Introduction
Polycystic ovarian syndrome (PCOS) is a common
endocrine disorder in women of childbearing age. Characteristic
clinical presentations include: Menstrual disorders, oligoovulation
or anovulation, infertility, hirsutism, obesity, ovarian
enlargement, and irregular serum hormone concentrations (1). Patients with PCOS account for 5–10% of
women of childbearing age, 70–80% of patients with ovulation
disorder, and 50% of patients who are undergoing assisted
reproductive technologies (2).
Due to the complex endocrine conditions associated
with PCOS, the induction of controlled ovulation for in
vitro fertilization (IVF) or intracytoplasmic sperm injection
(ICSI) is challenging. This is largely due to the very narrow
response range to gonadotropin (Gn), ovarian hyperstimulation
syndrome (OHSS), and a surge in endogenous luteinizing hormone
(LH), which may affect the quality of oocytes and reduce clinical
pregnancy rates (3–5). To date, no exact optimization scheme
exists for patients with PCOS (6).
Currently, the majority of reproductive centers use traditional
long protocols to inhibit an endogenous premature LH surge and
prevent premature luteinized follicles, in order to induce high
quality oocytes and successful pregnancies (7,8). As
such, the pregnancy rates of patients with PCOS who receive the
long pituitary downregulation protocol are not always ideal; some
patients exhibit poor outcomes due to insufficient downregulation,
and premature endogenous LH surge, even with a long duration of
high dose Gn. To date, the ultra-long protocol has predominantly
been used for infertile patients with endometriosis, with
noteworthy outcomes (9). In the
present study, a modified ultra-long protocol for patients with
PCOS was implemented. Data were retrospectively summarized between
September 2011 and April 2012 in our assisted reproductive
technology center in the Reproductive & Genetic Hospital of
Citic-Xiangya (Changchun, China). Patients with PCOS were divided
into modified ultra-long protocol or conventional long protocol
groups, and endogenous LH levels, endometrial receptivity, and the
clinical outcomes of the two groups were compared.
Materials and methods
Patients
A total of 147 patients admitted to the Reproductive
& Genetic Hospital of Citic-Xiangya were recruited between
September 2011 and April 2012 undergoing IVF/ICSI, were divided
into two groups: An ultra-long protocol group (n=75) and a long
protocol group (n=72). The exclusion criteria were: Related
disorders, >38 years old, endometriosis, uterine malformation,
untreated hydrosalpinx, intrauterine adhesions, and a history of
endometriosis. PCOS was diagnosed according to the Rotterdam
criteria (10) and patients were
selected based on at least two of the following three features: i)
Oligoovulation or anovulation, ii) clinical and/or biochemical
signs of hyperandrogenism, or iii) polycystic ovaries with
congenital adrenal hyperplasia, excluding Cushing's syndrome and
tumor-related testosterone concentration elevations. The research
protocol was approved by the Ethics Committee of the Central South
University (Changsha, China) and the Reproductive & Genetic
Hospital of Citic-Xiangya. All participants provided their written
informed consent to participate in the present study.
Treatments
The starting doses of Gn releasing hormone agonist
(GnRHa) were chosen according to the patient's age, history and
body weight. All patients received Dane-35 (Schering GmbH und Co.
Produktions KG, Bayer, Germany) for one complete menstrual cycle
from day 3.
Routine long pituitary downregulation
protocol
On day 20 of the patient's menstrual cycle 1.5–1.875
mg GnRHa was intramuscularly injected. After a period of 13–20
days, following confirmation of pituitary-ovarian suppression,
75U-150 IU/d recombinant follicle stimulating hormone (rFSH)
(Gonal-F or Puregon; Merck Serono S.A., Coinsins, Switzerland) was
administered for 4–5 days. Human chorionic Gn (hCG) was injected
following confirmation of adequate follicle stimulation by
ultrasound and hormone concentrations.
