Introduction
Anti-leucine-rich glioma-inactivated 1 (LGI1) limbic
encephalitis (LE) is characterized by faciobrachial dystonic
seizures (FBDSs), memory loss and antibodies against the
leucine-rich, glioma-inactivated subunit of the voltage-gated
potassium channel (VGKC)-complex. FBDSs precede the other symptoms
and prompt the diagnosis (1). The
clinical course is subacute (2).
Newly discovered antibodies have revealed that target neuronal cell
surface antigens, VGKCs and ligand-gated ion channels are present
in anti-LGI1 LE, despite not being commonly found in the presence
of malignancies (3). LGI1 has also
been identified as a specific target of potassium channel
antibodies (4). In the present case,
the patient presented with FBDSs and memory loss, but no nausea,
vomiting, insomnia or hyponatremia, which commonly accompany
anti-LGI1 LE.
The patient provided informed consent for the use of
her case data, and permission to present the case was obtained from
the ethics committee of the First Hospital of Jilin University
(Changchun, China).
Case report
A 41-year-old woman was admitted for anterograde
memory loss and right facial grimacing and arm posturing that had
started 1 month previously, in May 2014. The relatives of the
patient stated that her memory disturbances, particularly
short-term memory loss and disorientation, had developed gradually
over the last month. Episodes of involuntary movement of her right
arm and face, lasting between 10 and 30 sec, were occurring with
increasing frequency; while at first these episodes had occurred 2
or 3 times a day, they had progressed to >10 times a day after 2
