Introduction
In recent years, trauma and chronic wounds have been
caused by a greater number of reasons, with a 4% increase in the
number of patients suffering from soft tissue defects in
combination with bone or tendon exposure reported every year since
2000 (1–4). Reconstructing the defects is essential
but challenging, particularly for patients with a weak blood
supply. The most common therapeutic approach in the treatment of
these defects is a flap graft, while the conventional treatment of
vessels is end-to-end anastomoses of the grafting flap and
recipient area for the main supplying artery, with one or two
grafting veins also being anastomosed. However, conventional
surgery sacrifices the major vessel of the grafting flap; immediate
postoperative monitoring of circulation to the graft is also
required, demanding a surgical team skilled in this microvascular
technique, and involving a long operative time. The flow-through
technology was first introduced clinically by Soutar et al
(5) in 1983, and the procedure
involved using a radial arterial flap in the reconstruction of the
head and neck. The use of a flow-through compound radial artery
forearm flap in reconstruction of the extremities was reported in
1984 (6). This operative technology
was adopted to recover the integrity of the grafting trunk vessel
by anastomosing its two extremities; the surgery not only
reconstructs the defects, but also increases the blood supply to
the distal limbs, especially for patients with only one remaining
trunk vessel (7–9). Patients suffering soft tissue defects
combined with tendon or bone exposure are required to undergo
several surgical procedures, causing severe damage to the majority
of the trunk veins. Reconstructing such kinds of defects is
challenging, and the optimum treatment of the grafting veins
remains controversial. The present study was conducted with the aim
of exploring the effects of the preservation of different grafting
veins on flow-through flap survival.
Materials and methods
Experimental animal model and skin
flap measurement
All experiments were approved by the Institutional
Animal Care and Use Committee of Wuhan University (Wuhan, China),
and the animal procedures were performed in strict accordance with
institutional and national guidelines (10). All efforts were made to minimize
suffering.
A total of 50 Japanese white ear rabbits weighing
2.5–3.5 kg were purchased from and housed in the approved animal
care facility at the Center for Animal Experiments of Wuhan
University (Wuhan, China). Rabbits were randomly divided into five
groups (each n=10) and were anesthetized via intramuscular
injection of 1% pentobarbital sodium (30 mg/kg). Subsequently, the
inner thighs of the rabbits were shaved, treated with betadine
solution and sterilized with iodine followed by 75% ethanol. A
5×2-cm skin flap was then created on the left inner thigh of each
rabbit.
In each rabbit, a superficial femoral artery
perforator running through the flap was preserved. Skin flaps in
which all trunk veins were retained were used in the control group,
and various numbers/types of veins were retained in the four
experimental groups: No veins, one superficial vein (SV), one
accompanying vein (AV), one SV plus one AV. Each rabbit received
antibiotic therapy (8×105 IU) by intramuscular injection
following the surgery in order to prevent infection of the skin
flaps.
Survival was determined on day 10. Tissues were
collected from the central portion of the skin flaps on day 3, 5, 7
and 9 for immunohistochemical analysis to count the number of
microvessels. Western blotting was also carried out to evaluate the
expression levels of vascular endothelial growth factor (VEGF),
which serves as an indicator of the growth of new blood
microvessels. Rabbits were anesthetized via intramuscular injection
of 15% pentobarbital sodium (30 mg/kg) on day 10. All rabbits were
sacrificed via decapitation following the experiment.
Percentage survival of the flap
areas
The condition of each skin flap was checked every
day after the surgery. The percentage of the skin flap area that
survived (survival rate) on day 10 was determined with the use of
Image Pro-Plus software (version 7; Media Cybernetics, Inc.,
Rockville, MD, USA) due to retraction of the skin flaps. The
survival rates of the flap areas were analyzed and compared between
different groups with the Image Pro-Plus software.
