Introduction
Carcinoma of the lung has the highest incidence and
mortality rates among malignancies worldwide. Adenocarcinoma is the
most common histological type of non-small cell lung carcinoma,
accounting for approximately half of all lung carcinomas (1). The histological subtypes of lung
adenocarcinoma differ greatly in their clinical and imaging
features and genetics. The International Association for the Study
of Lung Cancer, American Thoracic Society and European Respiratory
Society jointly published an international multidisciplinary
classification of lung adenocarcinoma in 2011 (2). The previous classifications were
essentially pathological and based on histopathological type. The
new classification drew on the additional expertise of oncologists,
thoracic surgeons, radiologists, molecular biologists and others.
The aim was to provide a more meaningful classification for
planning treatment and guiding prognosis.
Enteric adenocarcinoma is a newly identified type of
primary lung adenocarcinoma, which shares certain morphological and
immunohistochemical features with colorectal adenocarcinoma, and is
characterized by a >50% intestinal differentiation of the tumor
(2). It may also contain features of
other histological subtypes of primary lung adenocarcinoma, such as
the alveolar subtype. The immune phenotype can include the
expression of one or more markers for colorectal carcinoma,
including caudal type homeobox transcription factor 2 (CDX2),
cytokeratin (CK)20 and mucin 2 (MUC2) (3–7). The
expression of CK7 and thyroid transcription factor-1 (TTF-1) has
been reported in up to 50% of intestinal adenocarcinomas. This can
be helpful in differentiating intestinal adenocarcinoma from
metastatic colorectal carcinoma (3).
The present study reports the case of a 53-year-old female patient
diagnosed with primary lung enteric adenocarcinoma, and includes a
review of the relevant literature. The protocol in the present
study was reviewed and approved by the Human Clinical and Research
Ethics Committees. This patient provided written informed
consent.
Case report
In early February 2014, a 53-year-old female was
admitted to the Peoples Hospital of Zhengding (Zhengding, China)
with a 1-year history of an intermittent dry cough, for which no
prior treatment had been received. The patient was not, and had
never been a smoker. She also reported pain in her right shoulder
and back, and had a poorly defined mass in the region of her right
sternoclavicular joint, which she reported to be growing slowly. At
presentation the mass measured 5 cm in diameter.
A computed tomography (CT) scan revealed a mass in
the mediastinum and more masses in the lungs, all of which were
suspected to be malignant. The bronchoscopy was normal. A biopsy of
the mediastinal mass was conducted under ultrasound control.
Histological examination revealed a solid, moderately
differentiated adenocarcinoma, which was infiltrating connective
tissue in the left side of the mediastinum. Immunohistochemical
staining was performed with a range of primary antibodies obtained
from Zymed Corporation, Inc. (San Francisco, CA, USA). The
neoplastic cells stained positively for CDX2 (rabbit; clone, EP25),
carcinoembryonic antigen (CEA; mouse; clone, CEA31), CK20 (rabbit;
clone, EP23), Villin (rabbit; clone, EP163) and cancer antigen 15–3
(CA15-3; mouse; clone, DF3), and negatively for TTF-1 (mouse;
clone, SPT24), noval aspartic proteinase of the pepsin family A
(mouse; clone, IP64), human epidermal growth factor-2 (Her-2;
mouse; clone, EP3), CK7 (mouse; clone, EP16), p63 (mouse; clone,
UMAB4) and CA19-9 (mouse; clone, C241; 5:1:1:4) (all dilutions,
1:100) (Fig. 1). On the basis of
these findings, metastatic colorectal carcinoma was excluded.
Cranial magnetic resonance imaging (MRI), pelvic CT (Fig. 2), gastroscopy, enteroscopy colorectal
colonoscopy and positron emission tomography-CT (PET-CT) were
performed. They failed to find evidence of a primary
gastrointestinal tumor. A bone scan revealed metastasis in the
right sternoclavicular joint. Abdominal MRI revealed a mass in the
upper pole of the right kidney and multiple nodules in both
kidneys. An abdominal PET-CT scan did not identify any abnormally
hypermetabolic lesions, double renal parenchyma, double multiple
high metabolic nodules or neoplasms in the gastrointestinal
tract.
