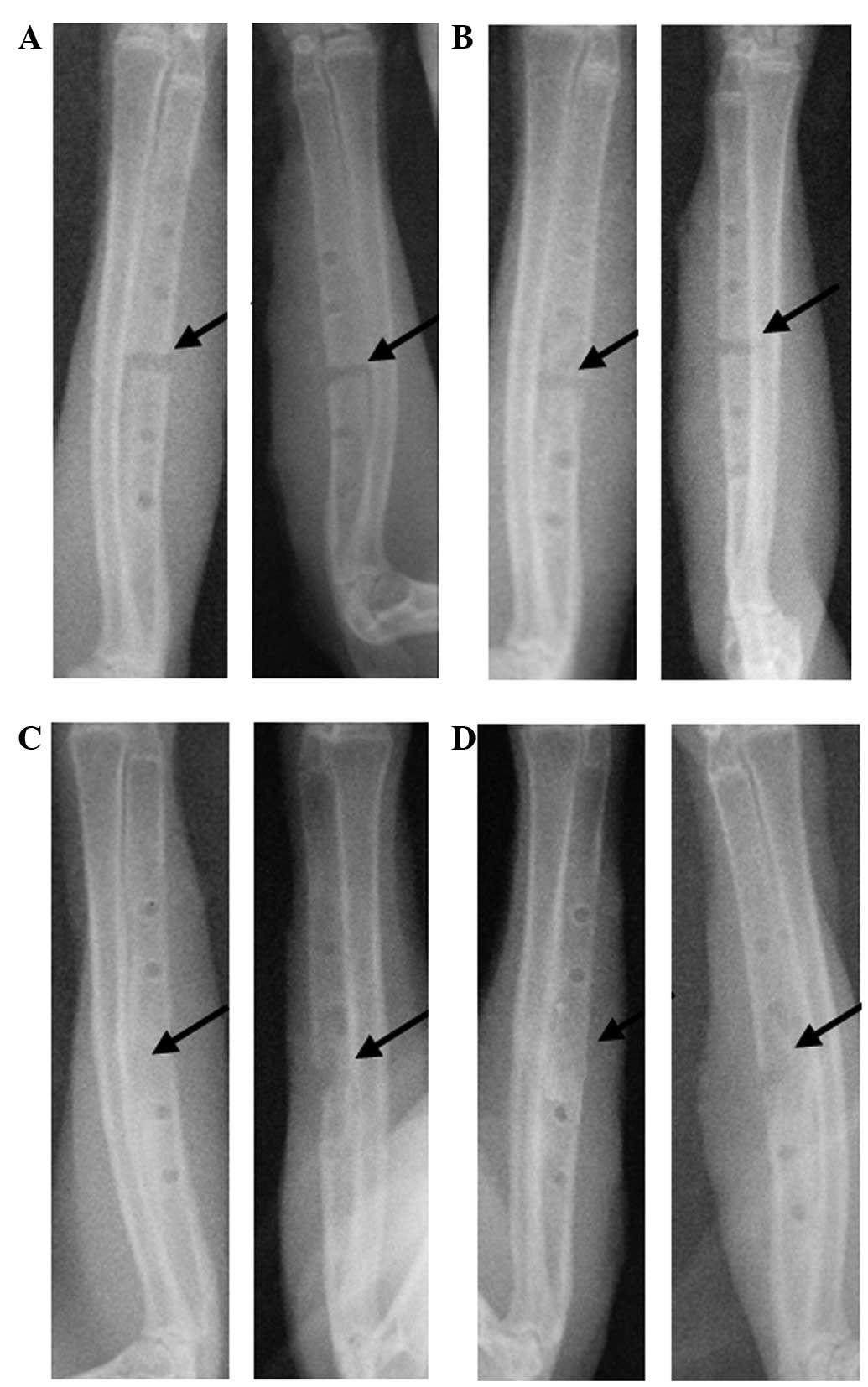
maintained. At 2 weeks after surgery, the experimental sides, which
contained the PDLLA-rhBMP-2, exhibited some callus formation, but
the fracture line remained evident; the control sides with the
normal PDLLA plate had no visible callus formation (Fig. 1A). At 4 weeks after surgery, the
experimental sides with the PDLLA-rhBMP-2 exhibited extensive
lamellar callus formation, and the fracture line was ambiguous;
however, the control sides with the normal PDLLA plate showed only
limited osteotylus formation, with a thinner callus and a visible
fracture line (Fig. 1B). At 8 weeks
after surgery, the experimental sides with the PDLLA-rhBMP-2
exhibited synostosis of the ulna and the disappearance of the
fracture line; by contrast, the control sides with the normal PDLLA
plate exhibited crumbly callus formation, and the fracture line was
cloudy and blurred (Fig. 1C). At 12
weeks after surgery, the experimental sides with the PDLLA-rhBMP-2
exhibited complete synostosis of the ulna and a mounded callus,
while the control sides with the normal PDLLA plate exhibited
extensive callus formation and the disappearance of fracture line
(Fig. 1D). The callus density of
fracture recovery for each side at different phases is shown in
Table I.
