Introduction
The co-occurrence of epilepsy and depression is
commonly observed in neurological psychiatry (1). Studies have revealed that the
co-occurrence of epilepsy with depression is associated with the
activation of 5-hydroxytryptamine 1A (5-HT1A) receptors (2,3). A
previous study indicated that the 5-HT1A receptor agonist
8-hydroxy-dipropylaminotetralin (8-OH-DPAT) exerts anti-epileptic
and anti-depressive effects in epileptic rats with depression,
which involve the enhancement of the growth of hippocampal neurons
(4). However, certain current
treatments possess shortcomings, such as the antidepressant
bupropion which may cause generalized seizures and central nervous
system depression if administered in excess (5). Chinese medicinal herbs, such as
Bupleurum chinense root and Pericarpium Citri Reticulatae appear to
exhibit limited adverse reactions and therapeutic efficacy
(6).
The present study employed chronic epileptic rats
with depression induced by a combination of pilocarpine and chronic
mild stress (CMS) to investigate the efficacy of Chaihu-Shugan-San
(CHSGS). Detection methods included behavioral analysis,
immunohistochemistry and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR). The expression levels of
5-HT1A receptor mRNA and numbers of 5-bromo-2′-deoxyuridine
(BrdU)-labeled cells in the hippocampus were detected to clarify
whether CHSGS improves cellular proliferation in the hippocampus by
promoting the expression of 5-HT1A receptor mRNA. The results of
this study may support the use of CHSGS as a novel treatment for
epilepsy with depression.
Materials and methods
Animals and experimental protocol
The present study was a randomized, controlled
animal study. The study was performed at the Anatomical Research
Laboratory of Hunan University of Chinese Medicine (Changsha,
China). A total of 60 male Sprague-Dawley (SD) rats (weight,
180–220 g; age, 8–9 weeks) were provided by the Laboratory Animal
Center of Hunan University of Chinese Medicine [License no.
SYXK(Xiang)2009-0001; Changsha, China]. Rats were separately housed
in plastic cages at 22°C and 60% humidity under a constant
light-dark cycle and were allowed free access to food and water.
The animals were acclimatized to the environment for 2 weeks, while
being trained to consume 1% (w/v) sucrose solution for taste
adaptation. Based on the baseline scores of sucrose consumption
test and the open-field test (OFT) during the adaptation period, 48
rats with similar behavior were randomly divided into four groups:
Control, model, fluoxetine and CHSGS groups (n=12 per group). All
procedures were performed in accordance with the Guidance
Suggestions for the Care and Use of Laboratory Animals, formulated
by the Ministry of Science and Technology of China (7).
Reagents
The CHSGS preparation contained the following: 6 g
Bupleurum chinense root, 6 g Pericarpium Citri Reticulatae,
4.5 g Rhizoma Chuanxiong, 4.5 g Rhizoma Cyperi, 4.5 g Fructus
Aurantii, 4.5 g Paeonia and 1.5 g Glycyrrhizae
uralensis root. All CHSGS herbal components were obtained from
the First Hospital of Hunan University of Chinese Medicine. The
herbal components were identified by an expert to fulfill the
quality requirements of the Pharmacopoeia of the People's Republic
of China (8). CHSGS and its
components were individually decocted in boiling water for 45 min,
concentrated and vacuum-dried to form a paste, and were
subsequently combined into a paste containing 8 g crude extracts
per gram. This CHSGS paste was diluted with distilled water to a
concentration of 1.0 g/ml for application. The CHSGS administration
dose was 2.7 g/kg/day in accordance with pharmacological
experiments (9).
Fluoxetine capsules (2061AA; 20 mg per capsule) were
purchased from Eli Lilly Suzhou Pharmaceutical Group Co., Ltd.,
(Suzhou, China). The administration dose of fluoxetine was 1.8
g/kg/day in accordance with previous pharmacological experiments
(9).
Sucrose consumption test
The sucrose consumption test was performed as
described previously (10). The test
was conducted over 3 days. On day 1, rats were housed individually
and had free access to two 100-ml bottles of sucrose solution (1%
w/v). Rats were trained to adapt to sucrose solution for 24 h. On
day 2, one bottle of sucrose solution was replaced with 100 ml
water for 24 h. On day 3, rats were deprived of water and food for
23 h, and subsequently received free access to two pre-weighed
bottles containing 100 ml sucrose solution (1% w/v) and 100 ml
water, respectively. After 1 h, the consumed volume from each
bottle was recorded. The sucrose preference rate was calculated
using the following formula: Sucrose preference rate (%) = Sucrose
consumption/(water consumption + sucrose consumption) × 100. This
test was conducted prior to division of the rats into groups, and
following the 28-day treatment period.
OFT
The OFT was performed according to a previously
described method (11), to measure
spontaneous activity in rodents. Briefly, the floor of a
100×100×40-cm square arena was divided into 16 equal 25×25-cm
squares. A single rat was placed in the center of the arena, and
after 30 sec of adaptation the frequency of vertical and horizontal
movement were recorded manually for 5 min. All rat movement was
recorded using an XZ-10 camera (Olympus Corporation, Tokyo, Japan)
located 40 cm above the arena. After each test, the arena was
cleaned with 90% alcohol solution.
