Introduction
Bone loss is caused by various congenital and
degenerative diseases, traumas or incorrect surgical procedures,
leading to severe problems, particularly in elderly individuals
(1). Tissue engineering is
frequently used for the reconstruction of mandible defects. Yamato
et al (2) have demonstrated a
novel cell sheet engineering method for tissue regeneration, which
uses temperature-responsive culture dishes (TRCDs). This technique
allows for different types of cultured cells to be noninvasively
harvested as intact sheets through simple temperature reduction,
without the use of proteolytic enzymes (3). Using this method, the noninvasive
transfer of these cell sheets can be achieved, while retaining the
typical distributions of Na+/K+-ATPase,
glucose transporter-1, sodium-glucose linked transporter-1,
aquaporin-1, neutral endopeptidase and dipeptidylendopeptidase IV
(4).
In the present study, scaffolds of
poly(lactic-co-glycolic acid) (PLGA) were produced, and composited
with recombination human bone morphogenetic protein-2 (rhBMP-2) and
vascular endothelial growth factor (VEGF). In addition, bone marrow
stem cells (BMSCs) were cultured in TRCDs to form BMSCs sheets.
PLGA/BMP-2/VEGF wrapped with BMSCs sheets were implanted into dogs
with mandibular defects. The aim of the present study was to
investigate the effects of tissue-engineered bones that have the
same structure as normal bones and can be used for the
reconstruction of bones with mandible defects.
Materials and methods
Animals
In this study, 16 healthy, adult, male mongrel dogs
(age, 14 months; weight, 18–23 kg) were used. All the animals were
obtained from the Laboratory Animal Center of the Affiliated
Hospital of Qingdao University (Qingdao, China) and treated under
the same standard laboratory conditions. The study was approved by
the Ethics Committee of the Affiliated Hospital of Qingdao
University.
Osteoinduction of BMSCs
Under general anesthesia (10 mg/kg ketamine and 5
mg/kg phenobarbital), 10 ml of bone marrow was collected from each
dog using a disposable syringe and then transferred into a
centrifuge tube containing 150 units heparin (Jiangsu Wanbang
Biochemical Pharmaceutical Co., Ltd., Xuzhou, China). BMSCs were
isolated by density gradient centrifugation (160 × g for 20 min at
4°C) and seeded into 50 ml culture flasks at a density of
1×107/ml. Next, the BMSCs were cultured in low-glucose
Dulbecco's modified Eagle's medium (DMEM; Hyclone Laboratories,
Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) and
incubated continuously at 37°C in a saturated humidified atmosphere
of 95% air and 5% CO2 (5). An inverted-phase contrast microscope
(IX 50; Olympus Corporation, Tokyo, Japan) was used to observe the
cells. When the BMSCs reached 80% confluence, the cells were
detached with 0.25% trypsin (including 0.02% EDTA) and subcultured
at a ratio of 1:2. Subsequently, the cells were cultured in a
different medium in order to induce differentiation into
osteoblasts. The medium used was changed from low-glucose DMEM to
high-glucose DMEM, which was supplemented with 10% FBS and an
osteogenesis-inducing reagent (50 µg/ml ascorbic acid, 10 mM
β-glycerophosphate, and 10−4 mM dexamethasone) (6–8).
Preparation of the BMSC sheets
The differentiation-induced BMSCs were seeded in a
TRCD (UpCell; Nunc, Thermo Scientific, Basingstoke, UK) and
incubated at 37°C in an atmosphere of 95% air and 5% CO2
(2). After 7–10 days, the cells had
spread over the entire TRCD. Next, the TRCD was placed at 20°C for
60 min. The BMSCs were then separated from the TRCD to be used as a
cell sheet (9–11).