weeks. No signs of insomnia or hyponatremia were observed. The
patient's temperature was normal and her past medical history was
unremarkable. The patient had no vomiting and headache at the onset
of her condition. No focal deficit was found on neurological
examination, with the exception of memory disturbance. Cranial
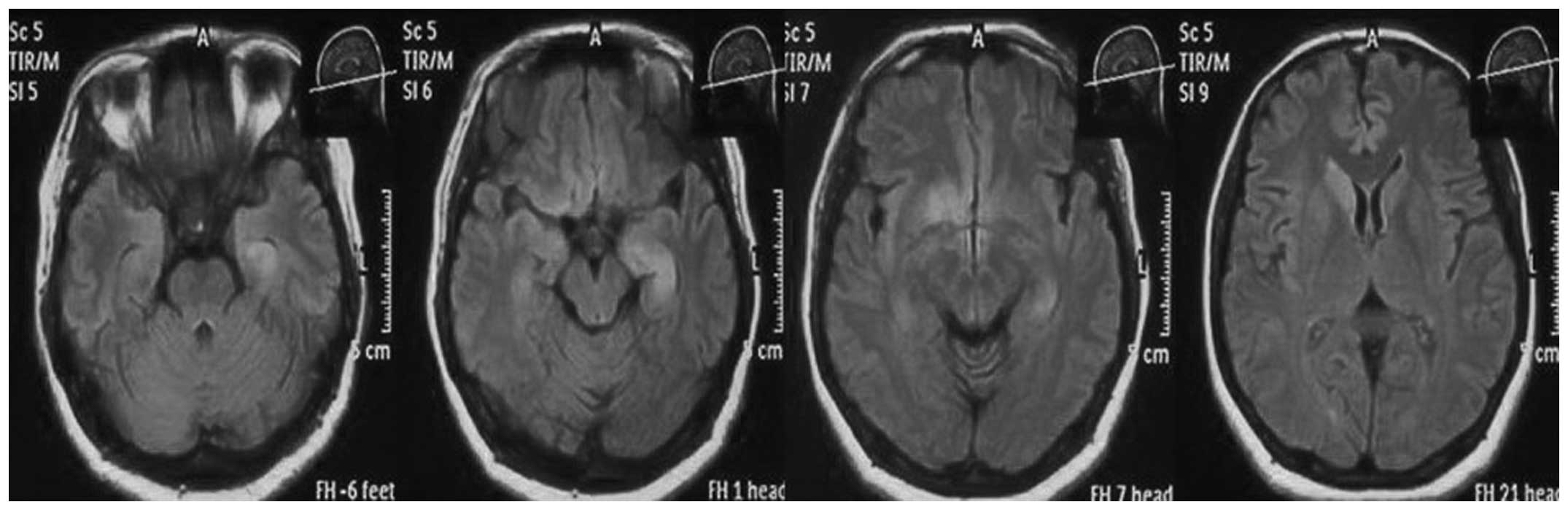
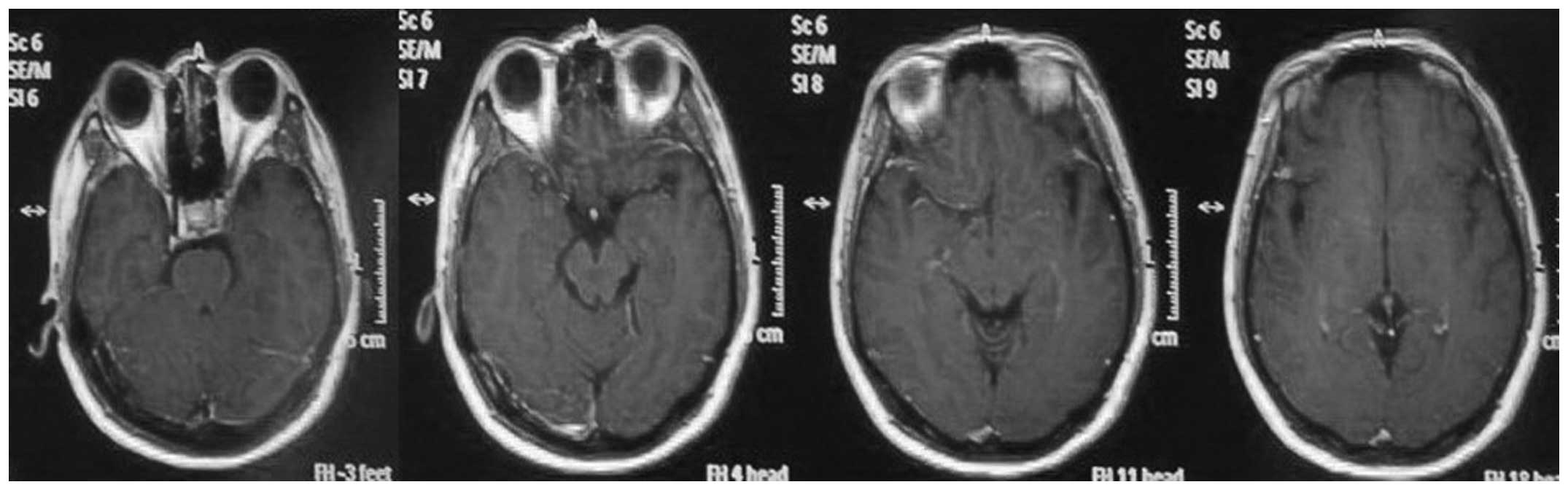
magnetic resonance (MR)-diffusion weighted imaging and -fluid
attenuated inversion recovery imaging revealed a hyperintense
signal in the left hippocampus and right basal ganglia, but without
contrast enhancement (Figs. 1 and
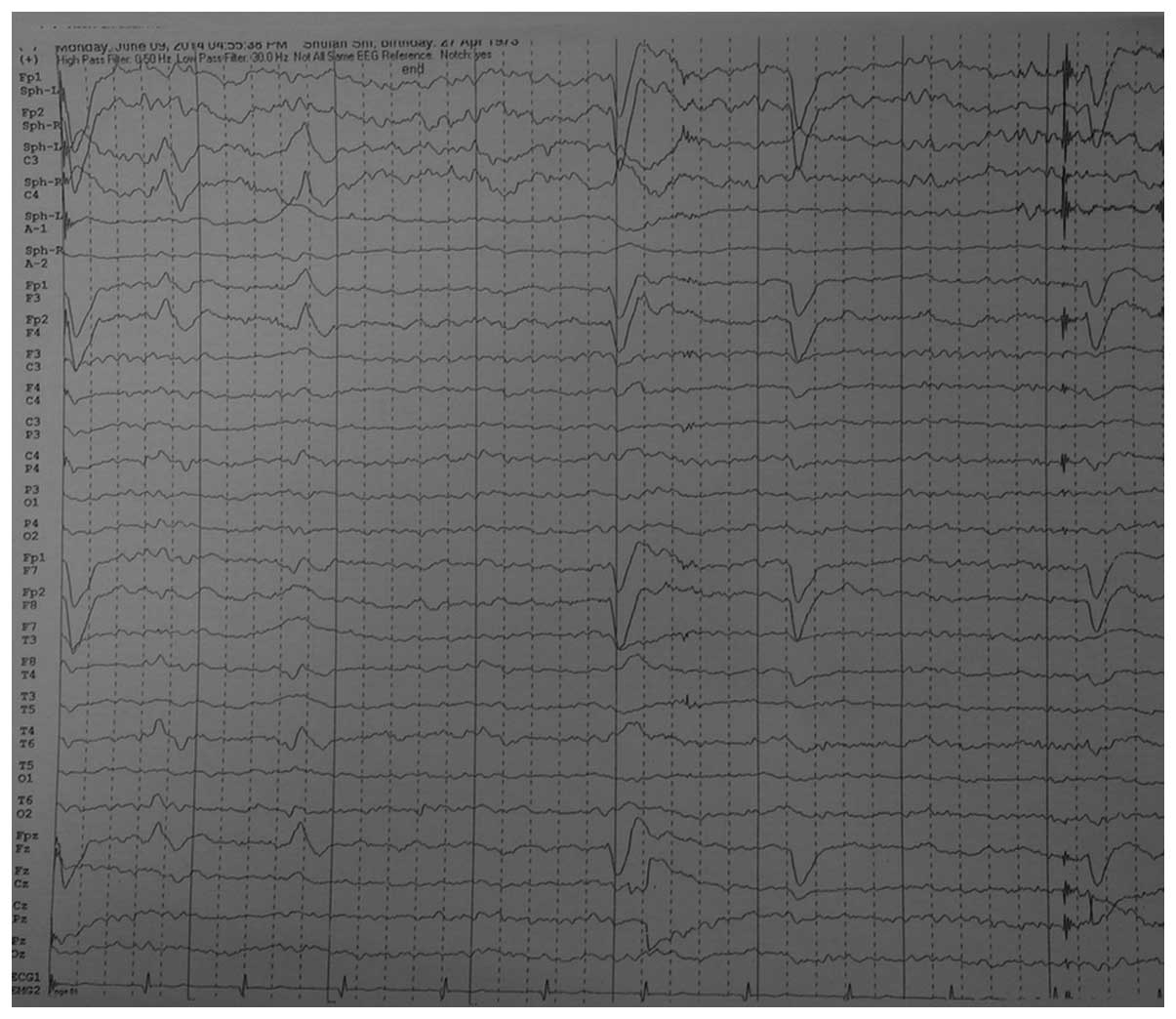
2). An electroencephalogram (EEG)
revealed rhythmic sharp and slow waves and rhythmic θ build-ups in
the left temporal area (Fig. 3).
