Introduction
For limited-stage small-cell lung cancer (LS-SCLC),
chemo-radiotherapy is a standard treatment and has been shown to
improve patient survival (1,2). However, maintenance of local control is
not reliably achieved with this treatment approach, which thus
contributes to the high morbidity and mortality rates observed for
patients with SCLC (3). Over the
past several decades, radiation dose and patterns of radiation have
been varied to optimize treatment (4–7).
Theoretically, the application of higher doses of radiation to a
tumor should improve local control rates, and studies have
confirmed that there is a positive association between tumor
control and higher radiation dose (5). However, in the Radiation Therapy
Oncology Group (RTOG)-0617 study of non-small cell lung cancer
(NSCLC) (8), the survival time of
patients receiving 74 Gy irradiation was found to be shorter than
that for patients receiving 60 Gy irradiation. While the reason for
this observation remains unclear, it may be due to adverse
radiation-induced effects. Thus, research is ongoing to optimize
radiation delivery to tumors while sparing surrounding normal
structures.
Of particular interest is the application of
simultaneous integrated dose reduction intensity-modulated
radiotherapy (SIR-IMRT) for the treatment of malignancies (9,10).
SIR-IMRT simultaneously delivers a relatively higher dose of
radiation to the primary disease, and a relatively lower dose to
the subclinical disease or other selected regions. However, the
outcome for SIR-IMRT in patients with LS-SCLC remains to be
determined.
Therefore, the goal of the present study was to
evaluate the feasibility of using SIR-IMRT for the treatment of
LS-SCLC, and to provide evidence in support of future clinical
studies.
Materials and methods
Patients
This retrospective clinical study was approved by
the institutional review board of Tianjin Medical University Cancer
Institute and Hospital (Tianjin, China). Between January 2010 and
March 2013, patients with LS-SCLC who accepted SIR-IMRT at the
hospital were included in this study. Two senior pathologists
specializing in lung carcinoma reviewed all biopsy specimens, and