Modified ultra-long pituitary
downregulation protocol
On day 20 of the patient's first menstrual cycle
1.5–1.875 mg GnRHa was intramuscularly injected. This was repeated
on day 21 of the following menstrual cycle. After 13–20 days,
following confirmation of pituitary-ovarian suppression, human
menopausal gonadotropin (hMG, 75 U-375 U/d, Menopur; Ferring
Pharmaceuticals, Kiel, Germany) was injected for 4–5 days. hCG was
injected following confirmation of adequate follicle stimulation by
ultrasound and hormone concentrations.
Evaluation criteria for follicle
stimulation
Follicle development status was determined by
ultrasonography, combined with serum hormone concentration
determinations. Controlled ovarian hyperstimulation (COH) was
adapted using a Gn step-up, step-down or withdrawal scheme
according to estrogen (E2), progestogen (P), and luteinizing
hormone (LH) levels, and vaginal ultrasound results. The criteria
for adequate pituitary-ovarian suppression were: E2 <40 pg/ml, P
<0.8 ng/ml and LH <3 mIU/ml serum concentrations; as well as
endometrium thickness <5 mm and, no follicle or corpus luteum
cyst ≥10 mm present in the ovary. rFSH or hMG was administered to
achieve egg stimulation until follicles ≥18 mm accounted for 60–70%
of follicles >14 mm, or follicles ≥20 mm accounted for 40–50% of
follicles >14 mm. Patients were scheduled for oocyte retrieval
35–36 h following hCG injection. E2 per every 14 mm follicle was
200–300 pg/ml. Indications and techniques for oocyte aspiration,
oocyte and embryo culture, insemination, ICSI, assisted hatching
and embryo transfer (ET) were based on the routine of the center
(ISO 9001 Certification) (11).
Determinations of endometrial
change
The endometrial thickness, morphology alterations,