Immunohistochemical analysis of
microvessels
Counting of the microvessels in the skin flaps was
conducted with staining of Factor VIII. Tissues collected from the
skin flaps were cut to 5-µm thickness and embedded in paraffin. For
analysis, the tissue sections were deparaffinized and then treated
with 20 mg/ml proteinase K (Roche Diagnostics, Basel, Switzerland)
for 15 min (3×5 min). Endogenous peroxidase was blocked by
incubating the sections with 3% hydrogen peroxide for ≥10 min. The
sections were then washed with phosphate-buffered saline (PBS) and
treated with blocking reagent (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Anti-rabbit anti-Factor VIII polyclonal antibody
(1:1,000; bs-0434R; Beijing Biosynthesis Biotechnology Co., Ltd.,
Beijing, China) was applied to the sections, which were then
incubated at room temperature for 1 h. The sections were rinsed
with PBS repeatedly and then incubated with fluorescein
isothiocyanate (FITC)-conjugated secondary antibodies (1:500;
HP1201; Amyjet Scientific, Inc., Wuhan, China) for 30 min at room
temperature. After treating the antibodies with avidin-biotinylated
enzyme complex in combination with peroxidase substrate solution
(both Roche Diagnostics) for 2 min, they were then visualized.
Mayer's hematoxylin was also used to counter-stain the sections for
5 min. The stained blood microvessels on each slide in the most
vascularized area of the section were counted. Tissue sections were
scanned under high magnification to identify the areas with the
greatest amount of vascularization; the specific number of
microvessels was counted in a high-power field of ×400 for five
non-overlapping areas.
Western blot analysis for VEGF
Skin flap tissues were homogenized in buffer with an
added protease inhibitor cocktail (Roche Diagnostics), which
consisted of 10 mM NaCl, 50 mM Tris, 1% NP-40 and 0.02% sodium
azide. Subsequently, homogenates were put into filtered centrifuge
tubes and centrifuged at 10,000 × g for 15 min at room temperature.
Following dissolution in Laemmli buffer (Santa Cruz Biotechnology,
Inc.), the proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride membranes. The membranes were blocked
with 5% milk for 5–10 min and incubated with primary antibody at
4°C overnight. The polyclonal primary antibodies targeted VEGF
(1:200; sc-507) or β-actin (1:10,000; sc-1616; both Santa Cruz
Biotechnology, Inc.) as a loading control. Blots were rinsed
several times with Tris-buffered saline containing 0.05% Tween-20
(TBST) and then incubated with horseradish peroxidase (HRP)-labeled
secondary antibodies (1:200; HP1201; Amyjet Scientific, Inc.).
Finally, the blots were washed again with TBST. Positive signals
were generated after the blots were incubated with a luminescent
HRP substrate (Roche Diagnostics) for 1–2 min. Using a
high-resolution flatbed scanner and ImageJ densitometry software
(version 1.45; National Institutes of Health, Bethesda, MD, USA),
the optical densities of at least three replicates of each group
were quantified and the densities were expressed for each group on
the basis of their intensities relative to β-actin.
Statistical analysis
Unpaired Student's t-tests were used to perform
between-group comparisonss using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
significant difference.