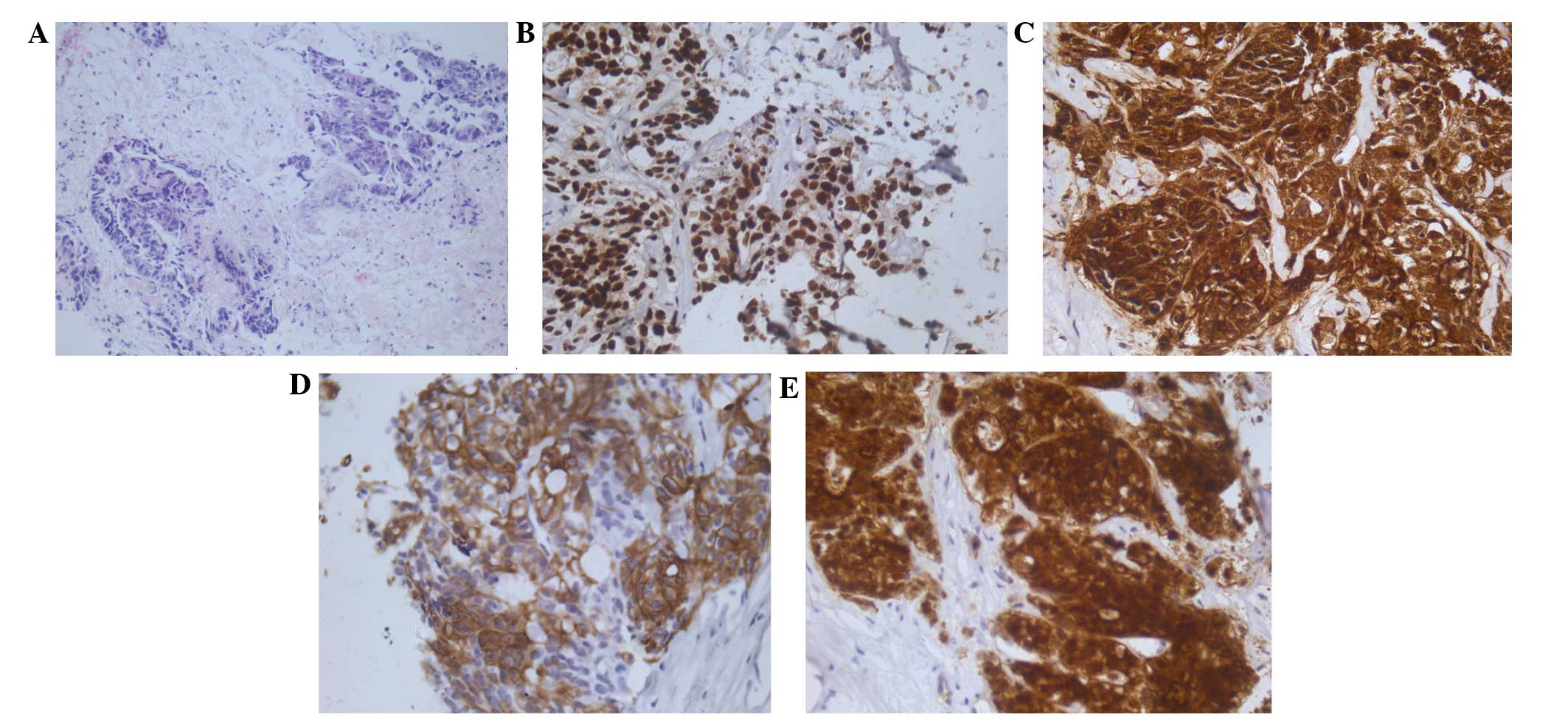 | Figure 1.Hematoxylin and eosin staining and
immunohistochemistry with CDX2, CEA, CK20 and villin. (A)
Histological examination showed a moderately differentiated
adenocarcinoma infiltrating fibrous connective tissues
(magnification, ×200); (B-E) Immunohistochemical staining for the
expression of CDX2, CEA, CK20 and villin in the neoplastic cells,
observed using anti-CDX2, anti-CEA, anti-CK20 with slight
hematoxylin counterstain (magnification, ×400). CDX2, caudal type
homeobox transcription factor 2; CEA, carcinoembryonic antigen; CK,
cytokeratin. |
Tumor histology was reviewed by pathologists at four
different institutions, who were in agreement that the primary
tumor was likely to be a malignancy of the digestive tract. The
institutions were the Departments of Pathology at the Cancer
Institute & Hospital, Chinese Academy of Medical Sciences;
Peking Union Medical College Hospital; Peoples Liberation Army
General Hospital; and the Affiliated Hospital Cancer Center,
Academy of Military Medical Sciences, respectively (all
institutions in Beijing, China). Cytogenetic studies of the tumor
tissue were undertaken using tumor markers obtained from Amoy
Diagnostics Co., Ltd. (Xiamen, China). The genotypic analysis
identified the expression of wild-type epidermal growth factor
receptor (EGFR), Kirsten rat sarcoma viral oncogene homolog
(K-ras), serine/threonine-protein kinase B-Raf (BRAF) and
UDP-glucuronosyltransferase 1–1 (UGT1A1). There was no expression
of echinoderm microtubule-associated protein-like 4-anaplastic
lymphoma kinase (EML4-ALK) and moderate expression of excision
repair cross-complementation group 1 (ERCC1),
ribonucleoside-diphosphate reductase large subunit (RRM1) and
tubulin β-3 chain (TUBB3). Strong expression of thymidylate
synthase (TYMS) and 677TC genotype expression of
methylenetetrahydrofolate reductase (MTHFR) was observed.