and blood flow of the two groups were assessed by vaginal color
ultrasound on the day of hCG injection and the day of ET. GE
V730-expert color Doppler ultrasonic diagnostic apparatus (GE
Healthcare Life Sciences, Little Chalfont, UK) was used with an
intra-cavity probe frequency of 5–9 MHz. The operator and settings
remained constant throughout the study, and test indices were
longitudinal endometrial thickness and endometrial morphology,
which were assessed using the classification system outlined by
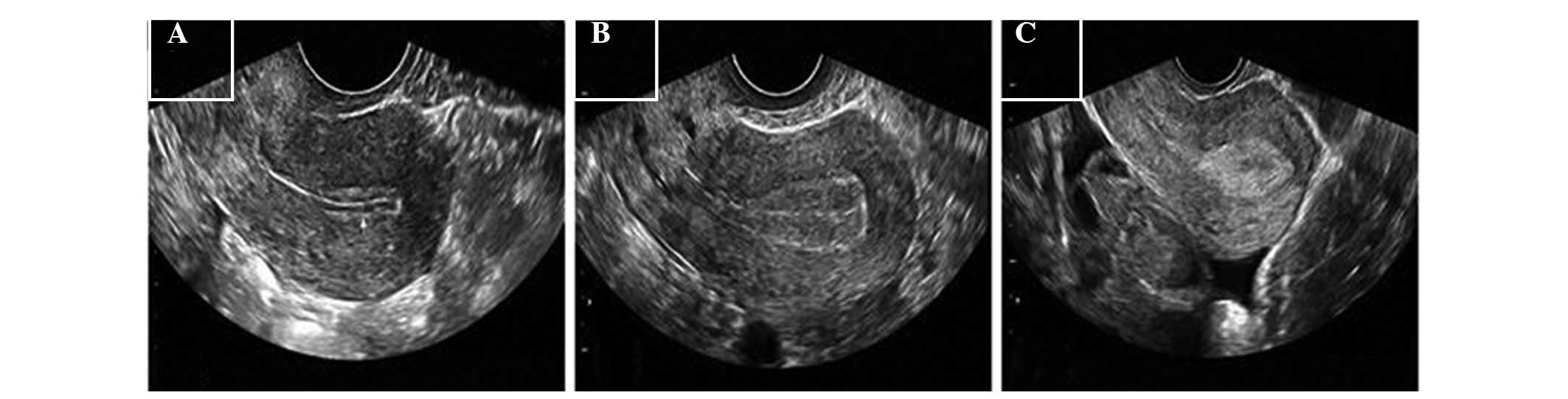
Gonen and Casper (12), as follows;
Type A, three linear or multilayer endometrium, echogenic outer and
central areas and hypoechoic or dark inner areas, and significant
uterine cavity midline echo; Type B, the middle area exhibits an
isolated echo-like uterine myometrium image, and the uterine cavity
midline echo is not clear; Type C, the endometrium is homogeneously
echogenic, without uterine cavity midline echo. Endometrial blood
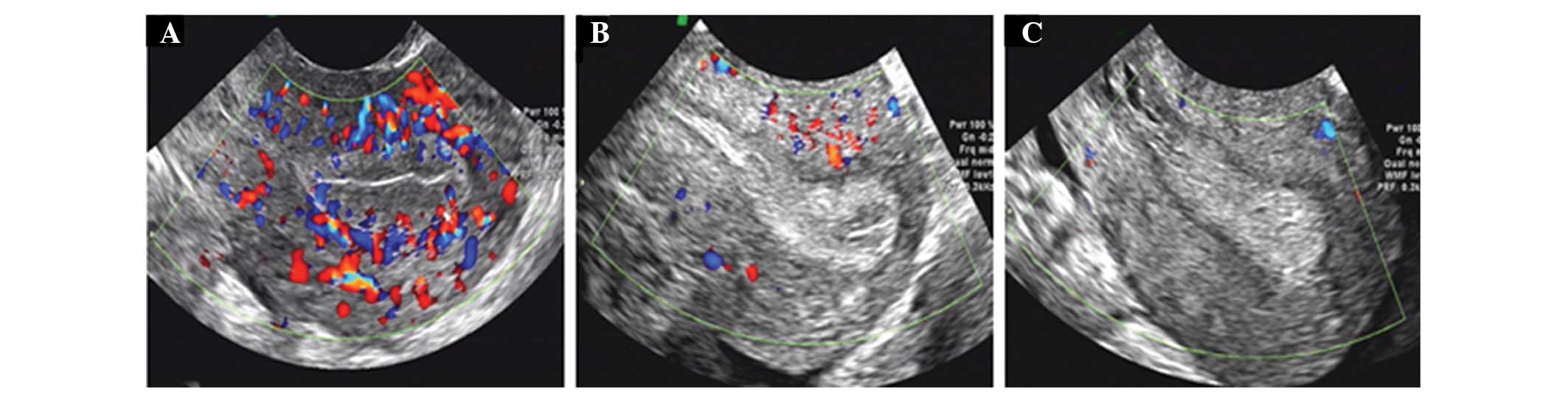
flow was categorized according to Chien et al (13); Type A, blood flow could be monitored
as endometrial and subendometrial; Type B, blood flow could only be
monitored as subendocardial blood flow; and Type C, Endometrium and
endometrial blood flow could not be detected.
Outcome parameters
Serum hormone concentrations of the both groups were
evaluated prior to GnRHa stimulation and on the day of hCG
administration. The mean number of retrieved oocytes, fertilization
rate, good quality embryo rate, cycle cancellation rate, the number
of transferred embryos, implantation rate, pregnancy rate,
miscarriage rate following transplantation, severe OHSS incidence,
the incidence of ectopic pregnancy, the GnRHa dosage, and the days
of drug administration were compared between the two groups.