Video-EEG captured eleptiform paradoxical discharge when the
patient was asleep at night. Single-photon emission computed
tomography (SEPCT) showed regional blood flow perfusion in the left
cerebral frontal lobe and right basal ganglia. Laboratory studies
revealed a normal serum sodium level, and the glucose, lactate and
protein levels in the cerebrospinal fluid (CSF) were normal. No
evidence of herpes simplex virus 1 or 2, Borrelia
burgdorferi or Treponema pallidum was found in the CSF
or the blood. The serum and CSF were positive for antibodies to
LGI1 but negative for anti-Yo, -Ri, -Hu, -Ma, -N-methyl-D-aspartate
receptor, -α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
receptor and -CV2 autoantibodies.
The patient was diagnosed with anti-LGI1 LE based on
the characteristic FBDSs, memory loss and positive LGI1 antibodies
in the blood and CSF. A treatment regimen of 500 mg/day IV
methylprednisolone for 3 days followed by 250 mg/day for 3 days and
125 mg/day for 3 days was initiated. This was followed by IVIg (0.4
g/kg/day) for 5 days and 8 weeks of tapered oral prednisolone. The
patient also received oxcarbazepine. The FBDSs of the patient
stopped completely 1 day after the initiation of treatment and her
memory deficits improved. At 3 months after treatment began, the
patient remained free from epileptic seizures and her memory had
been partially restored.
Discussion
LE is a well-recognized syndrome and is associated
with several different antibodies, including anti-Hu, anti-Ri,
anti-Yo, anti-Ma2, anti-amphiphysin and anti-CV2/collapsin response
mediator protein 5. These antibodies are expressed throughout the
nervous system and are also associated with less well-known
neurological disorders that affect wider brain systems (5). Numerous patients with LE do not have
detectable brain tumors.
Anti-LGI1 LE has been identified as an autoimmune
encephalitis. The disorder usually involves the medial temporal
area, which causes memory dysfunction and seizures, and has
distinctive clinical characteristics, including FBDSs, memory
disturbance and a subacute, progressive course (6). According to the literature,
hyponatremia is commonly found in patients with anti-LGI1 LE
(1); however, this is a non-specific
sign (7). The patient in the present
case exhibited the characteristic clinical symptoms, but no
hyponatremia, insomnia or abnormalities on cranial MR imaging,
video-EEG and SPECT. The patient's diagnosis was confirmed by the
presence of LGI1 antibodies in the blood and CSF.
The diagnosis of autoimmune LE is difficult and
often delayed. While certain cases involve the limbic system
exclusively, other systems may also be involved, confusing the
diagnostic picture (8). Clinicians
in Korea (5) described a case that
spontaneously went into remission prior to a definitive diagnosis
being made. The symptoms recurred in 2013, when the disorder was
identified. French researchers observed a 65-year-old anti-LGI1 LE
patient with insomnia in 2012 (9).
Only few reports have highlighted the presence of reversible
insomnia in autoimmune encephalitis (10), and the mechanisms by which LGI1
antibodies may cause insomnia remain unclear (9). German researchers were the first to
report the neuropathological characteristics of LGI1 LE and
suggested a CD8+ T-cell-mediated immune process directed
against hippocampal neurons (11).
Early diagnosis of this rare disease is important so
that treatment can be instituted as soon as possible. Treatment
delays can result in ongoing functional memory problems and other
lingering neurological deficits (12). FBDSs may be the earliest
manifestation of LE associated with the LGI1 antibody, so
recognizing this unique type of seizure at once is important and
allows treatment to start quickly, when it is most effective
(13).
Although there are no definitive therapeutic
guidelines for anti-LGI1 LE (14),
first-line treatment usually involves high doses of steroids in
combination with other immunosuppressive therapy, such as IVIg,
plasma exchange or mycophenolate (15). Failure to respond within 1–2 weeks
should prompt second-line therapy, such as three to five
plasmapheresis exchanges or the administration of cyclophosphamide
or rituximab (16). Response to
therapy is best judged by the patient's clinical status. Following
antibody titers alone is not useful, as they do not always
correlate with clinical progress. The effect of long-term,
steroid-sparing agents, such as mycophenolate or azathioprine, on
relapse rates is unclear (17).