Results
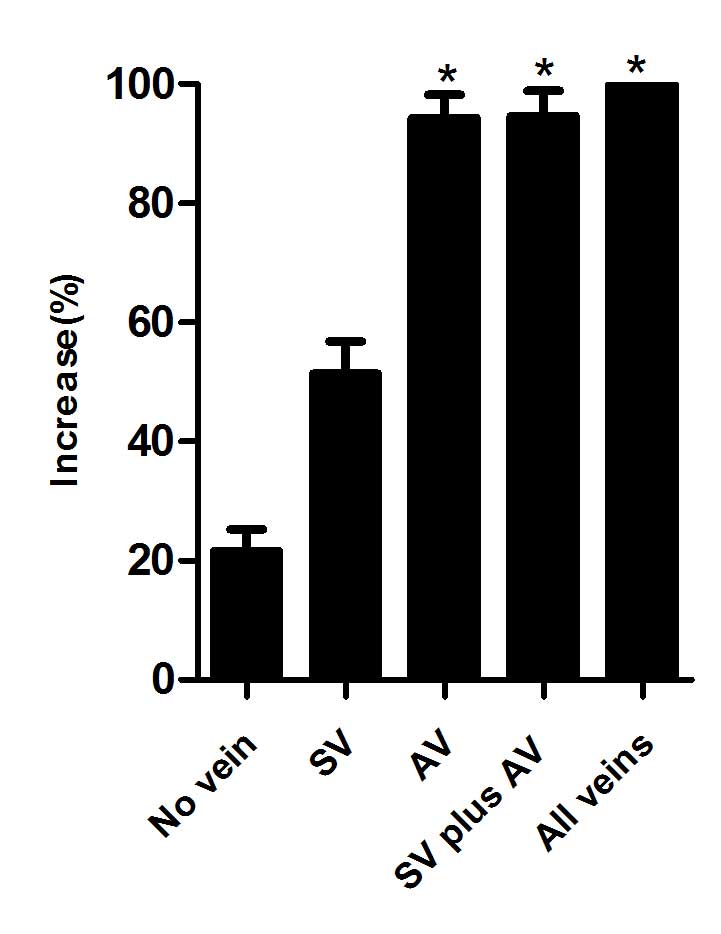
Survival rates of the skin flap
areas
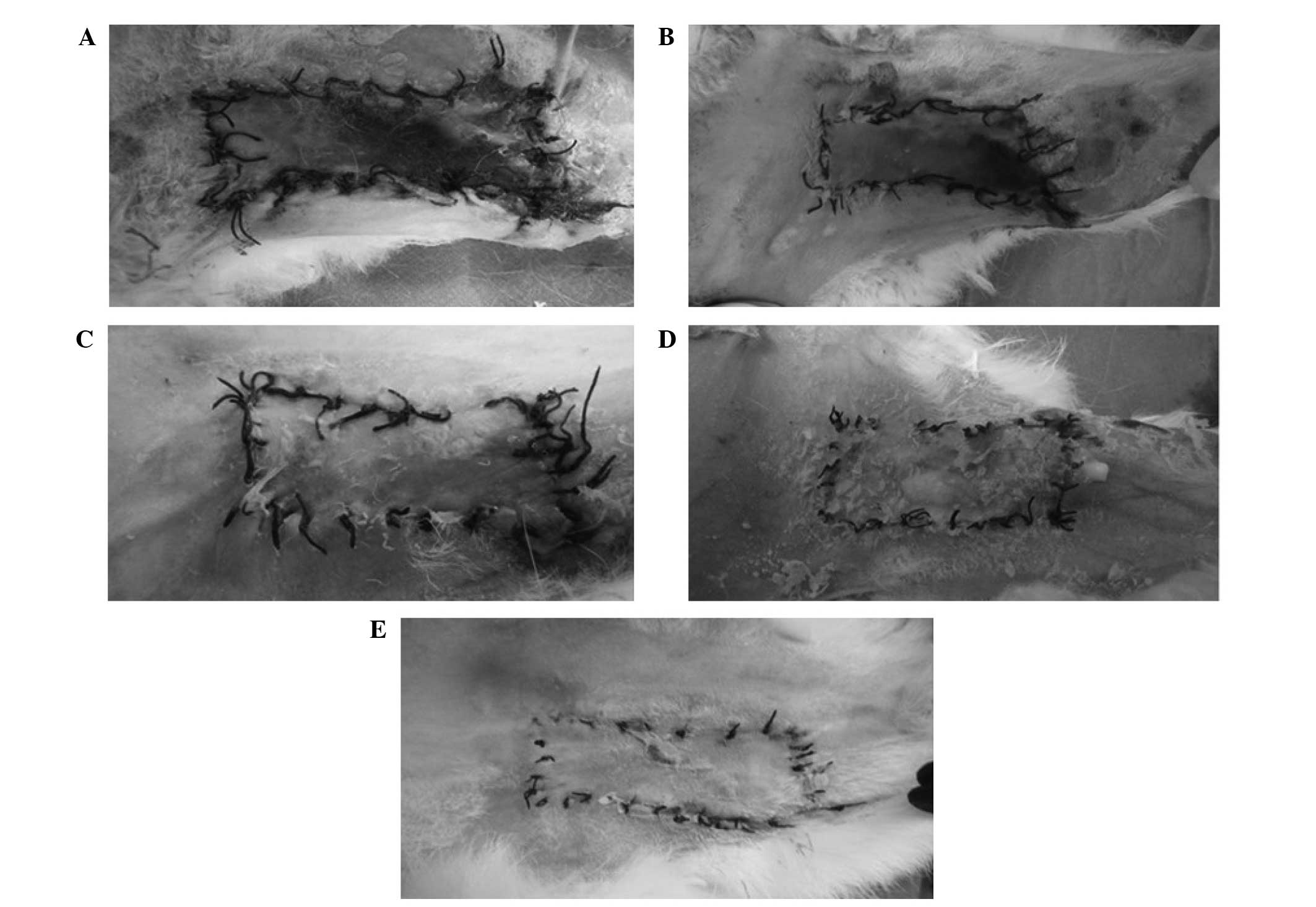
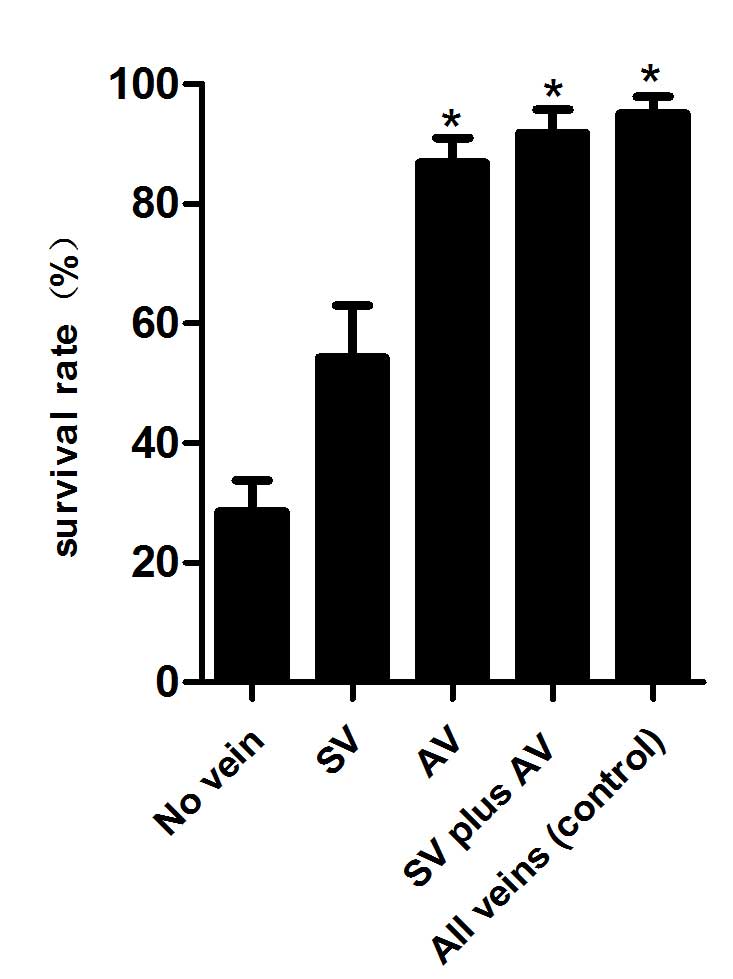
The survival status of each flap was assessed daily,
representative images were captured on day 10 (Fig. 1) and the survival rates were analyzed
(Fig. 2). The results of the present
study demonstrated that skin flaps with no veins faced more
difficult survival conditions and had a lower survival rate, as
compared with the other groups (P<0.01). Improved survival
states and rates were demonstrated in the AV, SV plus AV and all
veins (control) groups, as compared with the SV and no vein groups
(P<0.01); however, no significant differences were detected
between the former three groups (P>0.05).