Epilepsy model establishment
Adult male rats in the model, fluoxetine and CHSGS
groups received 127 mg/kg lithium chloride (62480; Sigma-Aldrich,
St. Louis, MO, USA) intraperitoneally (i.p.). On the following day,
1 mg/kg methylscopolamine bromide (W131102; i.p.; Shanghai Tongyong
Pharmaceutical Co., Ltd., Shanghai, China) was administered to
limit the peripheral effects of the convulsant. Status epilepticus
was induced by injecting 35 mg/kg pilocarpine (P1650000; i.p.;
Sigma-Aldrich) 30 min after the administration of methylscopolamine
bromide. Animals were monitored throughout status epilepticus
induction, and seizure severity was assessed according to the scale
described by Racine (12). Animals
that did not show clear signs of status epilepticus were excluded,
since ≥1 h of status epilepticus is required to develop spontaneous
recurrent epileptic seizures in the pilocarpine model of epilepsy.
Status epilepticus occurred within 15–60 min and was characterized
by continuous motor-limbic seizures accompanied by intermittent
rearing and falling and occasional wild running spells. At 1 h
after status epilepticus, the seizures were stopped with 4 mg/kg
diazepam (AH131203; Shanghai Sunrise Pharmaceutical Co. Ltd.,
Shanghai, China) administered intramuscularly and/or 3 ml/kg
chloral hydrate (i.p.; Anatomical Research Laboratory of Hunan
University of Chinese Medicine).
Epilepsy with depression model
establishment
Following the successful establishment of the
epilepsy model, CMS was induced in accordance with a previously
described method (13). The CMS
regimen included seven different stressors, which were arranged in
random order for 14 consecutive days as follows: Food and water
deprivation (20 h, followed by sucrose consumption test); water
deprivation (18 h, followed by 1 h exposure to empty bottles); 45°
cage tilt (17 h); overnight illumination (lights on for a total of
36 h); soiled cage (200 ml of water in 100 g sawdust bedding, 21
h); swimming in 41°C water (5 min); and paired caging (2 h). At day
14 after the establishment of CMS, the model, fluoxetine and CHSGS
groups were intragastrically perfused with 10 ml/kg normal saline,
1.8 g/kg fluoxetine and 2.7 g/kg CHSGS once per day for a total of
28 days.
5-Bromo-2′-deoxyuridine (BrdU)
labeling and immunofluorescence staining
The thymidine analog BrdU (EDll00; Sigma-Aldrich)
was dissolved in saline with 0.007 M NaOH. The BrdU solution (50
mg/kg) was administered twice per day for 7 days following the
experimental treatment with fluoxetine or CHSGS administration.
Subsequently, 6 rats in each group were sacrificed 24 h after the
final BrdU administration using 10% chloral hydrate, transfused
intraperitoneally. Rats were perfused transcardially with cold 0.1
M phosphate-buffered saline (PBS) and 4% paraformaldehyde. The
brains were removed, post-fixed for 18 h, and placed in 30% sucrose
until they sank. Frozen coronal sections were cut at 25 µm using a
cryostat (CM3050S; Leica Biosystems GmbH, Nußloch, Germany) and
stored at −20°C in a cryoprotectant solution containing 25%
ethylene glycol, 25% glycerol and 50% 0.1 M phosphate buffer (pH
7.4) for immunofluorescence staining.
The sections were subsequently incubated with 1%
bovine serum albumin to block non-specific interactions. Following
this step, the sections were incubated with mouse anti-rat BrdU
monoclonal antibody (1:100; BM0201; Wuhan Boster Biological
Technology, Ltd., Wuhan China) in PBS containing 0.3% Triton X-100
overnight at 4°C. Subsequently, the sections were incubated with
goat anti-mouse IgG-Cy2 (1:100; SA1021; Wuhan Boster Biological
Technology, Ltd.) for 2 h at room temperature. The stained sections
were observed using a BX-51 confocal microscope (Olympus
Corporation). BrdU-labeled cells were quantified using Image Pro
software, version 6.0 (Media Cybernetics, Inc., Bethesda, MD,
USA).
RT-qPCR to detect 5-HT1A receptor mRNA
expression levels in the rat hippocampus
Following the behavioral tests, 6 rats in each group
were sacrificed. Fresh hippocampus tissue was dissected and placed
on ice. Specimens were removed from the liquid nitrogen. Total RNA
was extracted using TRIzol (2214L; Beijing CoWin Biotech Co., Ltd.,
Beijing, China). RNA concentration was determined using a P-Class
NanoPhotometer (5622; Implen GmbH, München, Germany). The RT step
was conducted using a Go ScriptTM Reverse Transcription System
(0000112333; Promega Corporation, Madison, WI, USA), following the
manufacturer's instructions. Primers were synthesized by Wuhan
Boster Biological Technology, Ltd. (Table I), and a 2× SYBR GREEN-I Mix kit
(0020140910; Beijing Bioteke Biotechnological Co., Ltd., Beijing,
China) was used. Amplification reactions were conducted using a
qPCR system (TL988; Xi'an Tianlong Science and Technology Co.,
Ltd., Xi'an, China).
 | Table I.Primer sequences used for polymerase
chain reaction. |
Table I.
Primer sequences used for polymerase
chain reaction.
| Primer | Sequences
(5′-3′) | Product size
(bp) |
|---|
| 5-HT1A | Forward:
GCACCAGCTTAGGAACTTCG | 206 |
|
| Reverse:
CAGAGGAAGGTGCTCTTTGG |
|
| β-actin | Forward:
GTCAGGTCATCACTATCGGCAAT | 210 |
|
| Reverse:
AGAGGTCTTTACGGATGTCAACGT |
|
The PCR cycling conditions were as follows: i)
Pre-denaturation at 95°C for 10 min; ii) 40 cycles of denaturation
at 95°C for 15 sec and annealing/extension at 60°C for 1 min; and
iii) final extension at 60°C for 5 min. The specificity of PCR
products was monitored via melting curve analysis, and the standard
curves of the target and reference gene β-actin were prepared. The
ratio of 5-HT1A to β-actin was considered as the relative
expression of the target gene. Relative quantification of gene
expression was determined using the 2−ΔΔCq method.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Measurement
data are expressed as the mean ± standard error of the mean.