pathologic staging was conducted according to the current American
Joint Committee on Cancer (AJCC) criteria for NSCLC (11). All patients were evaluated for
hematologic, hepatic and renal function, and also underwent chest
computed tomography (CT), neck and abdomen ultrasound, brain
magnetic resonance imaging (MRI) and bone scan imaging prior to
receiving radiotherapy.
Therapy
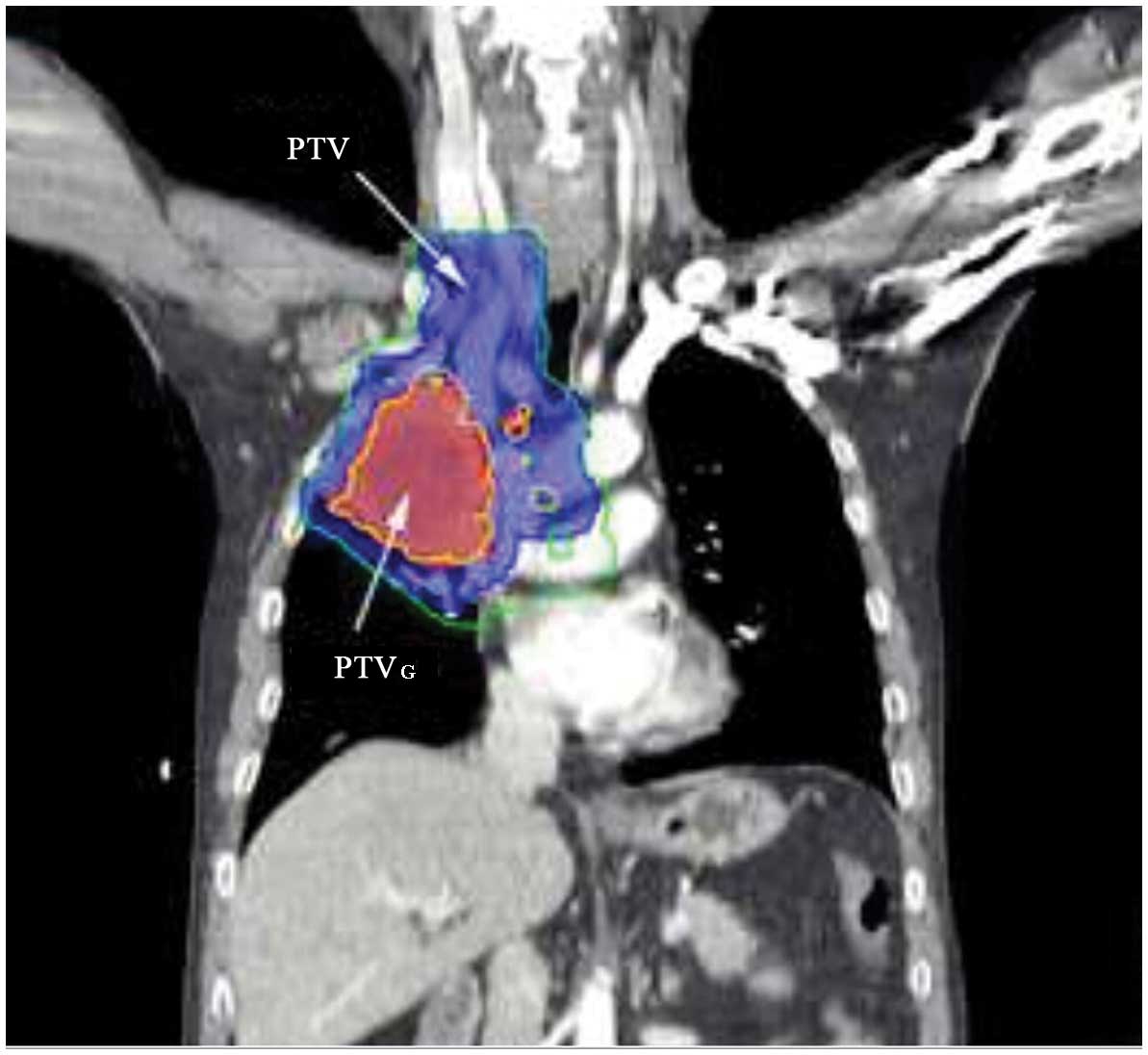
The primary tumor was delineated using a lung
window, while mediastinal windows were used to delineate the medial
border of centrally located primary tumors, involved lymph nodes
and adjacent normal organs. Gross tumor volume (GTV) was defined as
any visible primary lesion present on CT simulations. All lymph
nodes with a diameter ≥1 cm along their short axis were also
included. The planning gross tumor volume (PTVG) was established by
including a 0.5-cm margin around the GTV. Clinical target volume
(CTV) was defined as the high-risk lymph nodal regions, including
adjacent regions of involved lymph nodes and the ipsilateral hilar
[in accordance with the new lymph node map of the International
Association for the Study of Lung Cancer (12)], including the GTV with a 0.5-cm
margin. Another 0.5-cm margin was added to establish the planning
target volume (PTV). The prescribed radiation dose, 60 Gy to the
PTVG at 2 Gy/day and 54 Gy to the PTV at 1.8 Gy/day, was delivered
to ≥95% of the PTVG or PTV, respectively. The representative dose
distribution using SIR-IMRT is shown in Fig. 1. Each treatment plan consisted of
five static fields with the following normal tissue constraints: i)
total lung, Vlung5 (i.e., the percentage of lung volume receiving
≥5 Gy) was ≤60% and Vlung20 was ≤35%; ii) Vlung40 was ≤30%; iii)
Vesophagus50 was ≤50%, Vesophagus maximum was ≤60 Gy; and iv)
Vspinal cord maximum was ≤45 Gy. The definitive dose volume
parameter for the organ at risk (OAR) parameter for each subject is
listed in Table I. For patients who
achieved a complete response (CR) following thoracic radiotherapy,
prophylactic cranial irradiation (PCI) was recommended, with a dose
of 25 Gy administered over 10 fractions.
 | Table I.Dose volume parameter of organ at risk
(OAR; n=52). |
Table I.