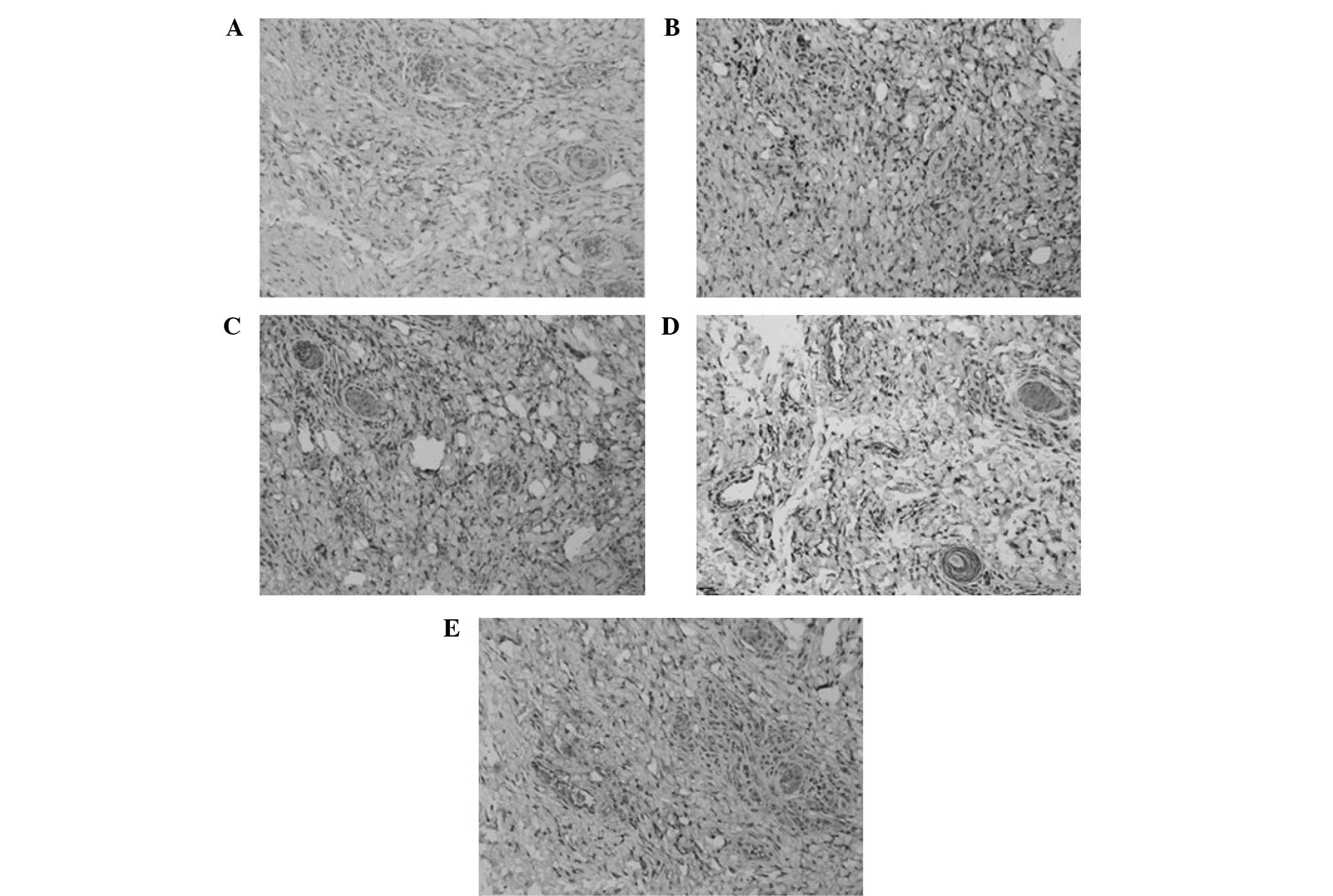
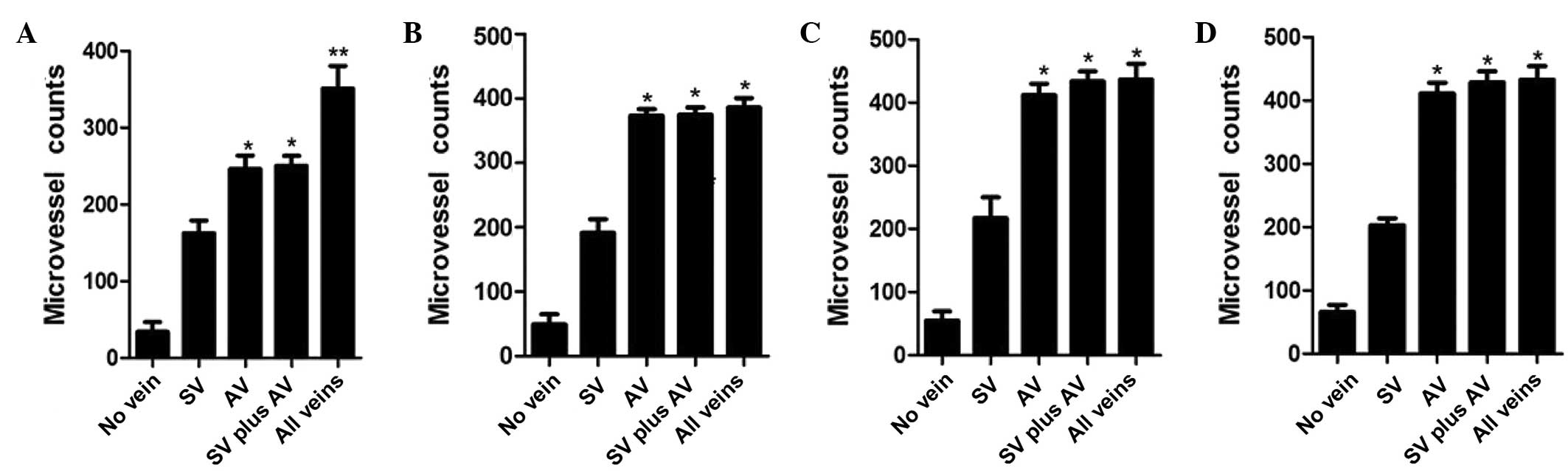
Density of microvessels
Microvessels in the skin flaps were counted by
staining for Factor VIII on days 3, 5, 7 and 9 (Figs. 3 and 4). On day 3, the flaps of the all veins
(control) group demonstrated the greatest microvessel count; the
microvessel counts of the AV and SV plus AV groups were greater
than those of the SV and no vein groups; and the flaps with a SV
had greater numbers of microvessels than those with no veins.
However, no significant differences were identified on days 5, 7
and 9 between the AV, SV plus AV and all veins (control) groups,
and the results for the remaining two groups were as described on
day 3.