Analysis of variance was used for intergroup comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Animal grouping and treatments
A total of 60 SD rats were used. The baseline scores
of the sucrose consumption test and OFT were determined, and then
48 SD rats with similar behavior were identified and randomly
divided into four groups: Control, model, fluoxetine and CHSGS
groups (n=12 per group).
Behavior of epileptic rats with
depression
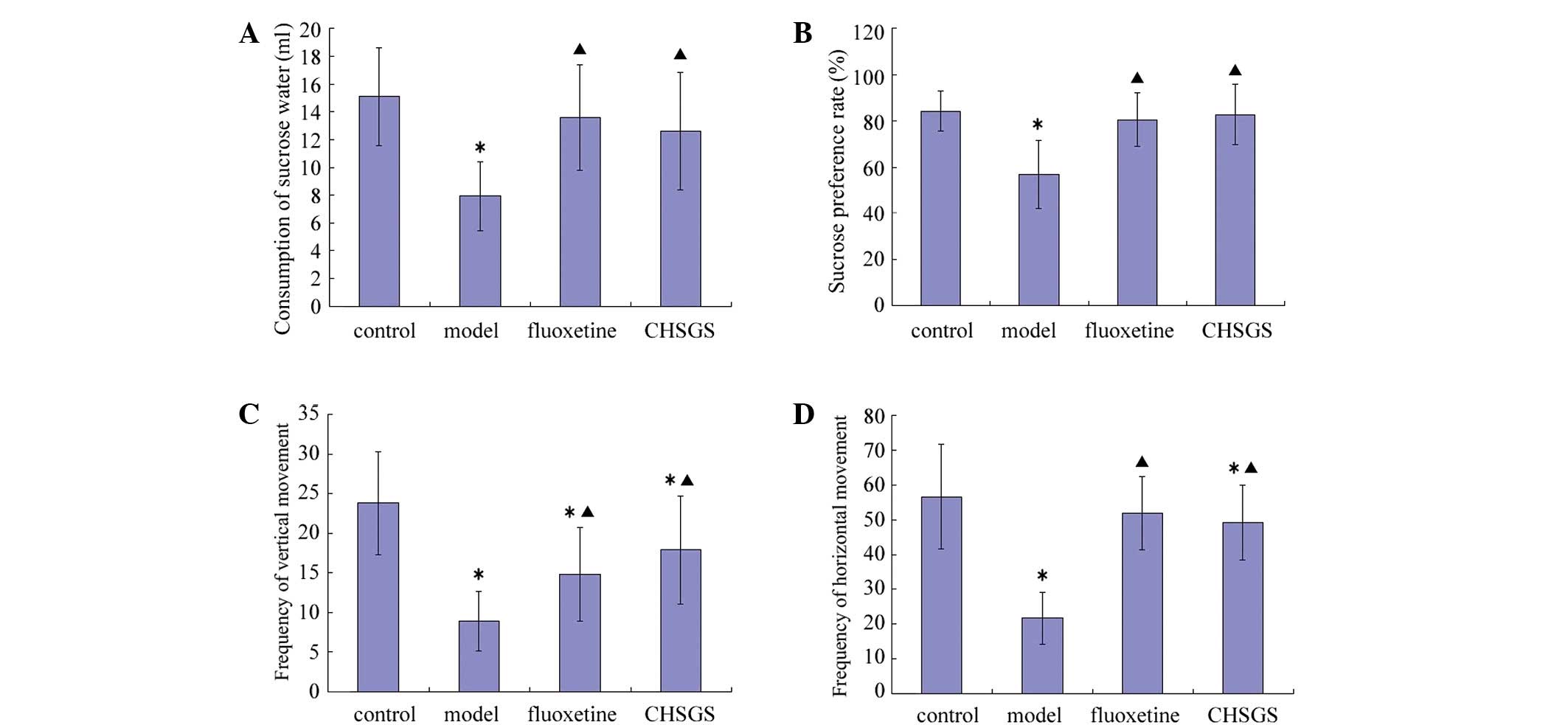
After 28 days of treatment, the consumption of
sucrose water, the sucrose preference rate, and frequency of
vertical and horizontal movement decreased in the model group
compared with the control group (P<0.05). Compared with the
control group, frequency of vertical movement decreased in the
fluoxetine group (P<0.05), while frequency of vertical and
horizontal movement decreased in the CHSGS group (P<0.05).
Compared with the model group, all sucrose and OFT test parameters
were increased in the fluoxetine and CHSGS groups (P<0.05).
However, no statistically significant differences were observed
between the fluoxetine and CHSGS groups (Fig. 1).