 | Table I.Results of the radiographic
examination. |
Table I.
Results of the radiographic
examination.
|
| Post-operative time
(weeks) |
|
|
|---|
|
|
|
|
|
|---|
| Group | 2 | 4 | 8 | 12 | F-value | P-value |
|---|
| Experimental | 39.22±2.48 | 48.79±1.26 | 63.78±1.78 | 78.60±1.25 | 10.963 | 0.003 |
| Control | 33.83±1.13 | 41.28±1.25 | 55.23±0.68 | 66.54±1.33 | 17.602 | 0.001 |
| t | 5.031 | 8.005 | 10.110 | 0.980 |
|
|
| P-value | 0.007 | 0.001 |
0.001 | 0.038 |
|
|
Visual analysis
At 1 week after surgery, the animals in each group
exhibited primary healing, and no red swelling of the skin,
effusion, wound dehiscence or histological material discharge was
observed. The two bioactive plates fixed stably, and the quantity
of new bone increased as time passed; in addition, the materials
degraded gradually. At 8 weeks after surgery, the experimental side
in 7 animals had recovered from the fracture, and the control side
in 2 animals had some callus formation. The bone defect in the
other sides was mainly filled with fibrous connective tissue. At 12
weeks after surgery, all animals showed defect recovery,
irrespective of the type of plate, and part of the surface of both
types of plate had begun to be degraded and absorbed; however, the
formation of new bone and the degradation of the plates in the
experimental side were notably faster. All the plates were far from
completely absorbed.
Histopathological examination
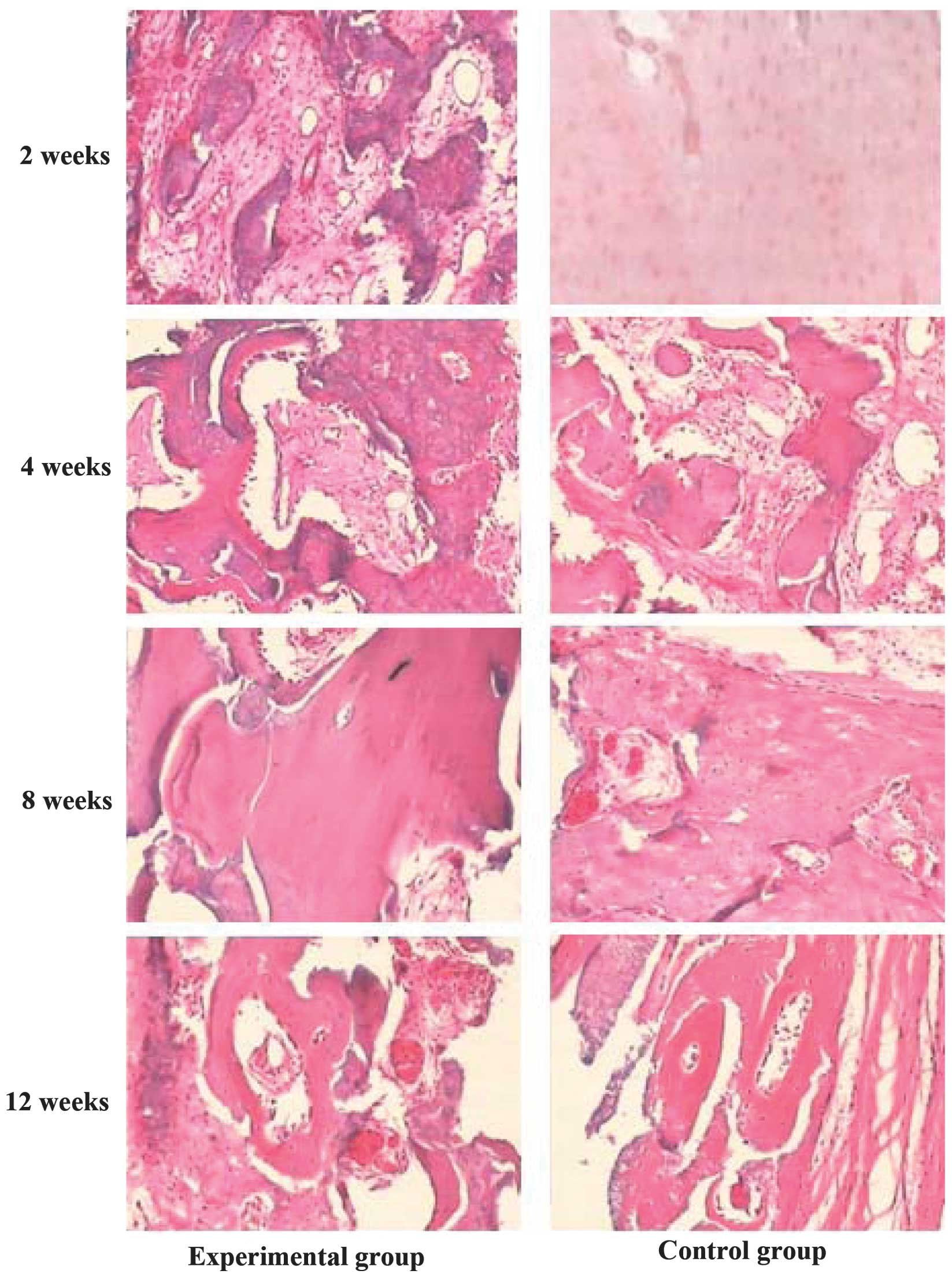
At 2 weeks after surgery, no obvious inflammatory
reaction was observed on either side; the experimental sides had a
small quantity of new bone, ground substance and cells, which
expanded across the surface between the bone and the materials.
Furthermore, blood capillaries appeared in the interface near the
soft tissue (Fig. 2). At 4 weeks
post-surgery, no obvious degradation or inflammatory reaction was
found on either side. The control sides had a small quantity of new
bone, ground substance and cells, which expanded across the surface