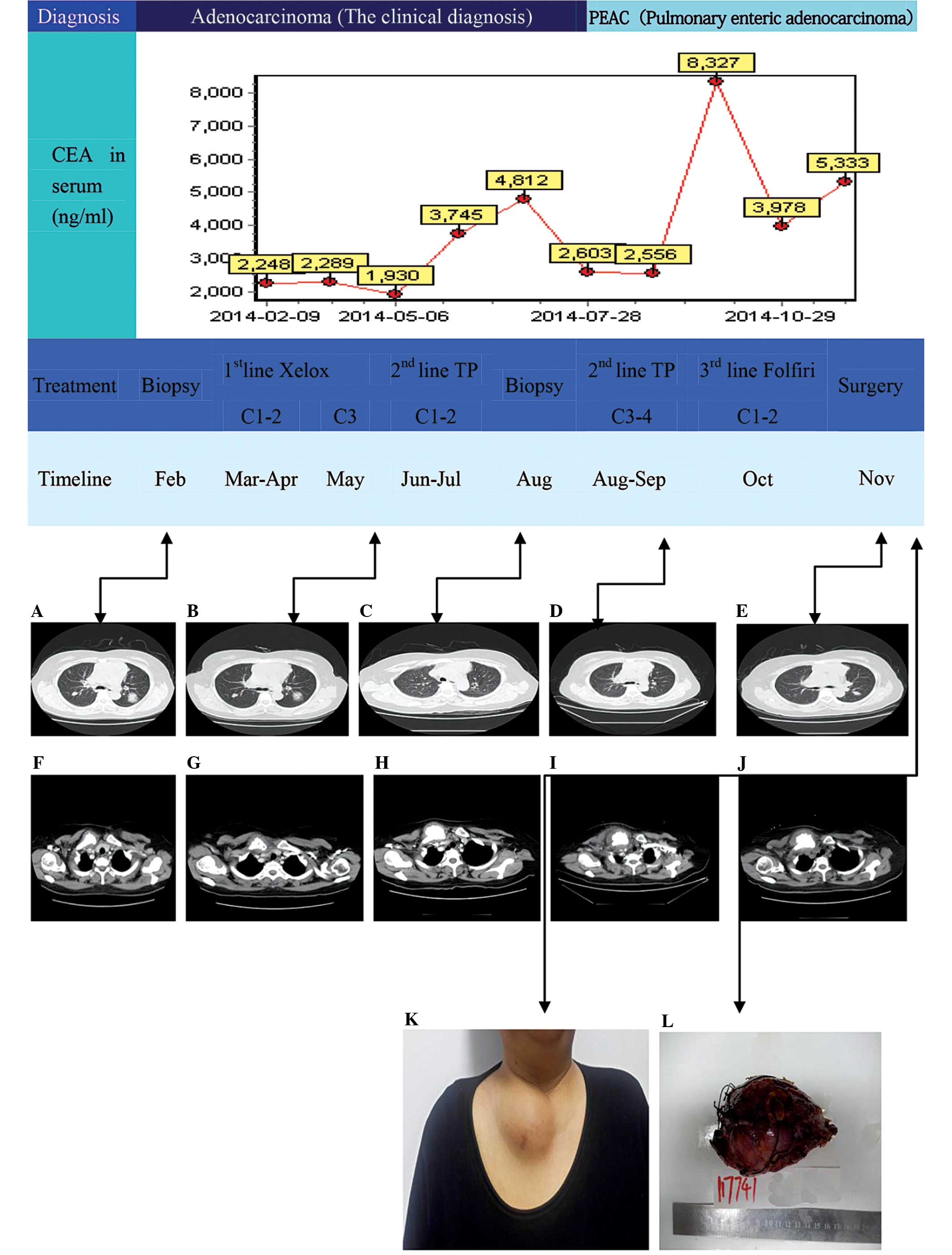
Initially, the patient was reluctant to undertake
targeted therapy instead of chemotherapy for economic reasons;
however, the tumor continued to develop despite the chemotherapy.