Statistical analyses
Sigmastat software, version 3.5 (Systat Software
Inc., San Jose, CA, USA) was used to carry out statistical
analyses. Values are expressed as mean ± standard deviation, or
number and percentage. Comparison of quantitative variables between
groups was achieved by one-way analysis of variance. Homogeneity of
variance and normality of data were estimated using Levene and
Kolmogorov-Smirnov tests, respectively. Qualitative variables were
analyzed by χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Age, infertility duration (years), body mass index
(BMI), and basal FSH and LH values were compared between the two
groups and no significant differences were detected (Table I). Table
II presents the comparisons between clinical outcomes of the
two protocols. Within the ultra-long protocol group, the LH level
on the day prior to Gn administration, and the P level (ng/ml) and
P/E2 (P (ng/ml) × 1,000/E2 (pg/ml)) ratio on the day of hCG
administration were significantly lower, as compared with that of
the long protocol group (P<0.05). Furthermore, the implantation
and clinical pregnancy rates of the ultra-long protocol group were
significantly higher, as compared with those of the long protocol
group, (P<0.05). Other parameters included: Gn dosage, duration
of Gn, levels of E2 and LH on the day of hCG, the mean number of
oocytes retrieved, fertilization rates, good quality embryo rates,
cycle cancellation rates, the number of transferred embryos,
implantation rates, pregnancy rates, miscarriage rates of
transplantations, severe OHSS incidences and the incidence of
ectopic pregnancies, all of which exhibited no significant
differences. The increase in clinical pregnancy rate observed in
the ultra-long protocol group may be caused by the optimal P level
and P/E2 ratio on the day of hCG administration, which resulted in
improved endometrial receptivity. Endometrial morphology and blood
flow are shown in Figs. 1 and
2, whereas Table III details the differences in
endometrial thickness, morphology, and blood flow between the two
protocols. The proportion of patients with type A and B endometrial
morphology on the day of hCG administration in the ultra-long
protocol group was 32.0 and 57.3%, respectively; thus greater than
that observed in the long protocol group (23.6 and 43.1%,
respectively); however significance was not reached. The proportion
of patients with type C endometrial morphology on the day of hCG
application in the ultra-long protocol group (10.7%) was
significantly lower as compared with that of the long protocol
group (33.3%) (P=0.001). The endometrial morphology of the patients
receiving both protocols was type C on the day of ET. The
proportion of patients with types A and B endometrial blood flow
and sub-endometrial tissue, on the days of hCG medication and of ET
was higher in the ultra-long protocol group compared with the long
protocol group; however, the difference was not statistically
significant. The proportion of patients with type C endometrial
morphology on the days of hCG administration and ET were
significantly lower in the ultra-long protocol group compared with
the long protocol group.
 | Table I.Basic clinical features of the
patients with polycystic ovarian syndrome. |
Table I.
Basic clinical features of the
patients with polycystic ovarian syndrome.
| Characteristics | Ultra-long protocol
(n=75) | Long protocol
(n=72) | P-value |
|---|
| Age (years) |
28.01±2.03 |
27.89±2.91 | 0.7652 |
| Infertility duration
(years) |
4.49±2.43 |
3.88±2.44 | 0.1262 |
| BMI
(kg/m2) |
22.61±2.85 |
21.71±2.85 | 0.0580 |
| Basal FSH
(mIU/ml) |
5.89±1.21 |
6.12±1.17 | 0.2460 |
| Basal LH
(mIU/ml) |
8.41±5.08 |
7.58±6.20 | 0.3753 |
 | Table II.Comparison of clinical outcomes of
patients with polycystic ovarian syndrome treated with two
protocols. |
Table II.
Comparison of clinical outcomes of
patients with polycystic ovarian syndrome treated with two
protocols.