Dose volume parameter of organ at risk
(OAR; n=52).
| OAR | Dose volume |
|---|
| MLD, cGy |
1,479.06±188.53 |
| Vlung5,
% |
49.33±7.08 |
| Vlung20,
% |
28.85±3.29 |
| Vlung30,
% |
20.17±3.13 |
| Esophagus
Dmax, cGy |
6,052.50±355.46 |
|
Vesophagus50, % |
42.98±14.88 |
| Cord Dmax,
cGy |
4,451.93±343.43 |
Follow-up
Patient follow-up started after the radiation
treatment was completed. Initially, patients were monitored 1 month
and 3 months after irradiation; they were monitored every 3 months
thereafter. Follow-up appointments included a chest X-ray or CT
scan and a color Doppler ultrasound of the abdomen. Cranial CT/MRI
and bone scans were also performed if necessary. However,
regardless of follow-up stage, any symptoms that developed were
immediately examined. By November 30, 2013, the follow-up rate for
this cohort was 100%, and the median follow-up period was 16.5
months (range, 7–42 months).
Response assessments and toxicity
Radiation-related toxicities for lung and esophagus
were assessed by two senior radiation therapists according to the
Common Terminology Criteria for Adverse Events (CTCAE) version 3.0
(13). Response to radiation was
first assessed 3 months after the completion of radiation based on
new guidelines designed to evaluate the treatment response of solid
tumors (14). These guidelines
include considerations of CR, partial response (PR), stable disease
(SD) and progressive disease (PD). Local recurrence was classified
as in-field relapse or out-of-field relapse. The former was defined
as recurrence within the 95% isodose curve of PTV. Correspondingly,
PTVG, GTV, CTV, and PTV recurrence were defined as being within the
95% isodose curve of each, respectively. Regarding out-of-field
recurrences, these were defined as lesions outside of the 95%
isodose curve of the PTV target area that were confined to the
lung, pulmonary, mediastinal and supraclavicular regions without
distant metastasis (DM). Recurrences beyond these areas were
considered DM events.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Using the
Kaplan-Meier method, OS, locoregional recurrence-free survival
(LRFS) and progression-free survival (PFS) were calculated using
the pathological diagnosis date as the starting point. The endpoint
for OS was the date of mortality or the date of the last follow-up;
the endpoint for LRFS was the date of primary tumor detection, the
date of regional lymph recurrence or the last follow-up date; and
the endpoint for PFS was the date that disease progression was
detected or the date of the last follow-up.
Results
Patients
Fifty-two LS-SCLC patients who received SIR-IMRT
were enrolled in the present study. Patient characteristics are
listed in Table II. All patients
completed thoracic radiotherapy. The chemotherapy regimens that
were administered included platinum-based doublets that were
combined with either etoposide (72%) or teniposide (28%). A total
of 42 patients accepted 2–4 cycles of induction chemotherapy before
SIR-IMRT was performed, while 9 patients accepted radiotherapy
after receiving 5–6 cycles of induction chemotherapy. In addition,
37 patients received chemotherapy after SIR-IMRT and 27 patients
received concurrent chemo-radiotherapy. The 25 patients (83.3%) who
achieved a CR after thoracic radiotherapy underwent PCI.
 | Table II.Patient characteristics (n=52). |
Table II.