between the bone and the materials, and numerous blood capillaries
appeared in the interface near the soft tissue; however, the
experimental sides exhibited the formation of new bone trabeculae,
blood capillaries and fibroblasts that almost filled the defect
between the interface of the bone and the implanted materials.
Furthermore, the initially formed cavitas medullaris and the broken
ends of the fractured bone were linked well by a fibrous callus
(Fig. 2). At 8 weeks after surgery,
the plates in both sides had very little degradation and absorption
and showed good biocompatibility, with no reaction of inflammatory
histocytes. The control sides with the normal PDLLA plate had a
large quantity of new bone, ground substance and cells, which
spread across the surface between the bone and the implanted
materials; blood capillaries appeared in the interface near the
soft tissue; woven bone formation was observed; and the broken ends
of the fractured bone were linked by a fibrous callus. The
experimental sides with the PDLLA-rhBMP-2 exhibited a larger area
of newly formed bone, dynamic rebuilding of the bone tissue and
neogenesis, numerous osteoblasts and blood capillaries around the
new bone tissue, mature bone tissue, fewer cellular components and
calcified nodules in certain regions (Fig. 2). At 12 weeks after surgery, the
plates in both sides had partly undergone degradation and
absorption. In the control sides with the normal PDLLA plates,
numerous osteoblasts and blood capillaries were observed around the
new bone tissue and woven bone. Furthermore, the appearance of
lamellar bone, reduced ingredient of cells and calcified nodules in
certain regions were observed. In the experimental sides, which
contained the PDLLA-rhBMP-2, there were numerous mature bone
tissues and lamellar bone. In addition, the plate absorption was
quicker than that in the control sides, showing enhanced
biocompatibility and bone healing, and there was a partial
appearance of the cavitas medullaris (Fig. 2).