CHSGS promotes cellular proliferation
in the hippocampal dentate gyrus
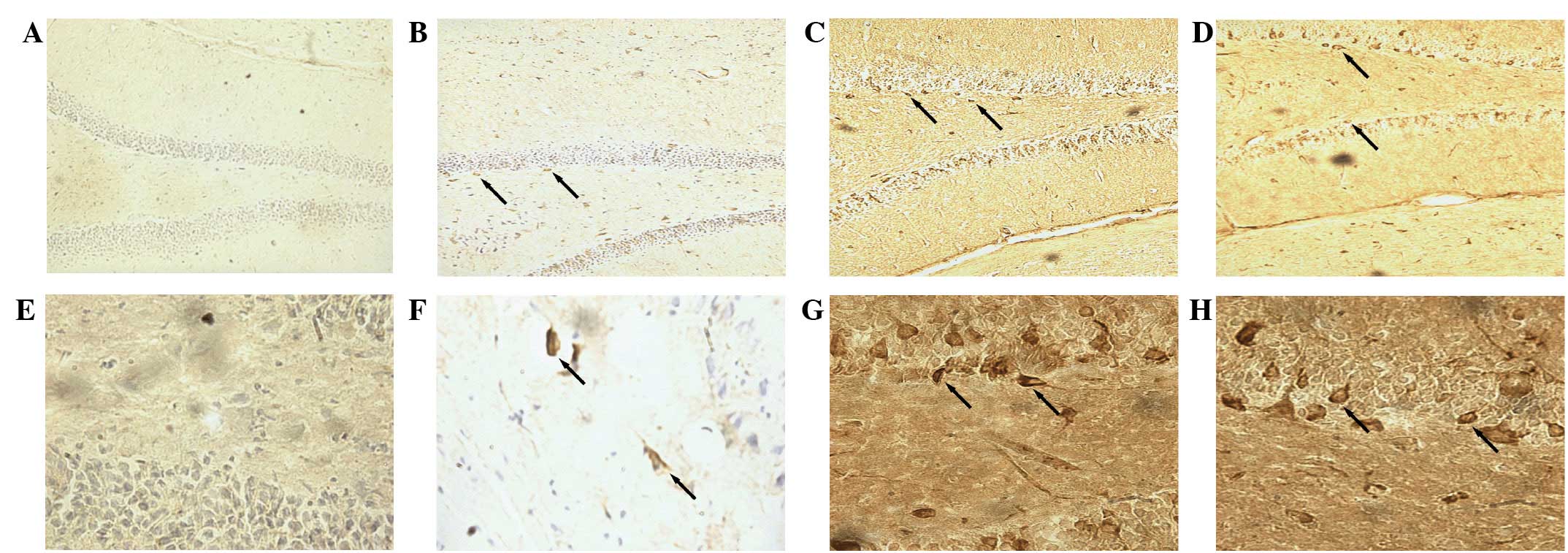
Immunofluorescence staining indicated the presence
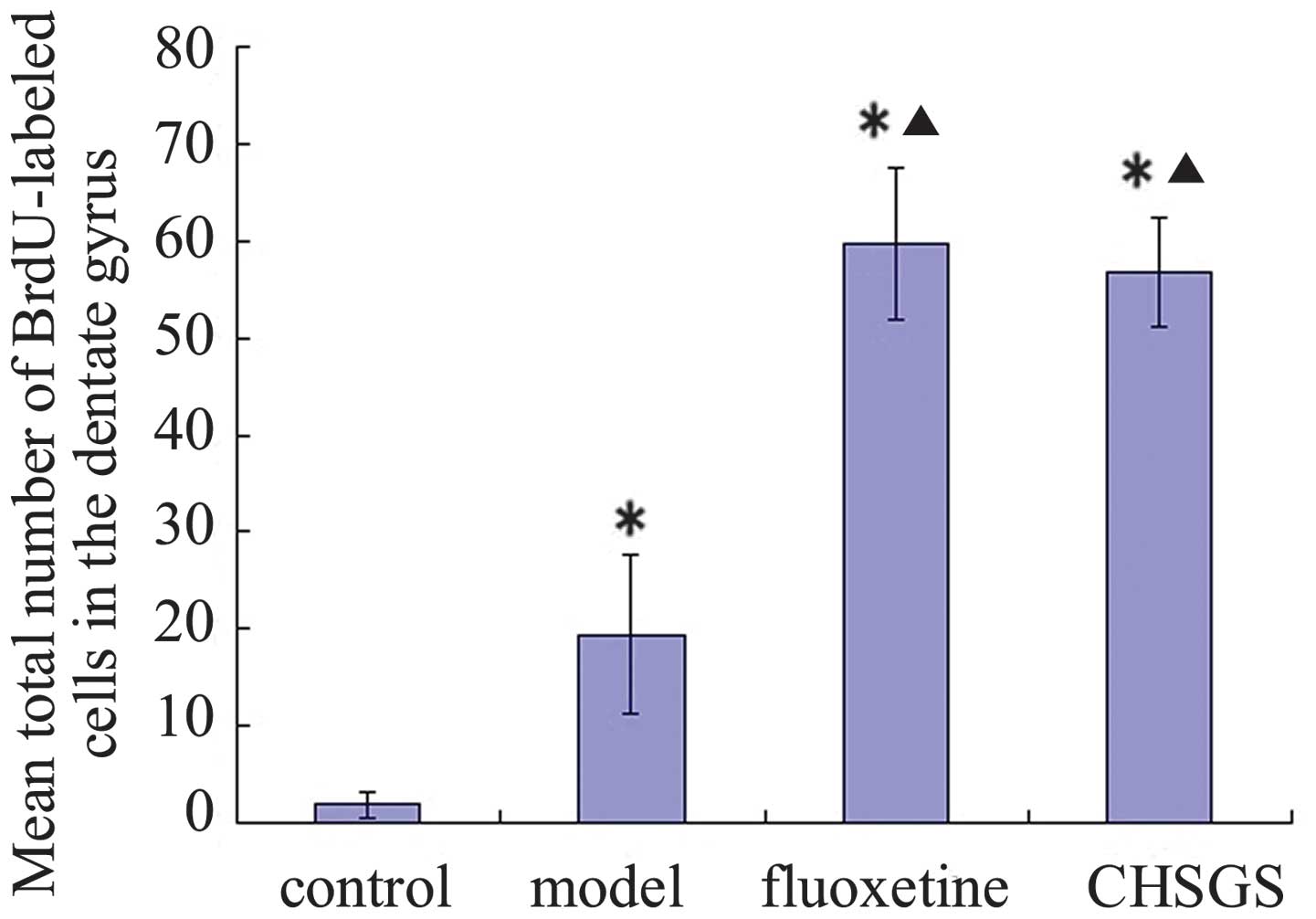
of BrdU-labeled cells in the hippocampal dentate gyrus (Fig. 2). After 28 days of treatment, the
number of BrdU-labeled cells increased significantly in the
hippocampal dentate gyrus of the model, fluoxetine and CHSGS groups
compared with the control group (P<0.05). Furthermore, compared
with the model group, the number of BrdU-labeled cells increased
significantly in the hippocampal dentate gyrus in the fluoxetine
and CHSGS groups (P<0.05). No significant difference was
identified between the fluoxetine and CHSGS groups (P>0.05;
Fig. 3).