Patient characteristics (n=52).
| Characteristics | No. (%) |
|---|
| Age (years) |
|
|
Median | 59 |
|
Range | 41–71 |
| Site |
|
| Left
lung | 19 (36.5) |
| Right
lung | 33 (63.5) |
| Type |
|
|
Peripheral | 6 (11.5) |
|
Central | 46 (88.5) |
| Gender |
|
| Male | 35 (67.3) |
|
Female | 17 (32.7) |
| Clinical T stage |
|
| T1 | 5 (9.6) |
| T2 | 29 (55.8) |
| T3 | 13 (25.0) |
| T4 | 5 (9.6) |
| Clinical N stage |
|
| N0 | 1 (1.9) |
| N1 | 0 (0.0) |
| N2 | 28 (53.9) |
| N3 | 23 (44.2) |
| Clinical stage |
|
| IIa | 1 (1.9) |
| IIIa | 25 (48.1) |
| IIIb | 26 (50.0) |
| Induction
chemotherapy |
|
| Yes | 51 (98.1) |
| No | 1 (1.9) |
| Adjuvant
chemotherapy |
|
| Yes | 37 (71.2) |
| No | 15 (28.8) |
| Concurrent radiation
with chemotherapy |
|
| Yes | 27 (51.9) |
| No | 25 (48.1) |
| Prophylactic cranial
irradiation |
|
| Yes | 25 (48.1) |
| No | 27 (51.9) |
Survival
Three months after completing the radiation
treatment, 30/52 (57.7%) patients and 20/52 (38.5%) patients
experienced CR and PR, respectively. The median OS for the
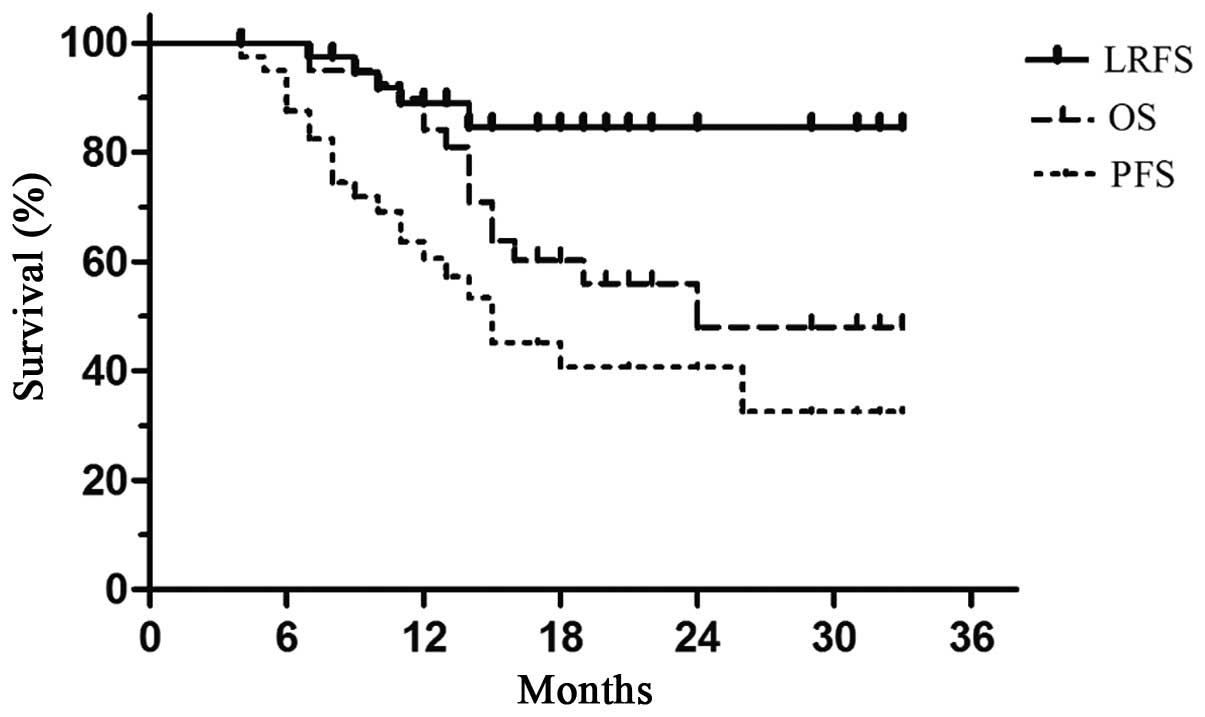
population was 24.0 months, and the median PFS was 18.0 months.