| Variables | Ultra-long protocol
(n=75) | Long protocol
(n=72) | P-value |
|---|
| LH level on the day
prior to Gn (mIU/ml) | 0.63±0.69 | 1.89±0.87 |
<0.0001a |
| Dosage of Gn
(IU) | 2104.9±908.92 | 2020.76±899.76 |
0.5737 |
| Duration of Gn
(days) | 11.52±2.15 | 11.85±2.13 |
0.3562 |
| P level on hCG day
(ng/ml) | 0.63±0.22 | 0.77±0.23 |
0.0002a |
| E2 level on hCG day
(pg/ml) | 3707.29±1307.97 | 3667.29±1621.14 |
0.8698 |
| Px1,000/E2 on HCG
day | 0.19±0.08 | 0.25±0.13 |
0.0006a |
| LH level on HCG day
(mIUml) | 1.40±0.45 | 1.29±0.51 |
0.1558 |
| Mean number of
oocytes retrieved | 14.63±5.96 | 15.58±5.00 |
0.2928 |
| Fertilization rate
(%) | 84.11±17.16 | 82.26±14.95 |
0.4865 |
| Good quality embryo
rate (%) | 71.13±25.10 | 66.52±25.15 |
0.2679 |
| Implantation rate
(%) | 59.56 (81/136) | 44.09 (56/127) |
0.012a |
| Cancel rate
(%) | 9.33 (7/75) | 13.89 (10/72) | 0.388 |
| Number of
transferred embryos | 2.0±0 | 2.05±0.28 |
0.1595 |
| Clinical pregnancy
rate per transfer (%) | 77.94 (53/68) | 61.29 (38/62) |
0.039a |
| Severe OHSS rate
(%) | 1.33 (1/75) | 2.78 (2/72) | 0.536 |
| Abortion rate
(%) | 4.76 (3/53) | 4.76 (2/38) | 1.000 |
| Ectopic pregnancy
rate (%) | 0 (0/53) | 2.38 (1/38) | 0.218 |
 | Table III.Endometrial thickness, morphology,
and blood flow following treatment with the two protocols. |
Table III.
Endometrial thickness, morphology,
and blood flow following treatment with the two protocols.
| Endometrial
characteristics | Ultra-long protocol
(n=75) | Long protocol
(n=72) | P-value |
|---|
| Day of hCG
administration |
|
|
|
|
Endometrial thickness
(mm) | 2.67±1.67 | 11.78±2.30 | 0.0092 |
|
Endometrial morphology
(%) |
|
|
|
|
A | 32.0 (24/75) | 23.6 (17/72) | 0.257 |
|
B | 57.3 (43/75) | 43.1 (31/72) | 0.083 |
|
C | 10.7 (8/75) | 33.3 (24/72) | 0.001 |
|
Endometrial blood flow
(%) |
|
|
|
|
A | 50.7 (38/75) | 36.1 (26/72) | 0.075 |
|
B | 49.3 (37/75) | 56.9 (41/72) | 0.278 |
|
C | 0 (0/75) | 6.9 (5/72) | 0.020 |
| Day of ET |
|
|
|
|
Endometrial thickness
(mm) | 12.91±2.01 | 11.97±2.85 | 0.0226 |
|
Endometrial morphology
(%) |
|
|
|
|
A | 0 (0/75) | 0 (0/72) | 1.000 |
|
B | 0 (0/75) | 0 (0/72) | 1.000 |
|
C | 100 (75/75) | 100 (72/72) | 1.000 |
|
Endometrial blood flow
(%) |
|
|
|
|
A | 32.0 (24/75) | 23.6 (17/72) | 0.257 |
|
B | 49.3 (37/75) | 37.5 (27/72) | 0.148 |
|
C | 18.7 (14/75) | 38.9 (28/72) | 0.007 |
Discussion
The present study demonstrated that patients with
PCOS treated with an ultra-long protocol pituitary downregulation
protocol benefited from improved clinical outcomes, as compared
with patients in the long protocol group; this may be associated
with a reduction in P level on the day of hCG administration. Bosch
et al (14) demonstrated that
serum P levels and the ratio of P/E2 on the day of hCG
administration may be indicators of an implantation window for
optimal endometrial receptivity, which is shifted forward in IVF
patients, as compared with women in natural menstruation cycles.