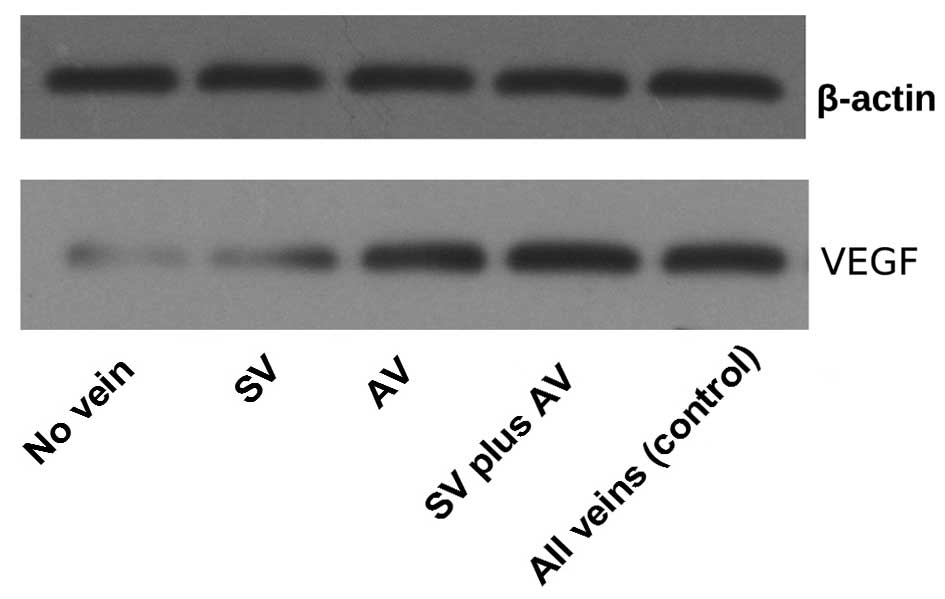
Expression levels of VEGF
VEGF expression is considered to be an important
indicator of the development of new microvessels which can also
promote angiogenesis in wound healing (11–13). In
order to test the effects of the various vein treatments on the
flow-through skin flap grafts, western blot analysis was performed
to determine the expression levels of VEGF on day 5 (Figs. 5 and 6). The expression levels of VEGF were
increased in the flaps of the AV, SV plus AV and all veins groups,
as compared with those in the SV and no vein groups; however no
significant differences were identified between the former three
groups. The flaps with the retention of a SV exhibited a higher
VEGF expression levels, as compared with that of the flaps in which
no veins were retained.
Discussion
Soft tissue defects in combination with bone or
tendon exposure are refractory, and patients suffering from them
are faced with a long period of hospitalization, high expense
during the hospital stay, and poor prognosis (14,15).
Reconstruction of the defect is rather difficult, and the most
common therapy is a flap graft, which in addition to reconstructing
the defect also provides the defect with a blood supply. The
conventional treatment of the blood vessels is end-to end
anastomoses of the donor and recipient area for the artery, which
help to provide the defects with a blood supply. Typically, 1 or 2
veins of the skin flaps are also anastomosed with veins near the
defect area, which facilitates the return of venous blood from the
grafting flaps, and improves the survival rate. However, the common
operative technology destroys the integrity and structure of the
grafting vessels; the blood supply is also weakened because of
ligation of the distal flap artery. The prognosis for patients with
a poor blood supply to the recipient area is poor if a conventional
flap grafting procedure is adopted to reconstruct the defects
(16,17).
The clinical use of flow-through flap grafting to
reconstruct a soft tissue defect in combination with bone or tendon
exposure has been widely accepted and good results are achieved,
particularly for patients with only one remaining trunk artery. The
surgical technology has been applied to various flaps with axial
vascular patterns and appropriate distal vessel sizes, including
free forearm flaps, free fibular flaps and anterolateral thigh
flaps (18–20). The application of this surgical
technology not only reconstructs the defects, but also recovers
continuity of the trunk vessels, and thereby stabilizes the blood
circulation in the flap. It has been demonstrated that flow-through
arterial anastomosis has a higher patency rate than end-to-end and
end-to-side anastomoses, and increases the flow rate through the
anastomotic sites (21). Miyamoto
et al (22) considered that
flow-through arterial anastomosis helped to maintain a high rate of
blood flow through the anastomotic site and facilitated early
mobilization for patients undergoing skin flap grafting.