Furthermore, the 1- and 2-year OS rates were 86.2 and 54.8%, the 1-
and 2-year LRFS rates were 91.6 and 83.0%, and the 1- and 2-year
PFS rates were 68.2 and 46.4%, respectively in each case (Fig. 2).
Patterns of failure
By the last follow-up, 21 (40.4%) patients
experienced treatment failure. Of these, locoregional recurrence
(LRR) developed in 6 (11.5%) patients, DM events at various sites
were detected in 18 (34.6%) patients, and 3 (5.8%) patients
experienced both LRR and DM. For the patients who developed LRR, 5
(9.6%) had in-field recurrences [4 (7.7%) within the GTV and 1
(1.9%) within the CTV], and 1 (1.9%) case involved an out-of-field
recurrence. Regarding the latter case, the cervical lymph nodes
were <1 cm in diameter prior to treatment. A clear diagnosis was
not obtained, and therefore, prophylactic neck irradiation was not
administered. Detailed data regarding DM events are listed in
Table III.
 | Table III.Patterns of failure for first
recurrence (n=52). |
Table III.
Patterns of failure for first
recurrence (n=52).
| Recurrence | No. (%) |
|---|
| Total | 21 (40.4) |
| Local regional
recurrence | 6
(11.5) |
|
In-field | 5 (9.6) |
| GTV | 4 (7.7) |
| CTV | 1 (1.9) |
|
Out-of-field | 1 (1.9) |
| Distant
metastasis | 18 (34.6) |
| Bone | 2 (3.8) |
|
Liver | 5 (9.6) |
| Celiac
lymph nodes | 4 (7.7) |
|
Brain | 9
(17.3) |
| Adrenal
gland | 2 (3.8) |
|
Heart | 1 (1.9) |
|
Pancreas | 1 (1.9) |
| Local
regional recurrence and distant metastasis | 3 (5.8) |
Treatment-related toxicity
Various grades of treatment-related toxicity were
observed in this study (detailed results are provided in Table IV). Grade 3 or higher
treatment-related pneumonia (TRP) was observed in 4/52 (7.6%)
patients, and grade 3 radiation-related esophagitis was experienced
by 2/52 (3.8%) of patients. In particular, 2/52 (3.8%) patients
experienced grade 5 TRP. Of these two patients, one succumbed due
to infectious pneumonia combined with TRP 69 days after completing
radiotherapy and the other patient succumbed to TRP that was
contracted 33 days after radiotherapy was completed. While the
first patient accepted six cycles of induced chemotherapy prior to
radiation therapy, the second patient accepted two cycles of
induced chemotherapy with two cycles of synchronous
chemotherapy.
 | Table IV.Treatment-related toxicity
(n=52). |
Table IV.
Treatment-related toxicity
(n=52).