The patient subsequently chose to undergo targeted therapy through
genetic testing. Unfortunately, the patient did not identify a
suitable candidate target for targeted therapy following genetic
testing, so the patient was not suitable for targeted therapy. As
an alternative treatment, the patients chemotherapy regimen was
altered. Therefore, the patient was treated with rescue
chemotherapy comprising 3 monthly cycles of oxaliplatin and
capecitabine (XELOX regimen). The response was poor, with
supraclavicular nodes being noted following the second cycle
(Table I and Fig. 1). The patient was switched to
docetaxel and cisplatin (the TP regimen) for 4 monthly cycles
(Table I and Fig. 1). The response was good in terms of
the pulmonary lesions and the level of CEA, which fell from a
pre-treatment level of 8,327 to 2,248 ng/ml; however, the
supraclavicular lesions progressed further and so the treatment of
the patient was changed again, this time to folinic acid,
florouracil and irinotecan (the FOLFIRI regimen), which was
administered in 2 monthly cycles. Neither the lung lesions nor the
supraclavicular lesions responded to this regimen. The latter was
associated with more severe upper limb pain and was treated with
surgical excision 9 months following the first presentation, which
relieved the pain. Two months following the surgery, lung lesion
(disease stable). The patient was subsequently treated with
docetaxel and cisplatin (the DP regimen) and continues to be
followed up (Table I and Fig. 1).
 | Table I.Details of XELOX, TP and FOLFIRI
chemotherapy. |
Table I.
Details of XELOX, TP and FOLFIRI
chemotherapy.
| Regimen | Dosage
(mg/m2) | Method of
administration | Day |
|---|
| XELOX |
|
|
|
|
Oxaliplatin | 130 | i.v. | 1 |
|
Capecitabine | 1,000 | p.o. b.i.d. | 1–14 |
| TP |
|
|
|
|
Paclitaxel | 175 | i.v. | 1 |
|
Cisplatin |
20 | i.v. | 1–3 |
| FOLFIRI |
|
|
|
|
Irinotecan | 180 | i.v. | 1 |
|
Leucovorin | 400 | i.v. | 1 |
| 5-Fu | 400 | bolus i.v. | 1 |
| 5-Fu | 2,400 | c.i.v. 46 h | 1–2 |
Discussion
Primary lung enteric adenocarcinoma is defined in
the 2011 classification of lung adenocarcinoma as a type of
invasive lung carcinoma (2). It is
characterized by >50% intestinal differentiation of the tumor,
with no evidence of a primary digestive tract tumor. Based on
immunohistochemistry, two types of lung adenocarcinoma with
intestinal differentiation and morphology can be distinguished:
Lung adenocarcinoma with intestinal differentiation and ≥1 positive
intestinal-type differentiation marker (CK20, CDX2, MUC-2 or
villin), and lung adenocarcinoma with intestinal morphology but
negative for intestinal-type differentiation markers (8–10).
Primary lung enteric adenocarcinoma has immunohistochemical
similarities with metastatic colorectal cancer. Its diagnosis
requires the exclusion of a primary enteric tumor, which, in turn,
requires gastroscopy and enteroscopy.
Details of the present and 30 previously reported
cases of primary lung intestinal adenocarcinoma (11–19) are
shown in Table II. All 31 cases
expressed CK7, CK20 or TTF-1; 26 (84%), 13 (42%) and 14 (45%)
cases, respectively, were positive for each marker. At least 20
cases were tested for the markers CDX2, napsin A and MUC2.
Seventeen out of 29 (59%), 3 out of 20 (15%) and 9 out of 26 (35%)
cases, respectively, exhibited a positive expression. Eleven out of
14 (79%) patients were positive for villin expression. All cases
positive for TTF-1 or napsin A expression were also positive for
CK7 expression. Eleven cases were positive for both CK20 and CDX2
expression. Seven out of 26 (27%) cases that were tested for the
intestinal-type markers CK20, CDX2 and MUC2 were negative for the
expression of all three proteins. Five out of 26 (19%) cases were
positive for the intestinal-type markers CK20 and CDX2, but
negative for the lung adenocarcinoma immune markers CK7 and TTF-1.
This indicates that, whilst the latter are important markers
assisting the diagnosis of lung adenocarcinoma, false negatives do
occur (20,21).
 | Table II.Review of all literature for primary
lung enteric adenocarcinoma (31 cases). |
Table II.