Preparation of the PLGA scaffold
The PLGA scaffold (aperture, 100–300 µm; porosity,
85%; molecular weight, 100,00; Shandong Institute of Medical
Instruments, Jinan, China) was shaped into a gengon and a
longitudinal groove was made in the gengon to prepare for vessels
embedded. The scaffold was examined under scanning electron
microscopy (SEM; JSM-840; JEOL, Ltd., Tokyo, Japan). Two growth
factors, rhBMP-2 (0.1 µg/ml) and VEGF (5 µg/ml), were added into
the PLGA scaffold by lyophilization (12). Following low-temperature plasma
sterilization, the scaffold was stored at 4°C (13) The scaffold and gengon were purchased
from the Shandong Institute of Medical Instruments (Jinan,
China).
Construction of BMSC sheets/PLGA
complex
The osteogenically induced BMSCs were detached from
the culture flasks using 0.25% trypsin (including 0.02% EDTA) and
seeded into the PLGA scaffolds at a density of 1×107/ml,
using a disposable syringe. Scaffolds wrapped with or without two
BMSC sheets at their surface were used. All the scaffolds were
incubated continuously at 37°C in an atmosphere of 95% air and 5%
CO2. After 3 days of incubation, these in vitro
scaffolds were fixed in 2% glutaric dialdehyde and then
characterized using SEM.
Implantation
The 16 dogs were divided into 4 groups (4 dogs per
group). Under general anesthesia, the dogs were placed on the
operating table in a supine position. The mandibular border was
exposed through a 5-cm submandibular skin incision and the
mandibular periosteum was carefully dissected. A defect with the
same shape as the PLGA scaffold was made in the two sides of the
dogs' mandible. Next, PLGA scaffolds wrapped with or without two
BMSC sheets were separately implanted into the defects in the two
sides. PLGA scaffolds wrapped with two BMSC sheets were implanted
into the right side of the mandible, serving as the experimental
side. PLGA scaffolds without BMSC sheets were implanted into the
left side of the mandible, serving as the control side. The
inferior alveolar neurovascular bundle was dissociated from the
mandible and embedded in the longitudinal groove of the scaffold.
The incision on the soft tissue was sutured to stabilise the
scaffold. Each dog was injected with penicillin (400 IU/day) for 7
days after the implantation.
X-ray analyses
After anesthesia, the dogs were sacrificed at 4, 8,
12 and 16 weeks after surgery. General observation and X-ray
examination were performed. The X-ray examination was performed
under the same conditions for all dogs (voltage, 60 kV; current,
2.51 mA; time, 0.04 sec; distance, 1 m). In the present study, the
optical density of the X-ray images, which is identified as the
gray value of the images and indicates the degree of bone
regeneration, was measured using the Image Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA) was measured and
analyzed using the Image Pro Plus 6.0 software. Specimens (5×5 mm)
were selected from the same part of the bone regeneration tissue at
both sides of the mandible and were embedded in paraffin for
preservation.
Hematoxylin and eosin (H&E)
staining
Tissue specimens were collected for H&E staining
(Beyotime Institute of Biotechology, Haimen, Jiangsu, China) and
observed under the inverted-phase contrast microscope. Three fields
under 100× magnification were randomly selected for measurement.
The initially repaired, the Haversian system and the new blood
vessel areas were measured. Then the percentages of the new bone
and new blood vessel areas were calculated in relation to the
total, initially repaired area, and were then analyzed. All
measurements and analyses were performed using the Image Pro Plus
6.0 software.
Bone hardness analysis
Bone tissue specimens were collected from the new
bone and surrounding area, and fixed in a gypsum base. The gypsum
base used in the present study was produced using ultra-anhydrite
power by the lab of the Qingdao University. The ultra-anhydrite
power (XSC-20) was purchased from Yuyao Xinshi Gypsum Products Co.,
Ltd. (Yuyao, China). The bone hardness was analyzed with a hardness
tester (HXD-1000TMC/LCD; Taiming Optical Instrument Co., Ltd.,
Shanghai, China). The hardness of each specimen was independently
determined three times (14).