|
| CTCAE 3.0 grade, n
(%) |
|---|
|
|
|
|---|
| Site | 0–1 | 2 | 3 | 4 | 5 |
|---|
| Lung | 36 (69.3) | 12 (23.1) | 2 (3.8) | 0 (0.0) | 2 (3.8) |
| Esophagus | 31 (59.7) | 19 (36.5) | 2 (3.8) | 0 (0.0) | 0 (0.0) |
Discussion
This study appears to be one of only a few clinical
reports to describe the treatment outcome for LS-SCLC following
SIR-IMRT. For LS-SCLC, chemo-radiation therapy is a standard
treatment. In 2013, the National Comprehensive Cancer Network
(NCCN) recommended that chemo-radiotherapy should include 1–2
cycles of chemotherapy followed by radiation therapy (15). The latter could include a 1.5 Gy dose
twice a day for a total dose of 45 Gy, or a 2 Gy dose once a day
for a total dose of 60–70 Gy. Currently, the optimal radiation dose
for SCLC remains unknown. However, certain studies suggest that an
appropriate increase in total dose may improve local control and
prolong OS. Correspondingly, in the RTOG 97-12 trial for SCLC
(5), the total doses were 50.4,
54.0, 57.6, 61.2 and 64.8 Gy, respectively, and the maximum
tolerated dose was 61.2 Gy. Furthermore, 54/62 (87%) patients
achieved a CR (68%) or PR (19%), and 61.2 Gy irradiation versus
50.4 Gy irradiation was found to improve 18- month OS rates (82%
vs. 25%, respectively). In another phase II study (16), the efficacy and feasibility of
accelerated radiotherapy involving a total dose of 61.2 Gy
concurrent with chemotherapy for SCLC was investigated. The median
survival period was 19.0 months, the 2-year OS rate was 46.4%, the
2-year PFS rate was 19.7%, and the median PFS period was 9.9
months. However, the results of the RTOG-0617 clinical trial showed
that a higher radiation dose did not improve the survival of NSCLC
patients compared to a traditional dose (8). While the reason for the latter
unexpected result remains unclear, treatment-related toxicities
associated with the high dose delivered to the PTV may play a role.
Correspondingly, the safe application and escalation of radiation
doses to a target while sparing and minimizing doses to adjacent
healthy organs may be key to improving the therapeutic outcome for
SCLC. At our institution, IMRT for SCLC patients includes a total
dose of 60 Gy applied to the PTVG and 54 Gy applied to
the PTV. Using this approach, the 2-year OS, LPFS, and PFS rates
for the present study were 54.8, 83.0 and 46.4%, respectively, and
these are consistent with the RTOG 9311 study (16). It should also be noted that the
present study achieved good results with a lower radiation dose,
and yet the LRR for the present study did not increase compared
with that observed in other studies, even though a relative lower
total dose (54 Gy) was delivered to an elective nodal area. Based
on these results, it appears that this dose of SIR-IMRT could
benefit the LRFS and OS of LS-SCLC patients.
Some studies have found that three-dimensional
conformal radiotherapy (3D-CRT) can result in a low elective nodal
failure rate. This may be due to incidental radiation received by
clinically uninvolved nodal regions (17,18).
Moreover, the amount of incidental radiation delivered to
non-targeted elective nodes may differ with IMRT, and thus, may be
a factor in the rate of elective nodal failure. Furthermore, it has
been observed that regional recurrence continues to occur in
low-dose areas (18). In the present
study, elective nodal regions received radiation therapy as a
preventative measure, while healthy adjacent organs were exposed to
tolerable doses. Elective nodal radiation of selected high risk
regions is standard for IMRT performed at our medical center.
Moreover, when elective nodal irradiation was applied, elective
nodal failure in the PTV occurred in only 6 patients. This suggests
that relatively lower doses of radiation delivered to elective
nodal regions can be sufficient to control subclinical lesions when
SIR-IMRT is used. Moreover, the overall results of the present
study confirm that treatment of SCLC with SIR-IMRT deserves further
consideration.
The toxic side-effects reported in the present study
were encouraging compared with those noted in other studies,
although IMRT has been associated with fewer side-effects (19,20). In
the present study, 4/52 (7.7%) cases involved TRP of grade 3 or
greater, and this is consistent with previous results (21). Moreover, in a recent study of IMRT
for NSCLC and SCLC, the incidence of acute esophagitis and acute
TRP (grade 3) ranged from 18–23% and from 7–11%, respectively
(22). In addition, only 3.8% of
patients experienced grade 3 or higher radiation-related
esophagitis, and this is a lower incidence rate than that
previously published (20). The use
of the SIR-IMRT technique also resulted in the application of a
dose gradient to the PTVG and PTV. This had the benefit of ensuring
tumor dose and providing protection for proximal normal organs.
Correspondingly, in a recent meta-analysis, symptomatic pneumonitis
increased 3% when lung V20 increased by 1% (23). In addition, predictors of fatal
pneumonitis were found to include a daily radiation dose >2 Gy,
V20 and the location of a tumor in the lower lobe
(11).