Review of all literature for primary
lung enteric adenocarcinoma (31 cases).
|
|
|
Immunohistochemical |
|
|---|
|
|
|
|
|
|---|
| First author (ref.),
gender/agea/smoking | Tumor size
(mm)/site | CK7 | CK20 | TTF-1 | CDX2 | Napsin A | MUC2 | Villin | Stage | Clinical follow-up
results (month) |
|---|
| Inamura et al
(11) |
|
|
|
|
|
|
|
|
|
|
|
M/NA/yes | 50/RUL | + | − | + | − | − | − | NA |
T3N1M0 | D (6) |
|
M/NA/yes | 40/LUL | + | − | + | + | − | p+ | NA |
T2N2M0 | D
(47) |
|
F/NA/yes | 26/LLL | + | p+ | − | p+ | − | − | NA |
T1N0M0 | A
(43) |
|
M/NA/yes | 34/RLL | + | p+ | − | p+ | − | − | NA |
T2N0M0 | A
(43) |
|
M/NA/yes | 23/RLL | + | + | − | + | − | p+ | NA |
T1N0M0 | A
(30) |
|
M/NA/yes | 17/RUL | + | − | p+ | p+ | − | − | NA |
T1N0M0 | A
(16) |
|
M/NA/yes | 39/LUL | + | − | − | − | − | p+ | NA |
T2N1M0 | A
(12) |
| Yousem (12) |
|
|
|
|
|
|
|
|
|
|
|
F/74/yes | 36/RUL | + | − | + | − | NA | − | NA |
T2N1M0 | D
(26) |
|
F/70/yes | 17/RUL | + | − | + | − | NA | − | NA |
T2N1M0 | D
(18) |
|
M/82/yes | 65/RUL | + | − | p+ | − | NA | − | NA |
T2N0M0 | D (5) |
|
F/63/yes | 15/RUL | + | − | + | − | NA | − | NA |
T1N0M0 | A (7) |
|
F/73/yes | 70/LLL | + | − | + | − | NA | p+ | NA |
T2N0M0 | A (3) |
|
F/57/yes | 20/RUL | + | − | + | − | NA | − | NA |
T2N0M0 | A (2) |
| Maeda et al
(13) |
|
|
|
|
|
|
|
|
|
|
|
M/69/NA | 25/RLL | + | − | + | NA | NA | NA | NA |
T1N0M0 | NA |
| Li et al
(14) |
|
|
|
|
|
|
|
|
|
|
|
F/51/yes | 33/LLL | − | + | − | + | NA | NA | NA |
T2N1M0 | A
(10) |
| Hatanaka et
al (15) |
|
|
|
|
|
|
|
|
|
|
|
F/51/no | 30/LLL and
10/RUL | − | + | − | + | − | p+ | NA |
T1N0M0 | A
(48) |
| Lin et al
(16) |
|
|
|
|
|
|
|
|
|
|
|
F/61/no | 50/RML | + | p+ | − | − | NA | NA | + |
T3N0M0 | A (6) |
| Stojsic et
al (17) |
|
|
|
|
|
|
|
|
|
|
|
M/24/NA | 60/LLL | − | + | − | + | − | − | + |
T2N0M0 | A
(23) |
|
F/26/NA | 60/LLL | − | + | − | + | − | − | + |
T3N0M0 | A
(31) |
| Qureshi et
al (18) |
|
|
|
|
|
|
|
|
|
|
|
F/61/NA | MB | + | + | − | + | NA | NA | NA |
TxNxM1 | NA |
|
F/61/no | 60/RML | + | p+ | − | NA | NA | NA | + |
T3N0M0 | A (6) |
| Wang et al
(19) |
|
|
|
|
|
|
|
|
|
|
|
M/65/no | 23/RLL | + | − | p+ | + | + | − | − |
T1N1M0 | D
(36) |
|
F/56/no | 30/RUL | + | − | + | + | − | + | − |
T1N0M0 | A
(27) |
|
M/60/yes | 35/RUL | + | − | + | + | − | − | + |
T2N0M0 | A
(39) |
|
F/63/yes | 27/RUL | + | − | − | − | + | p+ | + |
T1N0M0 | A
(43) |
|
F/65/yes | 20/RUL | + | − | + | − | − | − | + |
T2N1M0 | D
(12) |
|
M/74/yes | 15/LLL | + | − | − | − | + | + | − |
T1N0M0 | A
(23) |
|
M/61/yes | 60/RUL | + | p+ | − | + | − | − | + |
T2N3M0 | D (7) |
|
F/34/no | 48/RUL | + | − | − | + | − | p+ | + |
T2N1M0 | A
(22) |
|
F/63/yes | 33/RUL | + | + | − | + | − | − | p+ |
T2N0M0 | A (6) |
| Present case |
|
|
|
|
|
|
|
|
|
|
|
F/53/no | MB | − | + | − | + | − | − | + |
T2N1M1 | A
(12) |
The main symptoms of the present patient were an
intermittent dry cough and a right-sided supraclavicular mass. The
patient did not exhibit any gastrointestinal symptoms such as
nausea, vomiting, abdominal pain, diarrhea, constipation or bloody
stools. The chest CT scan revealed lesions in the mediastinum,
lungs and right supraclavicular region. CT and PET-CT scans of the
large and small intestine and pelvic cavity were performed. No
evidence of a primary gastrointestinal tract tumor was found,
although multiple metastases were observed in the right kidney.