Elgindy (15) reported that clinical
blastocyst pregnancy rates per transfer were reduced if, on the day
of hCG administration, the P serum levels and the ratio of P/E2
were higher than 1.5 ng/ml and 0.55, respectively. Previous studies
(16–18) have noted high P levels on the day of
hCG administration and resultant lower clinical and complete
pregnancy rates. The results of the present study demonstrated that
serum P levels and the ratio of P/E2 on the day of hCG
administration were significantly lower in the ultra-long protocol
group, as compared with those in the long protocol group; thus
inhibiting the forward shift of the uterine endometrium
implantation window and improving endometrial receptivity. LH
levels prior to the day of Gn stimulation reflect downregulation of
pituitary suppression. Prior to Gn stimulation, lower LH levels
were observed in the ultra-long protocol group compared with the
long protocol group. A prior study suggested that the optimum LH
threshold range of 0.5–1.5 IU/l (19). hMG was used to initiate ovulation in
the ultra-long protocol, which was a supplement for low LH levels
and obtained good pregnancy outcomes.
Previous studies have demonstrated that embryos may
be easier to implant, and exhibit improved development on type A
and B endometrial morphologies, as compared with that of type C
(6,20,21).
Järvelä et al (22) suggested
that COH multilayer (three linear) endometrial morphology is more
suitable for embryo implantation, with homogeneous endometrial
morphology associated with poor IVF outcomes, as non-three linear
endometrial morphology can indicate that premature transformation
of the endometrium into the secretory phase. In the present study,
the percentage of type C endometrial morphology on the day of hCG
medication in the ultra-long protocol group was significantly
lower, as compared with that observed in the long protocol group
(P=0.001). The majority of previous studies concur that appropriate
thickness of the endometrium is essential for embryo implantation.
It has been observed that clinical pregnancy rates increase once
the endometrial thickness increases to >14 mm on the day of hCG
application, whereas implantation and pregnancy rates were almost
zero when endometrial thickness was <6–7 mm (21,23–26). In
the present study, the endometrium of the ultra-long protocol group
was thicker on the day of hCG administration, as compared with that
of the long protocol group, and the endometrial morphologies on day
of ET were type C in both protocols. These findings demonstrate
that the ultra-long protocol led to adequate pituitary
downregulation and may have inhibited premature endometrium
transformation into the secretory phase, thus inhibiting the
forward shift of the endometrial implantation window.
In the present study, the proportion of patients
with type A endometrial blood flow and sub endometrial tissue on
the days of hCG medication and of ET was higher in the ultra-long
protocol group compared with the long protocol group. The
proportion of patients with type B endometrial blood flow and sub
endometrial tissue on the day of ET was higher in the ultra-long
protocol group compared with the long protocol group; however, the
difference was statistically insignificant. In previous studies,
good sub-endometrial and endometrial perfusion has been reported to
be important for successful embryo implantation (8,13,27).
Furthermore, previous studies have noted that endometrial blood
flow following oocyte retrieval in IVF/ICSI-ET pregnant patients,
and in spontaneous pregnancies post-ovulation, was higher than in
non-pregnant women (22,28).
Obesity and a high BMI negatively affect embryo
quality and endometrial receptivity in assisted reproduction.
Pregnancy rates have been shown to be significantly lower in
patients with BMI ≥27 kg/m2, as compared with in
patients with BMI ≤25 kg/m2 and 25–27 kg/m2
(27,29–31). The
ideal BMI of Chinese women is 18–23 kg/m2 and in the
present study, the BMI of patients in the modified ultra-long
protocol group was slightly higher, as compared with that in the
long protocol group. However, the implantation and pregnancy rates
were significantly higher in patients in the ultra-long protocol
group, as compared with the long protocol group; therefore, we
propose that patients with PCOS with BMIs >23 kg/m2
or obesity may be more suitable for the ultra-long protocol.
In conclusion, two injections of small GnRHa doses
prior to COH, combined with hMG, significantly increased pregnancy
rates. Lower serum P levels on the day of hCG administration in a
modified ultra-long pituitary downregulation protocol may improve
pregnancy rates and ameliorate the receptivity of the endometrium,
as assessed by endometrial morphology, thickness, and blood flow.