CHSGS enhances the mRNA expression of
5-HT1A receptor in the hippocampal dentate gyrus
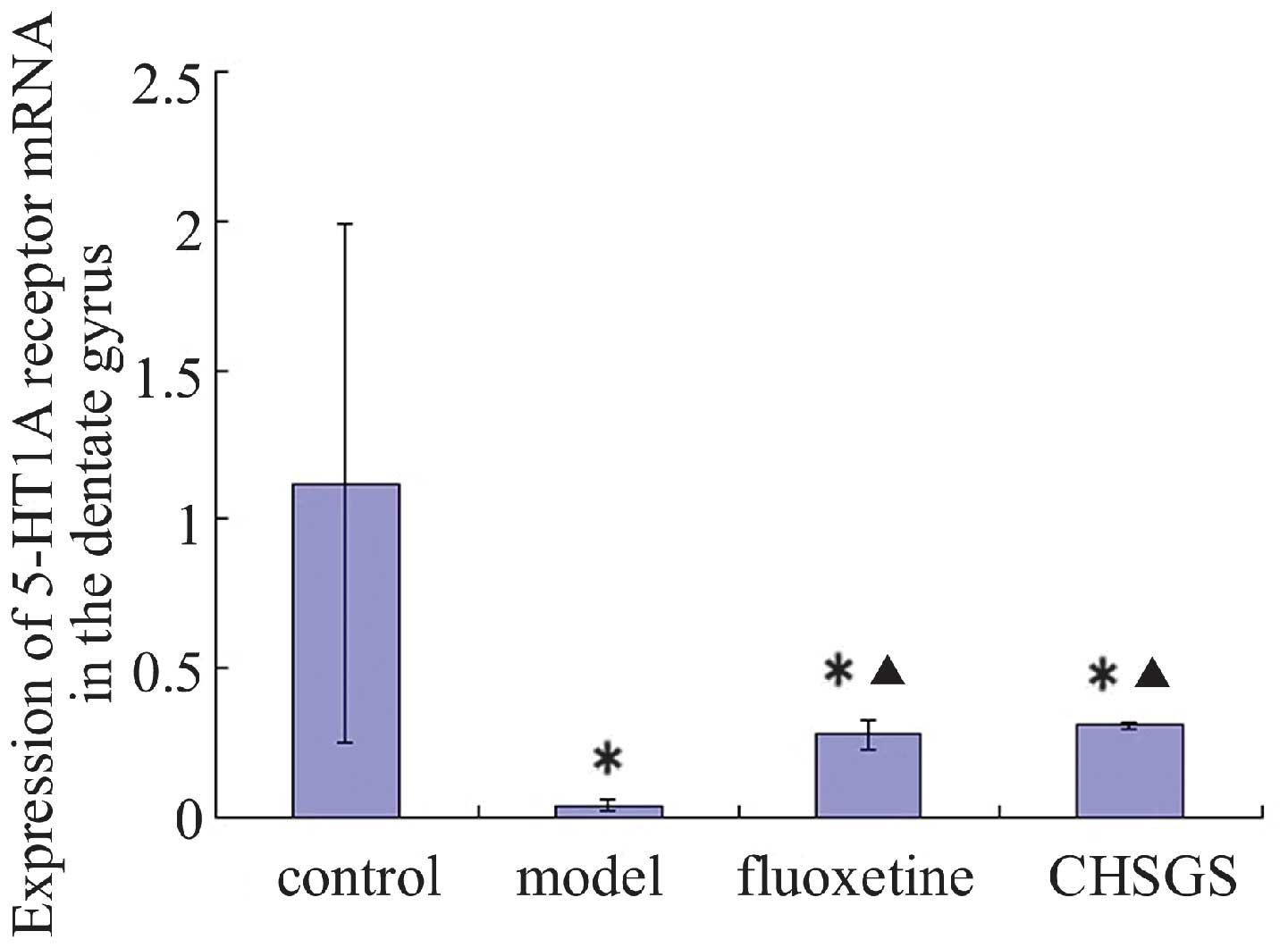
Following 28 days of treatment, the expression
levels of 5-HT1A receptor mRNA decreased in the model, fluoxetine
and CHSGS groups compared with the control group (P<0.05).
Compared with the model group, the expression levels of 5-HT1A
receptor mRNA were enhanced in the fluoxetine and CHSGS groups
(P<0.05). However, no difference in 5-HT1A expression levels was
observed between the fluoxetine and CHSGS groups (P>0.05;
Figs. 4 and 5).