Similarly, gastroscopy, enteroscopy and colorectal colonoscopy did
not find evidence of a gastrointestinal malignancy. The diagnosis
of a primary lung enteric adenocarcinoma was made on the basis of
the aforementioned clinical, imaging and pathological findings.
With regard to molecular markers for primary lung
enteric adenocarcinoma, the mutation rates for EGFR, K-ras,
EML4-ALK and BRAF are ~50, 20, 5 and 2%, respectively (22,23). In
colorectal cancer, the mutation rates for K-ras and BRAF are ~30
and 9%, respectively (24); however,
in the present case the EGFR, K-ras and BRAF genes that were
expressed were all wild-type and no expression of the EML4-ALK
fusion gene was identified. These findings indicate that the
patient was not a suitable candidate for targeted drug therapy.
To date, the only chemotherapy regimen reported to
be successful in the treatment of this tumor is pemetrexed and
carboplatin given for 4 cycles (14). The side-effects of this regimen have
been said to be tolerable, with a stable disease response. There
are no reports suggesting that genotyping helps predict the
response to chemotherapy. At the 2013 meeting of the American
Society of Clinical Oncology, the Spanish Lung Cancer Group
reported that the expression status of ERCC1 and RRM1 is not
helpful for individualizing chemotherapy regimens (25). At the 2013 World Conference on Lung
Cancer, Bonanno et al reported that neither breast cancer 1
nor receptor-associated protein 80 assisted in predicting the
response to individualized chemotherapy in phase III randomized
controlled studies (26). Therefore,
it is not currently possible to implement individualized
chemotherapy based on the expression of specific genes; however,
certain reports have suggested correlations between gene expression
and drug efficacy (27–29). Retrospective measurement of gene
expression in those sensitive to chemotherapy demonstrated moderate
expression of ERCC1, TUBB3 and RRM1, and high expression of TYMS,
wild-type UGT1A1 and MTHFR TC, microsatellite stable. These results
suggest the likelihood of a particular sensitivity to taxane and
platinum drugs, and reduced sensitivity to fluoropyrimidine.
Clinical practice tends to confirm this (27–29).
The majority of the previously reported cases of
primary lung intestinal adenocarcinoma had localized disease
without metastases and were treated surgically. The present case,
however, had advanced disease, which was not amenable to curative
surgery. The patient received palliative treatment instead, both
with surgery and with four different chemotherapy regimens. The
first and third chemotherapy regimens used in the present case are
usually selected for colorectal carcinomas, whilst the second and
fourth regimens are mainly used to treat non-small cell carcinomas
of the lung. Detailed evaluation showed that the TP regimen
produced an clear response, shown by a reduction in the level of
CEA.
Standard chemotherapy for colorectal carcinoma
comprises oxaliplatin, irinotecan and fluorouracil (30,31). The
patient of the present case had a poor response to chemotherapeutic
drugs used to treat metastatic colorectal cancer. Taxane combined
with platinum is commonly used as the first line chemotherapy for
non-small cell lung carcinoma. This has shown limited efficacy in
preclinical studies in colorectal carcinoma, and for that reason
the Federal Drug Administration does not recommend this regimen for
the treatment of colorectal carcinomas (32). In the present case, the patient's
lung lesions responded well to taxane combined with platinum, which
was shown by a significant reduction in their size. No change,
however, was observed in the size of the mediastinal and renal
metastases. The progression of the right supraclavicular mass,
despite chemotherapy, was probably a consequence of the
heterogeneity of the tumor.