Computer image analysis
The percentage area of newly formed bone at the same
side of bone defect was analyzed with image analysis software (show
in Table II). At 2, 4 and 8 weeks
after surgery, a significant difference was found in the area of
newly formed bone between the experimental and control sides
(P<0.01 for 2 and 4 weeks, P<0.05 for 8 weeks). It was
evident that the formation of new bone in the experimental side was
more rapid than that in the control side. At 12 weeks after
surgery, no significant difference in the area of newly formed bone
was found between the experimental and control sides
(P>0.05).
 | Table II.Percentage area of newly formed bone
at the same side of the bone defect analyzed using image analysis
software. |
Table II.
Percentage area of newly formed bone
at the same side of the bone defect analyzed using image analysis
software.
|
| Post-operative time
(weeks) |
|
|
|---|
|
|
|
|
|
|---|
| Group | 2 | 4 | 8 | 12 | F-value | P-value |
|---|
| Experimental | 0.106±0.015 | 0.292±0.019 | 0.457±0.048 | 0.574±0.047 | 130.285 | <0.001 |
| Control | 0 | 0.193±0.019 | 0.339±0.029 | 0.601±0.037 | 204.855 | <0.001 |
| t | 17.909 | 6.525 | 3.672 | 0.778 |
|
|
| P-value |
0.003 | 0.003 | 0.021 | 0.480 |
|
|
Discussion
In in vitro culture, rhBMP-2 facilitates the
differentiation of progenitor cells into osteoblasts, but has a
depressive effect on the differentiation of muscle plasma cells
(12). Previous studies have
demonstrated that rhBMP-2 can stimulate the differentiation of
cells derived from bone tissue, such as precursor cells of
osteoblasts, W-20-17 marrow stromal cells, as well as cell lines
derived from non-bone tissue, such as multipotent fibroblasts and
myoblasts, to osteoblasts (12,13).
rhBMP-2 can suppress the expression of genes for
myoblast and myotube formation, which explains the ability of
rhBMP-2 to promote the directional differentiation of precursor
cells into osteoblasts (14,15). In the present study, radiographic,
pathohistological and computer image analyses were performed, which
demonstrated that, during the first 2–8 weeks after surgery, the
broken ends of the fractured bone fixed with the PDLLA-rhBMP-2 grew
more rapidly than those of the control side. Since rhBMP-2 is a
type of hydrophobic glycoprotein, it is stable in a acidic,
hypothermal and desiccative environment (6,8,13); therefore, in the present study, the
hydrophobicity of the polymer was used to embed the rhBMP-2 and to
isolate it from the body fluid, resulting in delayed rhBMP-2
release at a constant concentration and time. This was demonstrated
to be an effective method of maintaining its osteoinductive
activity.
The osteoinductive activity of BMP is generally
accepted; however, its clinical use is not satisfactory. In
addition to the purification and the surgical procedure, the
preparation of the carrier is an important factor affecting the
efficacy of BMP. Using simple BMP to produce a marked effect is
challenging, as it dissolves in the body fluid; however, the use of
a slow-release carrier can enhance the effect of the BMP (16). The current slow-release carriers that
are commonly used include mineral salts (such as hydroxyapatite,
ceramic and gypsum), biological materials (such as collagen and
fibrin) and bioabsorbable polymerized materials; however, there are
disadvantages associated with the majority of these carriers:
Ceramic is fragile and easily smashed, hydroxyapatite cannot be
degraded, gypsum produces heat when reacting with foreign materials
and biological materials have immunity-related problems, which
cannot be completely overcome. Bioabsorbable polymerized materials
can, on the whole, solve the above problems, as their slow-release
rate can be adjusted, making it possible to adjust the rates of
degradation and release of BMP; therefore, bioabsorbable
polymerized materials are one of the most suitable carriers
(2,17,18).