Discussion
The reported prevalence of epilepsy in China ranges
between 3.6 and 7.0% (14). The
number of patients with active epilepsy was >9 million in 2010,
and increases by ~400,000 each year. Recurrent epileptic attacks
and long-term treatment can seriously reduce the life quality of
the patients, and affect mentality to a certain extent. Evident
psychological and behavioral disorders have been identified in
large numbers of patients with epilepsy (15). The prevalence of depression in
patients with epilepsy is reportedly 23.1%, which is higher
compared with that in the general population. In addition, the
lifetime prevalence of depression in patients with epilepsy is
high, approaching 13% (16). Thus,
the co-occurrence of epilepsy and depression is a common
phenomenon.
In our previous study, epileptic rats with
depression were used in a lithium pilocarpine-induced epileptic
model (4). Thus, a combined model of
epilepsy with depression was established. However, the model
exhibited a number of shortcomings, such as the low success rate of
model establishment of ~25%, high experimental expense and waste of
resources. In the present study, the establishment of the combined
model of epilepsy with depression has been improved compared with
the previous model. The revised model includes a combination of the
traditional lithium pilocarpine-induced epilepsy model and the
CMS-induced depression model. The success rate of model
establishment reached ~80% in the present study.
In our previous study, the 5-HT1A receptor agonist
8-OH-DPAT was used to improve depressive symptoms in epileptic rats
with depression. However, the clinical application of 8-OH-DPAT is
currently limited, which may be due to a number of factors.
Firstly, although 5-HT1A receptor agonists, such as tandospirone,
have good antidepressant effects (17), they are frequently used as
antidepressant synergists (18).
Secondly, the treatment compliance for traditional and newer
antidepressant drugs has been adversely affected by their defects,
including side effects and addictive properties. Certain
antidepressant agents in clinical use, such as tricyclic
antidepressants, bupropion and fluoxetine (19,20),
have been reported to cause seizures in certain patients.
Therefore, the selection of antidepressants for epileptic patients
with depression is controversial.
According to Traditional Chinese Medicine (TCM)
theory, epilepsy with depression belongs to the ‘depressed
syndrome’ category of diseases (21)
and the pathogenesis has a marked association with emotion. The
liver fails to maintain the normal flow of ‘Qi’, which affects
‘pneuma’ transportation to the five ‘zang’ organs and the six ‘fu’
organs, resulting in corresponding emotional symptoms and
potentially depression. The primary treatment method for depression
according to the TCM tradition is the herbal preparation Shu Gan,
which is hypothesized to conduct ‘Qi’ and release ‘liver
depression’. According to TCM theory, the movement of ‘Qi’ is able
to ‘free the blood’, and thus alleviate various forms of stagnancy.
Therefore, in the present study a representative decoction of the
‘Shu Gan Li Qi’ method (CHSGS) was selected as a candidate
treatment for epileptic rats with depression.
It has been reported that CHSGS reduces the
expression of microRNA-125a and microRNA-182, which are associated
with the onset of depression (22)
in the hippocampus. Furthermore, CHSGS increases brain-derived
neurotrophic factor and tropomyosin receptor kinase B levels in the
amygdaloid nucleus, hippocampus and frontal lobe (23) and upregulates the expression of
ERK1/2 mRNA in the hippocampus (24), thus improving the symptoms of
depression in rat depression models. CHSGS appears to enhance the
expression levels of B-cell lymphoma 2 (Bcl-2) and decrease those
of Bcl-2-like protein 4 in the hippocampus and frontal cortex, thus
inhibiting neuronal apoptosis in epileptic rats (25). In addition, CHSGS has been shown to
decrease the expression of multidrug resistance protein
P-glycoprotein and electroencephalograph epileptiform discharges in
the hippocampus and temporal lobe cortex (26), which are associated with
antiepileptic changes. In the clinical treatment of patients with
post-stroke depression, the curative effect of CHSGS was found to
be better compared with fluoxetine at relieving the symptoms of
depression and stabilizing patient emotional state (27). CHSGS combined with the conventional
antidepressant venlafaxine has been shown to significantly improve
neurological functions and alleviate depressive symptoms in stroke
patients (28). In addition, Wang
et al (29) conducted a
meta-analysis which confirmed the efficacy and safety of CHSGS for
the treatment of depression. In the present study, CHSGS was
administered to epileptic rats with depression. No statistically
significant difference in depressive behavior was observed between
the CHSGS and fluoxetine groups. By contrast, compared with the
model group, the behavioral data in CHSGS group exhibited
statistically significant differences. These results suggest that
CHSGS improves the symptoms of depression in epileptic rats with
depression.
However, the underlying mechanism by which CHSGS
ameliorates the depressive symptoms in epileptic rats with
depression requires clarification. Recent studies and our previous
study have indicated that the 5-HT1A receptor is associated with
the pathogenesis of epilepsy with depression (4,30). The
5-HT1A receptor has become a key drug target for the treatment of
epilepsy with depression (31).
Therefore, it is possible that CHSGS improves depressive symptoms
in rats by modifying the expression of the 5-HT1A receptor. In the
present study, compared with the control group, the expression of
5-HT1A receptor mRNA was decreased in the model, fluoxetine and
CHSGS groups. The expression of 5-HT1A receptor mRNA was enhanced
in the fluoxetine and CHSGS groups compared with the model group.
However, no significant difference in 5-HT1A receptor mRNA
expression was identified between the fluoxetine and CHSGS groups.
These results indicate that the expression of 5-HT1A receptor mRNA
is reduced in epileptic rats with depression. Fluoxetine and CHSGS
are able to increase the expression of 5-HT1A receptor mRNA;
however, there is no discernible difference in their therapeutic
efficacy.