In conclusion, the present study reports a case of
metastatic carcinoma with the primary tumor being enteric carcinoma
of the lung, and includes details of its response to chemotherapy
regimens. The patient presented with mediastinal, lung and right
supraclavicular masses, but with no evidence of a gastrointestinal
malignancy. She was treated with four different chemotherapy
regimens, namely, XELOX, TP, FOLFIRI and DP. A significant
reduction in the size of the lung lesions was observed during the
second regimen. Further consideration is required when deciding on
a chemotherapy regimen for metastatic enteric carcinoma of the
lung, and future reports of individual cases of this rare tumor
could considerably assist the decision-making process.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2013. View Article : Google Scholar
|
|
2
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International Association for the Study of
Lung Cancer/American Thoracic Society/European Respiratory Society:
International multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chhieng DC, Cangiarella JF, Zakowski MF,
Goswami S, Cohen JM and Yee HT: Use of thyroid transcription factor
1, PE-10 and, cytokeratins 7 and 20 in discriminating between
primary lung carcinomas and metastatic lesions in fine-needle
aspiration biopsy specimens. Cancer. 93:330–336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueno T, Linder S and Elmberger G: Aspartic
proteinase napsin is a useful marker for diagnosis of primary lung
adenocarcinoma. Br J Cancer. 88:1229–1233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barbareschi M, Murer B, Colby TV, Chilosi
M, Macri E, Loda M and Doglioni C: CDX-2 homeobox gene expression
is a reliable marker of colorectal adenocarcinoma metastases to the
lungs. Am J Surg Pathol. 27:141–149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ho SB, Shekels LL, Toribara NW, Kim YS,
Lyftogt C, Cherwitz DL and Niehans GA: Mucin gene expression in
normal, preneoplastic and neoplastic human gastric epithelium.
Cancer Res. 55:2681–2690. 1995.PubMed/NCBI
|
|
7
|
Lau SK, Weiss LM and Chu PG: Differential
expression of MUC1, MUC2 and MUC5AC in carcinomas of various sites:
An immunohistochemical study. Am J Clin Pathol. 122:61–69. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Min KW: Two different types of carcinoid
tumors of the lung: Immunohistochemical and ultrastructural
investigation and their histogenetic consideration. Ultrastruct
Pathol. 37:23–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger K, Yatabe Y, Ishikawa Y, Wistuba I, Flieder
DB, Franklin W, et al: Diagnosis of lung adenocarcinoma in resected
specimens: Implications of the 2011 International association for
the Study of lung Cancer/American Thoracic Society/European
Respiratory Society classification. Arch Pathol Lab Med.
137:685–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ou SH, Kawaguchi T, Soo RA and Kitaichi M:
Rare subtypes of adenocarcinoma of the lung. Expert Rev Anticancer
Ther. 11:1535–1542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inamura K, Satoh Y, Okumura S, Nakagawa K,
Tsuchiya E, Fukayama M and Ishikawa Y: Pulmonary adenocarcinomas
with enteric differentiation: Histologic and immunohistochemical
characteristics compared with metastatic colorectal cancers and
usual pulmonary adenocarcinomas. Am J Surg Pathol. 29:660–665.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yousem SA: Pulmonary intestinal-type
adenocarcinoma does not show enteric differentiation by
immunohistochemical study. Mod Pathol. 18:816–821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maeda R, Isowa N, Onuma H and Miura H:
Pulmonary intestinal-type adenocarcinoma. Interact Cardiovasc
Thorac Surg. 7:349–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li HC, Schmidt L, Greenson JK, Chang AC
and Myers JL: Primary pulmonary adenocarcinoma with intestinal
differentiation mimicking metastatic colorectal carcinoma. Am J
Clin Pathol. 131:129–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hatanaka K, Tsuta K, Watanabe K, Sugino K
and Uekusa T: Primary pulmonary adenocarcinoma with enteric
differentiation resembling metastatic colorectal carcinoma: A
report of the second case negative for cytokeratin 7. Pathol Res
Pract. 207:188–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin D, Zhao Y, Li H and Xing X: Pulmonary
enteric adenocarcinoma with villin brush border immunoreactivity: A
case report and literature review. J Thorac Dis. 5:E17–E20.
2013.PubMed/NCBI
|
|
17
|
Stojsic J, Kontic M, Subotic D, Popovic M,
Tomasevic D and Lukic J: Intestinal type of lung adenocarcinoma in
younger adults. Case Rep Pulmonol. 2014:2821962014.PubMed/NCBI
|
|
18
|
Qureshi A and Furrukh M: Enteric
adenocarcinoma lung: A rare presentation in an Omani woman. BMJ
Case Rep. 25:doi: 10.1136/bcr-2012-007667. 2013.
|
|
19
|
Wang CX, Liu B, Wang YF, Zhang RS, Yu B,
Lu ZF, Shi QL and Zhou XJ: Pulmonary enteric adenocarcinoma: A
study of the clinicopathologic and molecular status of nine cases.