This study had limitations. First, because the
patients were not prospectively followed, selection bias and loss
to follow-up may have contributed to underestimates of tumor
recurrence and mortality rates. Second, four-dimensional CT
examinations were not performed in this study, and this may have
influenced the clinical outcomes. However, most of the primary
tumors were located in the upper or middle lobes, or were central
type tumors. Despite these limitations, however, the results of the
present study indicate that SIR-IMRT improves patient survival and
reduces toxic side-effects for patients with LS-SCLC, and also
provides an intriguing justification for future studies of SCLC
treatment involving SIR-IMRT.
Acknowledgements
The authors thank Medjaden Bioscience for assisting
in the preparation of this manuscript. This study was supported by
Nature Science Foundation of China (grant. no. 81372429) and the
Project of Natural Science Fund of Tianjin (grant. no.
12JCQNJC06600)
References
|
1
|
Takada M, Fukuoka M, Kawahara M, Sugiura
T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T,
et al: Phase III study of concurrent versus sequential thoracic
radiotherapy in combination with cisplatin and etoposide for
limited-stage small-cell lung cancer: Results of the Japan Clinical
Oncology Group Study 9104. J Clin Oncol. 20:3054–3060. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turrisi AR III, Kim K, Blum R, Sause WT,
Livingston RB, Komaki R, Wagner H, Aisner S and Johnson DH:
Twice-daily compared with once-daily thoracic radiotherapy in
limited small-cell lung cancer treated concurrently with cisplatin
and etoposide. N Engl J Med. 340:265–271. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hann CL and Rudin CM: Management of
small-cell lung cancer: Incremental changes but hope for the
future. Oncology (Williston Park). 22:1486–1492. 2008.PubMed/NCBI
|
|
4
|
Aridgides PD, Movsas B and Bogart JA:
Thoracic radiotherapy for limited stage small cell lung carcinoma.
Curr Probl Cancer. 36:88–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Komaki R, Swann RS, Ettinger DS, Glisson
BS, Sandler AB, Movsas B, Suh J and Byhardt RW: Phase I study of
thoracic radiation dose escalation with concurrent chemotherapy for
patients with limited small-cell lung cancer: Report of Radiation
Therapy Oncology Group (RTOG) protocol 97-12. Int J Radiat Oncol
Biol Phys. 62:342–350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schild SE, Bonner JA, Hillman S, Kozelsky
TF, Vigliotti AP, Marks RS, Graham DL, Soori GS, Kugler JW, Tenglin
RC, et al: Results of a phase II study of high-dose thoracic
radiation therapy with concurrent cisplatin and etoposide in
limited-stage small-cell lung cancer (NCCTG 95-20-53). J Clin
Oncol. 25:3124–3129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schild SE, Bonner JA, Shanahan TG, Brooks
BJ, Marks RS, Geyer SM, Hillman SL, Farr GH Jr, Tazelaar HD, Krook
JE, et al: Long-term results of a phase III trial comparing
once-daily radiotherapy with twice-daily radiotherapy in
limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys.
59:943–951. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bradley J, Masters G, Hu C, Blumenschein
G, Bogart J, Schild S, Michalski JM, Kavadi V, Garces YI, Narayan
S, et al: An intergroup randomized phase III comparison of
standard-dose (60 Gy) vs high-dose (74 Gy) chemoradiotherapy (CRT)
+/- cetuximab (cetux) for stage III non-small cell lung cancer
(NSCLC): Results on cetux from RTOG 0617. Clin Adv Hematol Oncol.