Statistical analysis
Statistical analysis was performed using the SPSS
version 16.0 software (SPSS, Inc., Chicago, IL, USA). All the data
are presented as the mean ± standard deviation. Statistically
significant differences were indicated by P<0.05 and were
determined among the week 4, 8, 12 and 16 groups using analysis of
variance and the least significant difference test. Differences
(P<0.05) between the control and experimental groups were
detected using t-test.
Results
Establishment of BMSC sheets-PLGA
scaffolds complex
At 10 days after BMSCs were seeded, the cells spread
over the entire bottom of the TRCD and were closely-arranged
(Fig. 1A). The dishes were placed in
a new incubator with a humidified atmosphere of 5% CO2
at 20°C and the cell sheets were detached from the edge of the dish
(15). Approximately 60 min were
required for complete cell sheet detachment.
As shown in Fig. 1B,
the PLGA scaffolds had a porous three-dimensional structure
(aperture, 100–300 µm), as observed using SEM. At 3 days after
seeding, the BMSCs were well-adhered and adequately proliferated,
and had extended on the surface and pores of the three-dimensional
scaffold. Cell sheets were adhered to the surface of the scaffold
and secreted on a large part of the extracellular matrix (Fig. 1B).
Implantation
All dogs were healthy during the experiments. In the
experimental group, the outline of the scaffold was observed 4
weeks post-implantation, with fibrous connective tissues
surrounding the complex. From 8 weeks post-implantation, absorption
of the scaffolds was observed, while at 12 weeks, the majority of
the defects were reconstructed by the new engineered bones. At 16
weeks post-implantation, the areas of new bones were larger than at
12 weeks. Compact bones were observed at the lingual of the
mandible and the defects of the mandible had been completely
reestablished with the new engineered bones. In the experimental
group, substantial new bone was observed without a clear boundary
between the newly formed tissue-engineered bones and the native
bones. In the control group, the size of the new bone was
significantly smaller compared with that in the experimental group.
At 16 weeks, the percentage of the new bone area in relation to the
initially repaired area in the experimental group was 90.5±2.3,
significantly higher than that in the control group (79.3±3.4)
(P<0.05).
As shown in Table I,
the bone trabecula and OD of the new bone increased gradually
between weeks 4 and 16 post-implantation. The ODs in the
experimental group were significantly higher compared with those in
the control group. Furthermore, the ODs of bones in the
experimental group at 16 weeks (Fig.
1C) after implantation were higher in all the new bones, but
remained lower than those of the normal mandible. Higher optical
density values demonstrated by X-ray are associated with the more
mineral contents in the bone. In addition, the optical density
values also reflect the maturity of the new bone indirectly.
 | Table I.Optical density detected at 4, 8, 12
and 16 weeks after surgery. |
Table I.
Optical density detected at 4, 8, 12
and 16 weeks after surgery.