Int J Clin Exp Pathol. 7:1266–1274. 2014.PubMed/NCBI
|
|
20
|
Remo A, Zanella C, Pancione M, Astati L,
Piacentini P, Cingarlini S, Bonetti A, Micheletto C, Talamini A,
Chilosi M, et al: Lung metastasis from TTF-1 positive sigmoid
adenocarcinoma. Pitfalls and management. Pathologica. 105:69–72.
2013.PubMed/NCBI
|
|
21
|
Sullivan LM, Smolkin ME, Frierson HF Jr
and Galgano MT: Comprehensive evaluation of CDX2 in invasive
cervical adenocarcinomas: Immunopositivity in the absence of overt
colorectal morphology. Am J Surg Pathol. 32:1608–1612. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsao MS and Fraser RS: Primary pulmonary
adenocarcinoma with enteric differentiation. Cancer. 68:1754–1757.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Satoh Y, Hoshi R, Tsuzuku M, Ishikawa Y,
Inamura K and Horai T: Cytology of pulmonary adenocarcinomas
showing enteric differentiation. Acta Cytol. 50:250–256. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagasaka T, Sasamoto H, Notohara K,
Cullings HM, Takeda M, Kimura K, Kambara T, MacPhee DG, Young J,
Leggett BA, et al: Colorectal cancer with mutation in BRAF, KRAS,
and wild-type with respect to both oncogenes showing different
patterns of DNA methylation. J Clin Oncol. 22:4584–4594. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moran T, Wei J, Cobo M, Qian X, Domine M,
Zou Z, Bover I, Wang L, Provencio M, Yu L, et al: Spanish Lung
Cancer Group; French Lung Cancer Group; Comprehensive Cncer Centre
of Drum Tower Hospital in Nanjing: Two biomarker-directed
randomized trials in European and Chinese patients with
nonsmall-cell lung cancer: The BRCA1-RAP80 expression customization
(BREC) studies. Ann Oncol. 25:2147–2155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bonanno L, Costa C, Majem M, Favaretto A,
Rugge M and Rosell R: The predictive value of BRCA1 and RAP80 mRNA
expression in advanced non-small-cell lung cancer patients treated
with platinum-based chemotherapy. Ann Oncol. 24:1130–1132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olaussen KA, Dunant A, Fouret P, Brambilla
E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH,
et al: IALT Bio Investigators: DNA repair by ERCC1 in
non-small-cell lung cancer and cisplatin-based adjuvant
chemotherapy. N Engl J Med. 355:983–991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reynolds C, Obasaju C, Schell MJ, Li X,
Zheng Z, Boulware D, Caton JR, Demarco LCO, Rourke MA, Shaw Wright
G, et al: Randomized phase III trial of gemcitabine-based
chemotherapy with in situ RRM1 and ERCC1 protein levels for
response prediction in non-small-cell lung cancer. J Clin Oncol.
27:5808–5815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bepler G, Sommers KE, Cantor A, Li X,
Sharma A, Williams C, Chiappori A, Haura E, Antonia S, Tanvetyanon
T, et al: Clinical efficacy and predictive molecular markers of
neoadjuvant gemcitabine and pemetrexed in resectable non-small cell
lung cancer. J Thorac Oncol. 3:1112–1118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
André T, Bensmaine MA, Louvet C, François
E, Lucas V, Desseigne F, Beerblock K, Bouché O, Carola E, Merrouche
Y, et al: Multicenter phase II study of bimonthly high-dose
leucovorin, fluorouracil infusion, and oxaliplatin for metastatic
colorectal cancer resistant to the same leucovorin and fluorouracil
regimen. J Clin Oncol. 17:3560–3568. 1999.PubMed/NCBI
|
|
31
|
Saltz LB, Cox JV, Blanke C, Rosen LS,
Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta
N, et al: Irinotecan plus fluorouracil and leucovorin for
metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med.
343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang K, Vaughn DJ, Shaw LM, Recio A,
Bonner HS and Haller DG: A phase II trial and pharmacokinetic
analysis of 96-hour infusional paclitaxel in patients with
metastatic colorectal cancer. Am J Clin Oncol. 21:548–552. 1998.
View Article : Google Scholar : PubMed/NCBI
|