We have studied the bioabsorbable polymerized
materials polyglycolic acid (PGA) and polylactic acid (PLA). Due to
the fact that PLA maintains its strength for longer and is
associated with lower degradation and tissue reaction rates and
response strength than PGA, PLA has become the focus of the study
of the use of bioabsorbable materials in internal fixation. PLA
contains self-enhanced poly-L-lactic acid (SR-PLLA), poly-L-lactic
acid (PLLA) and PDLLA and can therefore be completely absorbed by
bone tissue. The degradation of PLA relies on water, and it is
transformed to carbon dioxide and water by the citric acid cycle,
prior to being expelled out of the body through respiration
(19). Bergsmaju et al
(20) found that PLA had not been
completely absorbed in the body after 5–7 years and caused a
tardive tissue reaction, which may have been due to the higher
crystallinity of SR-PLLA and PLLA. PDLLA is an amorphous material
and can be completely absorbed by the body in between 24 weeks and
18 months due to its good histocompatibility, biodynamic
performance and adequate absorption rate; therefore, PDLLA is
considered to be the most suitable and effective candidate material
for use in bone technology (11,21). The
present study showed that it is possible to control the release
rate of rhBMP-2 by controlling the aperture size and porosity of
the synthesized materials to adjust the material strength and
degradation time. The PDLLA-rhBMP-2 had good incipient mechanical
fixation strength and slow degradation, with the constant formation
of new bone, and avoided the stress-shielding effect, which can
affect the healing of the fracture. In addition, the PDLLA-rhBMP-2
exhibited an enhanced fracture-healing ability, improved
compatibility with the surrounding tissue, faster bone formation,
an increased bone regeneration mass and enhanced medullary canal
structure compared with the normal PDLLA plate.
Traditional materials for internal and external
fixation are metal. These metal materials have certain
disadvantages (5): i) Electrolytic
tarnishing in the body; ii) higher rigidity and lower elasticity
creating a stress that can protect the broken ends of fractured
bone and affect the union of a fracture; iii) high weight, which
can affect functional exercise and activity; iv) interference with
magnetic resonance imaging and v) requirement of further surgery to
remove the implant, increasing the burden and pain of the patients.
Previous investigations have demonstrated that bioabsorbable
polymerized materials can be used to prepare screws and fixation
sticks for treating fractures (22,23);
however, the strength and osteoinductive activity of these
materials are insufficient and the cost is high, which prevents the
generalized application of these materials. In the present study,
PDLLA-rhBMP-2, which exhibited good bending and tensile strength,
and no conspicuous absorption and looseness, was designed. The
material showed good biocompatibility and osteoinductive activity,
making it an ideal fixation material for fracture repair. This
plate has no requirement for further surgery to be removed, and it
can enhance fracture healing; therefore, PDLLA-rhBMP-2 shows
potential for treating fracture or nonunion at non-weight-bearing
sites, such as the acetabulum pelvis, skull and jaw facial bones.
Further research is required prior to its clinical application.
Acknowledgements
This study was supported by the Science and
Technology Project of Shanghai Pudong New Area Committee (no.
pkj2012-y10).
References
|
1
|
Urist MR and Strates BS: Bone
morphogenetic protein. J Dent Res. 50:1392–1406. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Whang K, Goldstick TK and Healy KE: A
biodegradable polymer scaffold for delivery of osteotropic factors.
Biomaterials. 21:2545–2551. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohgushi H, Goldberg VM and Caplan AI:
Heterotopic osteogenesis in porous ceramics induced by marrow
cells. J Orthop Res. 7:568–578. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai XD, Li G, Zhao C, Duan H and Qu F:
BMP7 induces the differentiation of bone marrow-derived mesenchymal
cells into chondrocytes. Med Biol Eng Comput. 49:687–692. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Na YH, He Y, Shuai X, Kikkawa Y, Doi Y and
Inoue Y: Compatibilization effect of
poly(epsilon-caprolactone)-b-poly (ethylene glycol) block
copolymers and phase morphology analysis in immiscible
poly(lactide)/poly(epsilon-caprolactone) blends. Biomacromolecules.