|
| Optical density at
different times after surgery |
|---|
|
|
|
|---|
| Groups | 4 weeks | 8 weeks | 12 weeks | 16 weeks |
|---|
| Experimental |
0.319±0.001a |
0.362±0.020a |
0.378±0.009a |
0.616±0.044a |
| Control | 0.231±0.005 | 0.256±0.020 | 0.326±0.019 | 0.572±0.042 |
Bone hardness analysis
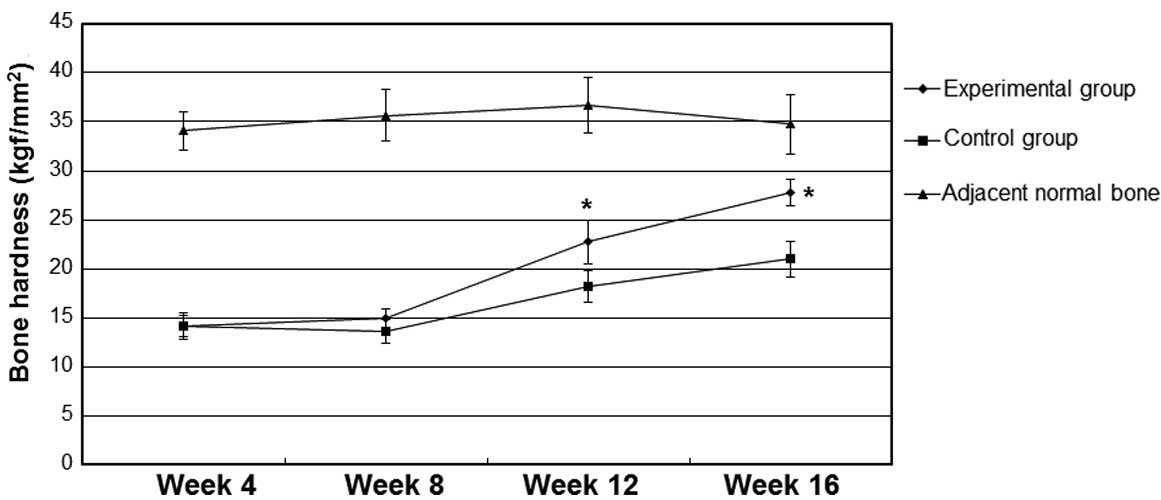
As shown in Fig. 2,
bone hardness values in the control and experimental groups were
not detectably increased at 4 and 8 weeks after implantation
(P>0.05). By contrast, at 12 and 16 weeks after implantation,
the values of bone hardness in the experimental group were
significantly higher compared with the values in the control group
(P<0.05). The bone hardness at 12 and 16 weeks after
implantation were increased when compared with the values at 4 and
8 weeks after implantation, but were lower compared with the values
of hardness of the adjacent normal bones (data not shown).
Histological examination and
histomorphological analysis
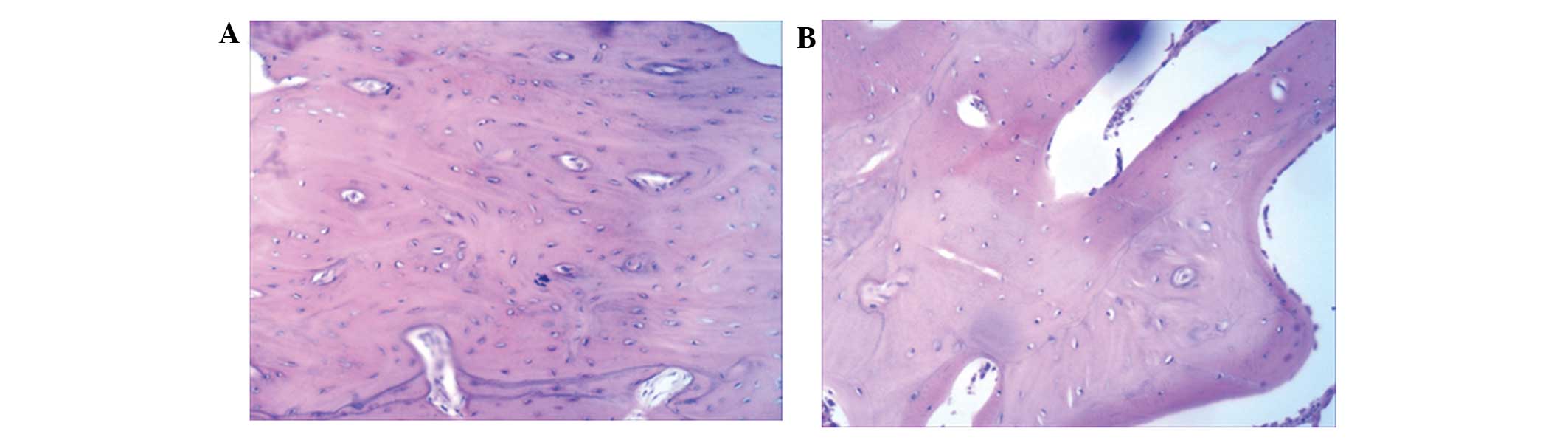
As shown in Fig. 3A,
at 16 weeks after implantation, thick bone trabecula was found to
be connected in the experimental side. Several Haversian bone
systems and a few lamellar bones were observed at the surrounding
region. Bone trabecula and red bone marrow were observed at the
center of the new engineered bone (Fig.