If clinicians suspect anti-LGI1 LE due to symptoms
of FBDSs and memory loss, immunotherapy should begin at once, even
before the laboratory results have confirmed the presence of LGI1
antibodies in the CSF and blood, to limit the duration of the
illness.
References
|
1
|
Irani SR, Michell AW, Lang B, Pettingill
P, Waters P, Johnson MR, Schott JM, Armstrong RJS, Zagami A,
Bleasel A, et al: Faciobrachial dystonic seizures precede Lgi1
antibody limbic encephalitis. Ann Neurol. 69:892–900. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asztely F and Kumlien E: The diagnosis and
treatment of limbic encephalitis. Acta Neurol Scand. 126:365–375.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tüzün E and Dalmau J: Limbic encephalitis
and variants: Classification, diagnosis and treatment. Neurologist.
13:261–271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lancaster E, Huijbers MG, Bar V, Boronat
A, Wong A, Martinez-Hernandez E, Wilson C, Jacobs D, Lai M, Walker
RW, et al: Investigations of caspr2, an autoantigen of encephalitis
and neuromyotonia. Ann Neurol. 69:303–311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JJ, Lee ST, Jung KH, Chu K and Lee SK:
Anti-lGI1 Limbic encephalitis presented with atypical
manifestations. Exp Neurobiol. 22:337–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Machado S, Pinto AN and Irani SR: What
should you know about limbic encephalitis? Arq Neuropsiquiatr.
70:817–822. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gulati S and Kumar L: ‘Chest epilepsy’ in
a child. Postgrad Med J. 68:369–370. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Irani SR, Bien CG and Lang B: Autoimmune
epilepsies. Curr Opin Neurol. 24:146–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peter-Derex L, Devic P, Rogemond V, Rheims
S, Mauguière F and Honnorat J: Full recovery of agrypnia associated
with anti-Lgi1 antibodies encephalitis under immunomodulatory
treatment: A case report with sequential polysomnographic
assessment. Sleep Med. 13:554–556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Montiel P, Sellal F, Clerc C, Richard P
and Batailard M: Limbic encephalitis with severe sleep disorder
associated with voltage-gated potassium channels (VGKCs)
antibodies. Rev Neurol (Paris). 164:181–184. 2008.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schultze-Amberger J, Pehl D and Stenzel W:
LGI-1-positive limbic encephalitis: A clinicopathological study. J
Neurol. 259:2478–2480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Irani SR, Stagg CJ, Schott JM, Rosenthal
CR, Schneider SA, Pettingill P, Pettingill R, Waters P, Thomas A,
Voets NL, et al: Faciobrachial dystonic seizures: The influence of
immunotherapy on seizure control and prevention of cognitive
impairment in a broadening phenotype. Brain. 136:3151–3162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sen A, Wang J, Laue-Gizzi H, Lee T,
Ghougassian D and Somerville ER: Pathognomonic seizures in limbic
encephalitis associated with anti-LGI1 antibodies. Lancet.
383:20182014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thieben MJ, Lennon VA, Boeve BF, Aksamit
AJ, Keegan M and Vernino S: Potentially reversible autoimmune
limbic encephalitis with neuronal potassium channel antibody.
Neurology. 62:1177–1182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lancaster E, Martinez-Hernandez E and
Dalmau J: Encephalitis and antibodies to synaptic and neuronal cell
surface proteins. Neurology. 77:179–189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishiura H, Matsuda S, Higashihara M,
Hasegawa M, Hida A, Hanajima R, Yamamoto T, Shimizu J, Dalmau J and
Tsuji S: Response of anti-NMDA receptor encephalitis without tumor
to immunotherapy including rituximab. Neurology. 71:1921–1923.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Irani SR and Vincent A: NMDA receptor
antibody encephalitis. Curr Neurol Neurosci Rep. 11:298–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|