3:1179–1186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qian G, Dong YH, Yang WC and Wang M:
Injectable calcium phosphate cement and fibrin sealant recombined
human bone morphogenetic protein-2 composite in vertebroplasty: An
animal study. Bosn J Basic Med Sci. 12:231–235. 2012.PubMed/NCBI
|
|
7
|
Bessho K: Ectopic osteoinductive
difference between purified bovine and recombinant human bone
morphogenetic protein. Bone Morphogenetic Proteins: Biology,
Biochemistry and Reconstructive Surgery. Lindholm TS: (Ney York,
NY). Academic Press. 105–111. 1996.
|
|
8
|
Guan Y, Wang Q, Cheng Y, Teng W and Huang
H: Study on gene transfection in bone marrow mesenchymal stem cells
mediated by plasmid of bone morphogenetic protein 2 loaded
lipopolysaccharide-amine nanopolymersomes. Zhongguo Xiu Fu Chong
Jian Wai Ke Za Zhi. 28:1292–1297. 2014.(In Chinese). PubMed/NCBI
|
|
9
|
Langer R: New methods of drug delivery.
Science. 249:1527–1533. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma J, Cao H and Li Y and Li Y: Synthesis
and characterization of poly(DL-lactide)-grafted gelatins as
bioabsorbable amphiphilic polymers. J Biomater Sci Polym Ed.
13:67–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma Z, Chen F, Zhu YJ, Cui T and Liu XY:
Amorphous calcium phosphate/poly(D,L-lactic acid) composite
nanofibers: Electrospinning preparation and biomineralization. J
Colloid Interface Sci. 359:371–379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vukicevic S and Sampath KTB: one
Morphogenetic Proteins: From Laboratory to Clinical Practice.
Berlin: Birkhäuser Verlag. 60–126. 2002.
|
|
13
|
Cirano FR, Togashi AY, Marques MM,
Pustiglioni FE and Lima LA: Role of rhBMP-2 and rhBMP-7 in the
metabolism and differentiation of osteoblast-like cells cultured on
chemically modified titanium surfaces. J Oral Implantol.
40:655–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luangphakdy V, Shinohara K, Pan H, Boehm
C, Samaranska A and Muschler GF: Evaluation of
rhBMP-2/collagen/TCP-HA bone graft with and without bone marrow
cells in the canine femoral multi defect model. Eur Cell Mater.
29:57–69. 2015.PubMed/NCBI
|
|
15
|
Kandziora F, Pflugmacher R, Scholz M,
Knispel C, Hiller T, Schollmeier G, Bail H, Schmidmaier G, Duda G,
Raschke M and Haas NP: Comparison of BMP-2 and combined
IGF-I/TGF-ss1 application in a sheep cervical spine fusion model.
Eur Spine J. 11:482–493. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmidmaier G, Wildemann B, Cromme F,
Kandziora F, Haas NP and Raschke M: Bone morphogenetic protein-2
coating of titanium implants increases biomechanical strength and
accelerates bone remodeling in fracture treatment: A biomechanical
and histological study in rats. Bone. 30:816–822. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kandziora F, Scholz M, Pflugmacher R,
Krummrey G, Schollmeier G, Schmidmaier G, Schnake KJ, Duda G,
Raschke M and Haas NP: Experimental fusion of the sheep cervical
spine. Part II: Effect of growth factors and carrier systems on
interbody fusion. Chirurg. 73:1025–1038. 2002.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Illi OE and Feldmann CP: Stimulation of
fracture healing by local application of humoral factors integrated
in biodegradable implants. Eur J Pediatr Surg. 8:251–255. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Böstman OM: Absorbable implants for the
fixation of fractures. J Bone Joint Surg Am. 73:148–153.
1991.PubMed/NCBI
|
|
20
|
Bergsmaju E, Bruijn W, Roxema FR, Bos RR
and Boering G: Late degradation tissue response to poly(L-lactide)
bone plate and screws. Biomaterials. 16:25–31. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suuronen R, Pohjonen T, Hietanen J and
Lindqvist C: A 5-year in vitro and vivo study of biodegradation of
polylactide plates. J Oral Maxillofac Surg. 56:604–614. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ashammakhi N, Mäkelä EA, Vihtonen K,
Rokkanen P, Kuisma H and Törmälä P: Strength retention of
self-reinforced polyglycolide membrane: An experimental study.
Biomaterials. 16:135–138. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Manninen MJ, Päivärinta U, Taurio R,
Törmälä P, Suuronen R, Räihä J, Rokkanen P and Pätiälä H:
Polylactide screws in the fixation of olecranon osteotomies. A
mechanical study in sheep. Acta Orthop Scand. 63:437–442. 1992.
View Article : Google Scholar : PubMed/NCBI
|