3A). In the control side, a large number of blood vessels was
observed (Fig. 3B). The control
group had fewer Haversian bone systems compared with the
experimental group, and no lamellar bones were identified (Fig. 3B). These results suggest that
tissue-engineered bones with the structure of lamellar bones can be
generated using BMSC sheets.
Discussion
BMSCs can be induced to generate various types of
cells, including osteoblast and fat cells (16). Due to this property, BMSCs have been
used in numerous fields. In the experiments of the present study,
differentiation of dog BMSCs into osteoblasts was induced in
vitro, and then the osteoblasts were implanted into porous
biodegradable scaffold materials. In the cell sheets obtained, the
BMSCs were arranged compactly, similar to their arrangement in
normal bones. Subsequently, porous scaffold materials were wrapped
with the cell sheets composed of osteoblasts.
Scaffolds are able to provide cells with an
environment required for their differentiation, defining the
ultimate shape of the engineered cartilage. They must be
biocompatible, biodegradable, highly porous, mechanically strong
and malleable (17). In the present
study, PLGA scaffolds were used, which are biocompatible and have
been approved by the Food and Drug Administration for certain human
clinical applications (18). These
were three-dimensional porous scaffolds with a pole diameter of
300–400 µm, which was beneficial to the growth of cells and blood
vessels. In addition, their 80% porosity was beneficial to the
growth of osteoblasts (19). The
PLGA scaffolds used in the current study resembled inverted
trapezia and had a groove on the back side in order to accommodate
mandible nerves and vessels. This not only ensures the stability of
the complex, but also provides large pieces of tissue-engineered
bone with blood supply.
In the current study, after 16 weeks of the
scaffold-BMSC sheet complex implantation, the mandibular border
defect was almost completely repaired and the characteristics of
the new engineered bone were similar to those of the normal
mandible in the experimental groups; however, this was not achieved
in the control group. The new engineered bone had a Haversian
system structure, with thick bone trabecula and red bone marrow.
The hardness of the new engineered bone was similar to that of the
normal surrounding mandible in the experimental groups; however,
these characteristics were not observed in the control group. The
current study demonstrated a novel method for the reconstruction of
large mandibular defects.
In conclusion, through the combination of a PLGA
scaffold and BMSC sheets, engineered bone was successfully
generated and the mandibular defect was reconstructed with a
significant effect. Cell sheet transplantation was found to enhance
bone formation at the reconstruction of mandibular defect.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 30872896) and the Natural
Science Foundation of Shandong Province of China (no. Y2008C7).
References
|
1
|
d'Aquino R, De Rosa A, Lanza V, Tirino V,
Laino L, Graziano A, Desiderio V, Laino G and Papaccio G: Human
mandible bone defect repair by the grafting of dental pulp
stem/progenitor cells and collagen sponge biocomplexes. Eur Cell
Mater. 18:75–83. 2009.PubMed/NCBI
|
|
2
|
Yamato M, Akiyama Y, Kobayashi J, Yang J,
Kikuchi A and Okano T: Temperature-responsive cell culture surfaces
for regenerative medicine with cell sheet engineering. Prog Polym
Sci. 32:1123–1133. 2007. View Article : Google Scholar
|
|
3
|
Sekiya S, Shimizu T, Yamato M, Kikuchi A
and Okano T: Bioengineered cardiac cell sheet grafts have intrinsic
angiogenic potential. Biochem Biophys Res Commun. 341:573–582.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kushida A, Yamato M, Isoi Y, Kikuchi A and
Okano T: A noninvasive transfer system for polarized renal tubule
epithelial cell sheets using temperature-responsive culture dishes.