The mechanism underlying the antiepileptic and
antidepressive effect of increased 5-HT1A receptor expression
remains unclear. Our previous study indicated that the 5-HT1A
receptor is associated with neural plasticity and is crucially
involved in the pathogenesis of epilepsy with depression (4). Therefore, we hypothesize that the
5-HT1A receptor exerts its antiepileptic and antidepressive effect
by promoting neural plasticity. The adult hippocampus has the
capability to generate new neurons throughout life, and this
property contributes to functional plasticity under physiological
and pathological conditions (32).
Further research has shown that neural stem cells are present in
the subgranular zone of the dentate gyrus throughout the lifetime
of an individual (33). These cells
proliferate and differentiate into neurons and glial cells, and
subsequently migrate into the granule cell layer and finally into
the molecular layer (33). Various
antidepressants, including tricyclic antidepressants and selective
serotonin reuptake inhibitors, are able to increase neuronal
proliferation in the hippocampal dentate gyrus in adult rodents
(34). A previous study has
indicated that the activation of the 5-HT1A receptor is a critical
step in the activation of seizure-induced cellular proliferation
and survival in the dentate gyrus (35). This effect may attenuate depressive
symptoms in epilepsy by enhancing neuronal plasticity.
In the present study, no statistically significant
difference was detected between the CHSGS and fluoxetine groups
with regard to the number of BrdU-labeled cells, indicating that
the two agents have similar effects on cell proliferation. Cell
proliferation in the CHSGS group was significantly greater than
that in the model group, and the expression of 5-HT1A receptor mRNA
was increased accordingly. Thus, the results of the present study
indicate that CHSGS regulates neurogenesis in epileptic rats with
depression by adjusting the expression levels of the 5-HT1A
receptor. However, the exact mechanism by which CHSGS exerts its
anti-epileptic and anti-depressive effect against epilepsy with
depression remains unclear and requires further study.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (No. 81302899 and 81373551),
Key Science and Research Program of Hunan Department of Science and
Technology (No. 2012TF-1005), Foundation of Shanxi Province for
Returnees (No. 2008-52) and the Project of Hunan Provincial
Education Department (No. 15C1051).
Glossary
Abbreviations
Abbreviations:
|
CHSGS
|
Chaihu-Shugan-San
|
|
CMS
|
chronic mild stress
|
|
OFT
|
open-field test
|
|
5-HT1A
|
5-hydroxytryptamine 1A
|
|
8-OH-DPAT
|
8-hydroxy-dipropylaminotetralin
|
|
SD
|
Sprague-Dawley
|
References
|
1
|
Peng WF and Wang X: Comorbidity of
epilepsy and depression: From clinical to basic research. Shi Jie
Lin Chuang Yao Wu. 33:13–17. 2012.(In Chinese).
|
|
2
|
Pineda EA, Hensler JG, Sankar R, Shin D,
Burke TF and Mazarati AM: Plasticity of presynaptic and
postsynaptic serotonin 1A receptors in an animal model of
epilepsy-associated depression. Neuropsychopharmacology.
36:1305–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Theodore WH, Wiggs EA, Martinez AR, Dustin
IH, Khan OI, Appel S, Reeves-Tyer P and Sato S: Serotonin 1A
receptors, depression, and memory in temporal lobe epilepsy.
Epilepsia. 53:129–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang P, Sun MZ, Li L and Shen YH:
8-hydroxy-dipropylaminotetralin promotes neural plasticity in
epileptic rats with depression. Neural Regen Res. 7:565–571.
2012.PubMed/NCBI
|
|
5
|
Al-Abri SA, Orengo JP, Hayashi S, Thoren
KL, Benowitz NL and Olson KR: Delayed bupropion cardiotoxicity
associated with elevated serum concentrations of bupropion but not
hydroxybupropion. Clin Toxicol (Phila). 51:1230–1234. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang S, Hu SY, Zhang CH, Qiu J and Li YH:
Antidepressant-like activity of Chaihu-Shugan-San aqueous extract
in rats and its possible mechanism. Pharmacogn Mag. 10(Suppl 1):
S50–S56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
The Ministry of Science and Technology of
the People's Republic of China: Guidance Suggestions for the Care
and Use of Laboratory Animals. Beijing: 2006.
|
|
8
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China. 1:(10th).
(Beijing). Chinese Medical Science and Technology Press. 38–263.
2010.
|
|
9
|
Tan YZ: Pharmacology Experiment. Beijing:
People's Medical Publishing House. 141–142. 2008.
|
|
10
|
Wang SH, Zhang ZJ, Guo YJ, Teng AJ and
Chen BA: Expression of 5-hydroxytryptamine 1A receptor protein and
message RNA in the dentate gyrus in post-stroke depression rats.
Zhong Hua Jing Shen Ke Za Zhi. 41:107–110. 2008.(In Chinese).
|
|
11
|
Meng H, Wang Y, Huang M, Lin W, Wang S and
Zhang B: Chronic deep brain stimulation of the lateral habenula
nucleus in a rat model of depression. Brain Res. 1422:32–38. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Racine RJ: Modification of seizure
activity by electrical stimulation. II. Motor seizure.
Electroencephalogr Clin Neurophysiol. 32:281–294. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang SH, Zhang ZJ, Guo YJ, Sui YX and Sun
Y: Notch1 signaling related hippocampal neurogenesis in adult
poststroke depression rats: A valid index for an efficient combined
citalopram and WAY100635 pharmacotherapy. Behav Pharmacol.