It may be hypothesized that a modified ultra-long protocol, coupled
with hMG ovarian stimulation, is a preferable treatment for
patients with PCOS, especially those with elevated BMIs. Further
studies should focus on biological endometrial changes and
associated mechanisms in an ultra-long protocol.
References
|
1
|
Sheehan MT: Polycystic ovarian syndrome:
Diagnosis and management. Clin Med Res. 2:13–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hart R: Polycystic ovarian syndrome -
prognosis and treatment outcomes. Curr Opin Obstet Gynecol.
19:529–535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen ZJ and Shi Y: Polycystic ovary
syndrome. Front Med China. 4:280–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elnashar AM: Progesterone rise on the day
of HCG administration (premature luteinization) in IVF: An overdue
update. J Assist Reprod Genet. 27:149–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pundir J, Sunkara SK, El-Toukhy T and
Khalaf Y: Meta-analysis of GnRH antagonist protocols: Do they
reduce the risk of OHSS in PCOS? Reprod Biomed Online. 24:6–22.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orvieto R, Meltcer S, Liberty G, Rabinson
J, Anteby EY and Nahum R: Does day-3 LH/FSH ratio influence in
vitro fertilization outcome in PCOS patients undergoing controlled
ovarian hyperstimulation with different GnRH-analogue? Gynecol
Endocrinol. 28:422–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rickes D, Nickel I, Kropf S and Kleinstein
J: Increased pregnancy rates after ultralong postoperative therapy
with gonadotropin-releasing hormone analogs in patients with
endometriosis. Fertil Steril. 78:757–762. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Surrey ES, Silverberg KM, Surrey MW and
Schoolcraft WB: Effect of prolonged gonadotropin-releasing hormone
agonist therapy on the outcome of in vitro fertilization-embryo
transfer in patients with endometriosis. Fertil Steril. 78:699–704.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mijatovic V, Florijn E, Halim N, Schats R
and Hompes P: Adenomyosis has no adverse effects on IVF/ICSI
outcomes in women with endometriosis treated with long-term
pituitary down-regulation before IVF/ICSI. Eur J Obstet Gynecol
Reprod Biol. 151:62–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rotterdam ESHRE/ASRM-Sponsored PCOS
Consensus Workshop Group: Revised 2003 consensus on diagnostic
criteria and long-term health risks related to polycystic ovary
syndrome (PCOS). Hum Reprod. 19:41–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alper MM, Brinsden PR, Fischer R and
Wikland M: Is your IVF programme good? Hum Reprod. 17:8–10. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gonen Y and Casper RF: Prediction of
implantation by the sonographic appearance of the endometrium
during controlled ovarian stimulation for in vitro fertilization
(IVF). J In Vitro Fert Embryo Transf. 7:146–152. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chien LW, Au HK, Chen PL, Xiao J and Tzeng
CR: Assessment of uterine receptivity by the
endometrial-subendometrial blood flow distribution pattern in women
undergoing in vitro fertilization-embryo transfer. Fertil Steril.
78:245–251. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bosch E, Valencia I, Escudero E, Crespo J,
Simón C, Remohí J and Pellicer A: Premature luteinization during
gonadotropin-releasing hormone antagonist cycles and its
relationship with in vitro fertilization outcome. Fertil Steril.
80:1444–1449. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elgindy EA: Progesterone level and
progesterone/estradiol ratio on the day of hCG administration:
Detrimental cutoff levels and new treatment strategy. Fertil
Steril. 95:1639–1644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Z, Li R, Ma Y, Deng B, Zhang X, Meng Y,
Chen X, Liu P and Qiao J: Effect of HCG-day serum progesterone and
oestradiol concentrations on pregnancy outcomes in GnRH agonist
cycles. Reprod Biomed Online. 24:511–520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Papanikolaou EG, Pados G, Grimbizis G,
Bili E, Kyriazi L, Polyzos NP, Humaidan P, Tournaye H and Tarlatzis
B: GnRH-agonist versus GnRH-antagonist IVF cycles: Is the
reproductive outcome affected by the incidence of progesterone
elevation on the day of HCG triggering? A randomized prospective
study. Hum Reprod. 27:1822–1828. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Venetis CA, Kolibianakis EM, Bosdou JK and
Tarlatzis BC: Progesterone elevation and probability of pregnancy
after IVF: A systematic review and meta-analysis of over 60 000
cycles. Hum Reprod Update. 19:433–457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Humaidan P, Bungum L, Bungum M and
Andersen CY: Ovarian response and pregnancy outcome related to
mid-follicular LH levels in women undergoing assisted reproduction
with GnRH agonist down-regulation and recombinant FSH stimulation.