Eur Cell Mater. 10:23–30; discussion 23–30. 2005.PubMed/NCBI
|
|
5
|
Bi W, Deng JM, Zhang Z, Behringer RR and
de Crombrugghe B: Sox9 is required for cartilage formation. Nat
Genet. 22:85–89. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vortkamp A, Pathi S, Peretti GM, Caruso
EM, Zaleske DJ and Tabin CJ: Recapitulation of signals regulating
embryonic bone formation during postnatal growth and in fracture
repair. Mech Dev. 71:65–76. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferguson CM, Miclau T, Hu D, Alpern E and
Helms JA: Common molecular pathways in skeletal morphogenesis and
repair. Ann NY Acad Sci. 857:33–42. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vortkamp A, Lee K, Lanske B, Segre GV,
Kronenberg HM and Tabin CJ: Regulation of rate of cartilage
differentiation by Indian hedgehog and PTH-related protein.
Science. 273:613–622. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashida Y, Nishida K, Yamato M, Watanabe
K, Maeda N, Watanabe H, Kikuchi A, Okano T and Tano Y: Ocular
surface reconstruction using autologous rabbit oral mucosal
epithelial sheets fabricated ex vivo on a temperature-responsive
culture surface. Invest Ophthalmol Vis Sci. 46:1632–1639. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimizu T, Yamato M, Kikuchi A and Okano
T: Cell sheet engineering for myocardial tissue reconstruction.
Biomaterials. 24:2309–2316. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura A, Akahane M, Shigematsu H,
Tadokoro M, Morita Y, Ohgushi H, Dohi Y, Imamura T and Tanaka Y:
Cell sheet transplantation of cultured mesenchymal stem cells
enhances bone formation in a rat nonunion model. Bone. 46:418–424.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao YH, Yang Q, Xia Q, Peng J, Lu SB, Guo
QY, Ma XL, Xu BS, Hu YC, Zhao B, et al: In vitro cartilage
production using an extracellular matrix-derived scaffold and bone
marrow-derived mesenchymal stem cells. Chin Med J (Engl).
126:3130–3137. 2013.PubMed/NCBI
|
|
13
|
Galiano RD, Tepper OM, Peb CR, Bhatt KA,
Callaghan M, Bastidas N, Bunting S, Steinmetz HG and Gurtner GC:
Topical vasular endothelial growth factor acceleretes diabetic
wound healing through increased angiogenesis and by mobilizing and
recruiting bone marrow derived cells. Am J Pathol. 164:1935–1947.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang LJ and Jin Y: Immunohistochemical
observations on bone morphogenetic protein in normal and abnormal
conditions. Clin Orthop Relat Res. 257:249–256. 1990.PubMed/NCBI
|
|
15
|
Hata H, Bär A, Dorfman S, Vukadinovic Z,
Sawa Y, Haverich A and Hilfiker A: Engineering a novel
three-dimensional contractile myocardial patch with cell sheets and
decellularised matrix. Eur J Cardiothorac Surg. 38:450–455. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bianco P, Robey PG and Simmons PJ:
Mesenchymal stem cells: Revisiting history, concepts, and assays.
Cell Stem Cell. 2:313–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Ran W, Wang GL and Jing XD:
Biocompatibility of new bone tissue engineering scaffolds in vivo.
Hua Xi Kou Qiang Yi Xue Za Zhi. 27:447–450. 2009.(In Chinese).
PubMed/NCBI
|
|
18
|
Chen G, Sato T, Ushida T, Ochiai N and
Tateishi T: Tissue engineering of cartilage using a hybrid scaffold
of synthetic polymer and collagen. Tissue Eng. 10:323–330. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu WF and Liao XL: Evaluation of cell
compatibility for porous 3D CF/PLA/CS composites scaffold in
vitro. Ying Yong Hua Xue. 28:214–218. 2011.(In Chinese).
|