12:2–4. 2014.
|
|
9
|
Studer G, Peponi E, Kloeck S, Dossenbach
T, Huber G and Glanzmann C: Surviving hypopharynx-larynx carcinoma
in the era of IMRT. Int J Radiat Oncol Biol Phys. 77:1391–1396.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McCammon R, Rusthoven KE, Kavanagh B,
Newell S, Newman F and Raben D: Toxicity assessment of pelvic
intensity-modulated radiotherapy with hypofractionated simultaneous
integrated boost to prostate for intermediate- and high-risk
prostate cancer. Int J Radiat Oncol Biol Phys. 75:413–420. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Groome PA, Bolejack V, Crowley JJ, Kennedy
C, Krasnik M, Sobin LH and Goldstraw P: Cancer Research and
Biostatistics; Observers to the Committee; Participating
Institutions: The IASLC lung cancer staging project: Validation of
the proposals for revision of the T, N and M descriptors and
consequent stage groupings in the forthcoming (seventh) edition of
the TNM classification of malignant tumours. J Thorac Oncol.
2:694–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rusch VW, Asamura H, Watanabe H, Giroux
DJ, Rami-Porta R and Goldstraw P: The IASLC lung cancer staging
project: A proposal for a new international lymph node map in the
forthcoming seventh edition of the TNM classification for lung
cancer. J Thorac Oncol. 4:568–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalemkerian GP, Akerley W, Bogner P,
Borghaei H, Chow LQ, Downey RJ, Gandhi L, Ganti AK, Govindan R,
Grecula JC, et al: Small cell lung cancer. J Natl Compr Canc Netw.
1:78–98. 2013.
|
|
16
|
Bradley J, Graham MV, Winter K, Purdy JA,
Komaki R, Roa WH, Ryu JK, Bosch W and Emami B: Toxicity and outcome
results of RTOG 9311: A phase I–II dose-escalation study using
three-dimensional conformal radiotherapy in patients with
inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol
Phys. 61:318–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sulman EP, Komaki R, Klopp AH, Cox JD and
Chang JY: Exclusion of elective nodal irradiation is associated
with minimal elective nodal failure in non-small cell lung cancer.
Radiat Oncol. 4:52009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kimura T, Togami T, Nishiyama Y, Ohkawa M
and Takashima H: Impact of incidental irradiation on clinically
uninvolved nodal regions in patients with advanced non-small-cell
lung cancer treated with involved-field radiation therapy: Does
incidental irradiation contribute to the low incidence of elective
nodal failure? Int J Radiat Oncol Biol Phys. 77:337–343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi NC, Herndon JN II, Rosenman J, Carey
RW, Chung CT, Bernard S, Leone L, Seagren S and Green M: Phase I
study to determine the maximum-tolerated dose of radiation in
standard daily and hyperfractionated-accelerated twice-daily
radiation schedules with concurrent chemotherapy for limited-stage
small-cell lung cancer. J Clin Oncol. 16:3528–3536. 1998.PubMed/NCBI
|
|
20
|
Jiang ZQ, Yang K, Komaki R, Wei X, Tucker
SL, Zhuang Y, Martel MK, Vedam S, Balter P, Zhu G, et al: Long-term
clinical outcome of intensity-modulated radiotherapy for inoperable
non-small cell lung cancer: The MD Anderson experience. Int J
Radiat Oncol Biol Phys. 83:332–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu HH, Wang X, Dong L, Wu Q, Liao Z,
Stevens CW, Guerrero TM, Komaki R, Cox JD and Mohan R: Feasibility
of sparing lung and other thoracic structures with
intensity-modulated radiotherapy for non-small-cell lung cancer.
Int J Radiat Oncol Biol Phys. 58:1268–1279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shirvani SM, Komaki R, Heymach JV,
Fossella FV and Chang JY: Positron emission tomography/computed
tomography-guided intensity-modulated radiotherapy for
limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys.
82:e91–e97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Palma DA, Senan S, Tsujino K, Barriger RB,
Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB, et al:
Predicting radiation pneumonitis after chemoradiation therapy for
lung cancer: An international individual patient data
meta-analysis. Int J Radiat Oncol Biol Phys. 85:444–450. 2013.
View Article : Google Scholar : PubMed/NCBI
|