Hum Reprod. 17:2016–2021. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharara FI, Lim J and McClamrock HD:
Endometrial pattern on the day of oocyte retrieval is more
predictive of implantation success than the pattern or thickness on
the day of hCG administration. J Assist Reprod Genet. 16:523–528.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Chen CH, Confino E, Barnes R,
Milad M and Kazer RR: Increased endometrial thickness is associated
with improved treatment outcome for selected patients undergoing in
vitro fertilization-embryo transfer. Fertil Steril. 83:336–340.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Järvelä IY, Sladkevicius P, Kelly S, Ojha
K, Campbell S and Nargund G: Evaluation of endometrial receptivity
during in-vitro fertilization using three-dimensional power Doppler
ultrasound. Ultrasound Obstet Gynecol. 26:765–769. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kinay T, Tasci Y, Dilbaz S, Cinar O, Demir
B and Haberal A: The relationship between endometrial thickness and
pregnancy rates in GnRH antagonist down-regulated ICSI cycles.
Gynecol Endocrinol. 26:833–837. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okohue JE, Onuh SO, Ebeigbe P, Shaibu I,
Wada I, Ikimalo JI and Okpere EE: The effect of endometrial
thickness on in vitro fertilization (IVF)-embryo
transfer/intracytoplasmic sperm injection (ICSI) outcome. Afr J
Reprod Health. 13:113–121. 2009.PubMed/NCBI
|
|
25
|
Rashidi BH, Sadeghi M, Jafarabadi M and
Tehrani Nejad ES: Relationships between pregnancy rates following
in vitro fertilization or intracytoplasmic sperm injection and
endometrial thickness and pattern. Eur J Obstet Gynecol Reprod
Biol. 120:179–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Traub ML, Van Arsdale A, Pal L, Jindal S
and Santoro N: Endometrial thickness, Caucasian ethnicity, and age
predict clinical pregnancy following fresh blastocyst embryo
transfer: A retrospective cohort. Reprod Biol Endocrinol. 7:332009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lintsen AM, Pasker-de Jong PC, de Boer EJ,
Burger CW, Jansen CA, Braat DD and van Leeuwen FE: Effects of
subfertility cause, smoking and body weight on the success rate of
IVF. Hum Reprod. 20:1867–1875. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raine-Fenning NJ, Campbell BK, Kendall NR,
Clewes JS and Johnson IR: Quantifying the changes in endometrial
vascularity throughout the normal menstrual cycle with
three-dimensional power Doppler angiography. Hum Reprod.
19:330–338. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jungheim ES, Lanzendorf SE, Odem RR, Moley
KH, Chang AS and Ratts VS: Morbid obesity is associated with lower
clinical pregnancy rates after in vitro fertilization in women with
polycystic ovary syndrome. Fertil Steril. 92:256–261. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Metwally M, Cutting R, Tipton A, Skull J,
Ledger WL and Li TC: Effect of increased body mass index on oocyte
and embryo quality in IVF patients. Reprod Biomed Online.
15:532–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sneed ML, Uhler ML, Grotjan HE, Rapisarda
JJ, Lederer KJ and Beltsos AN: Body mass index: Impact on IVF
success appears age-related. Hum Reprod. 23:1835–1839. 2008.
View Article : Google Scholar : PubMed/NCBI
|