21:47–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Liu M and Liang WN: Progress on
the epidemiological study of epilepsy. Zhonghua Liu Xing Bing Xue
Za Zhi. 28:92–94. 2007.(In Chinese). PubMed/NCBI
|
|
15
|
Lacey CJ, Salzberg MR and D'Souza WJ: Risk
factors for depression in community-treated epilepsy: Systematic
review. Epilepsy Behav. 43:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fiest KM, Dykeman J, Patten SB, Wiebe S,
Kaplan GG, Maxwell CJ, Bulloch AG and Jette N: Depression in
epilepsy: A systematic review and meta-analysis. Neurology.
80:590–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao YL and Yang X: Comparative
observation on synergistic effect of tandospirone antidepressant.
Lin Chuang Jing Shen Yi Xue Za Zhi. 18:402008.(In Chinese).
|
|
18
|
Mago R, Mahajan R and Thase ME: Medically
serious adverse effects of newer antidepressants. Curr Psychiatry
Rep. 10:249–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma L and Wu HF: A case of epileptic state
caused by fluoxetine. Lin Chuang Wu Zhen Wu Zhi. 21:802008.(In
Chinese).
|
|
20
|
Pineda EA, Hensler JG, Sankar R, Shin D,
Burke TF and Mazarati AM: Interleukin-1β causes fluoxetine
resistance in an animal model of epilepsy-associated depression.
Neurotherapeutics. 9:477–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang GQ, Wang JM, Fan MY, Lv JJ and Zhang
HK: Progress on pathogenesis and treatment of epilepsy with
depression in integrative medicine. Zhong Xi Yi Jie He Xin Nao Xue
Guan Bing Za Zhi. 9:1247–1249. 2011.(In Chinese).
|
|
22
|
Cao MQ, Chen DH, Zhang CH and Wu ZZ:
Screening of specific microRNA in hippocampus of depression model
rats and intervention effect of Chaihu Shugan San. Zhongguo Zhong
Yao Za Zhi. 38:1585–1589. 2013.(In Chinese). PubMed/NCBI
|
|
23
|
Deng Y, Zhang CH and Zhang HN: Effects of
chaihu shugan powder on the behavior and expressions of BDNF and
TrkB in the hippocampus, amygdala and the frontal lobe in rat model
of depression. Zhongguo Zhong Xi Yi Jie He Za Zhi. 31:1373–1378.
2011.(In Chinese). PubMed/NCBI
|
|
24
|
Wang S, Hu S, Zhang C, Qiu J and Li Y:
Effect of Chaihu Shugan San and its components on expression of
ERK1/2 mRNA in the hippocampus of rats with chronic mild
unpredicted stress depression. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
36:93–100. 2011.(In Chinese). PubMed/NCBI
|
|
25
|
Huang YS, Zhuo Y and Liu ZL: Effect of
Chaihu-Shugan-San on expression of bc1–2 and bax in
cardiazole-induced epileptic rats' hippocampus and frontal cortex.
Zhonghua Lin Chuang Yi Shi Za Zhi (Dian Zi Ban). 6:1574–1575.
2012.(In Chinese).
|
|
26
|
Xie W, Shi GJ, Li CZ, Bao Y, Yu L, Yu YH
and Du NN: Effect of Chaihu Shugan Tang on electroencephalogram and
expression of multidrug resistance protein p-glycoprotein of
refractory epilepsy. Zhong Guo Shi Yan Fang Ji Xue Za Zhi.
17:128–131. 2011.(In Chinese).
|
|
27
|
Liu J and Zhong C: 40 cases of post-stroke
depression treated by Chaihu-Shugan-San. Zhong Guo Zhong Yi Ji
Zheng. 21:7882012.(In Chinese).
|
|
28
|
Zou LH, Li H, Zeng ZQ, Chen XD and Deng
ZH: Chaihu Shugan powder combined with venlafaxine for treatment of
post-stroke depression. Zhong Xi Yi Jie He Xin Nao Xue Guan Bing Za
Zhi. 9:1330–1332. 2011.(In Chinese).
|
|
29
|
Wang Y, Fan R and Huang X: Meta-analysis
of the clinical effectiveness of traditional Chinese medicine
formula Chaihu-Shugan-San in depression. J Ethnopharmacol.
141:571–577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanner AM: Depression and epilepsy: A
bidirectional relation? Epilepsia. 52(Suppl 1): S21–S27. 2011.
View Article : Google Scholar
|
|
31
|
Hasler G, Bonwetsch R, Giovacchini G,
Toczek MT, Bagic A, Luckenbaugh DA, Drevets WC and Theodore WH:
5-HT1A receptor binding in temporal lobe epilepsy patients with and
without major depression. Biol Psychiatry. 62:1258–1264. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Covic M, Karaca E and Lie DC: Epigenetic
regulation of neurogenesis in the adult hippocampus. Heredity
(Edinb). 105:122–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ming GL and Song H: Adult neurogenesis in
the mammalian brain: Significant answers and significant questions.
Neuron. 70:687–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taciany Bonassoli V, Micheli Chassot J,
Longhini R, Milani H, Mello JC and de Oliveira RM: Subchronic
administration of Trichilia catigua ethyl-acetate fraction
promotes antidepressant-like effects and increases hippocampal cell
proliferation in mice. J Ethnopharmacol. 143:179–184. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Radley JJ and Jacobs BL:
Pilocarpine-induced status epilepticus increases cell proliferation
in the dentate gyrus of adult rats via a 5-HT1A receptor-dependent
mechanism. Brain Res. 966:1–12. 2003. View Article : Google Scholar : PubMed/NCBI
|