Application of venous flow-through flaps in the
reconstruction of soft tissue defects has been widely used, which
can involve flow-through flaps as a vessel carrier or arterialized
venous flaps (23,24). The difference between venous
flow-through flaps and conventional flaps is that arterial
inflow-capillary-venous outflow is replaced by venous inflow-venous
outflow (25). There are three main
theories that may explain the possible mechanisms by which the
venous flaps survive. The first is ‘reverse flow’, which means
intermittent flow with perfusion of the blood from the flap vein to
the capillary and then back to the vein (26), while the second theory is ‘A-V
shunting’, which means retrograde flow from the venous system to
the arterial system via paralyzed arterial-venous shunts (27). The third theory is ‘capillary
bypass’, which means that the flow is through the venous system
without entry into the arterial system until neovascularization has
occurred (28).
The present study was conducted in order to explore
the effects of different treatments of trunk veins on grafting flap
survival. The results demonstrated that a flow-through flap without
the retention of any veins retention exhibited the ability to
survive, although the survival state was poorer than that of skin
flaps in which one or two veins were retained. The processes
underlying the survival remain controversial, however there are
several hypotheses. Anastomosing the two extremities of the artery
creates a channel, which helps to facilitate venous drainage from
the grafting flap, relieving much of the hydrostatic pressure of
the area; the perforating veins, which forms in the early stage of
grafting, combine with the capillary network, which forms in the
late stage, to create blood circulation between the donor and
recipient areas, resulting in the drainage of venous blood from the
grafting flap; the bone marrow cavity beneath the graft provided a
venous channel, which helps to drain the majority of the venous
blood from the flap (7,9,29,30).
Thoracic negative pressure induced when a respiratory pump is used,
and the inherent venous pressure of the flap may also be important
forces that drive the return of venous blood (22).
Certain surgeons have hypothesized that performing
two venous anastomoses (AV and SV) is imperative for the survival
of flow-through flap grafts because multiple draining veins result
in improved blood flow through the flap (31,32).
Hanasono et al (33)
considered that the blood velocity of a flap graft with two venous
anastomoses did not increase significantly and resulted in a better
prognosis when compared with one venous anastomosis (AV) in
patients who underwent a flap graft with one or two anastomoses,
due to the hypothesis that thrombosis is associated with a
low-velocity state and performing two anastomoses theoretically
increased the risk of thrombosis. The results of the present study
are consistent with this, with regard to the survival condition on
day 3, the survival rate on day 10, the microvessel counts and VEGF
expression levels. Ross et al (34) found that flaps with two venous
anastomoses did not have a higher survival rate than flaps with one
venous anastomosis (93.6 and 98.6%, respectively).
Aside from the risk of an anastomotic thrombosis,
which may result in failure of the graft, it may be hypothesized
that performing a single venous anastomosis could result in the
loss of the flap graft as a result of insufficient drainage of the
flap, because the anastomosed vein drains only a specific area of
the grafting flap. However, there is little evidence in the
literature to support this hypothesis, and multiple
interconnections between the AV and SVs over the course of the
pedicle and multiple microcirculatory communications create a
channel that can help to transport blood from superficial to deep
portions of the grafting flap (33).
Therefore, we believe that just one deep venous anastomosis near
the defect is adequate for survival of the grafting flap. It has
been observed clinically and experimentally in anatomical studies
that venous insufficiency caused by limited communication between
the superficial and deep vascular networks may affect the survival
of the grafting flap; however, this applies only to patients
undergoing replants or in flaps where trauma to the donor or
recipient vessel cannot be ruled out (35,36). The
results of the present study also indicate that the retention of a
SV is not sufficient for the complete survival of the grafting
flap, because the interconnection between the AV and SV is
unidirectional, which means that blood from the SV can flow to the
accompanying (deep) vein, but the reverse cannot occur.
A limitation of this study is that the animal model
may have some differences from clinical conditions in the aspects
of blood velocity, patency rate of the blood vessels and state.
Increases in the operative time of vascular anastomosis may also
increase the risk of thrombosis; however, this was not examined in
the present experimental study. Furthermore, the present study
indicates that one SV is not sufficient for survival of the
grafting flap. Further study is required to investigate whether the
retention of a greater number of SVs is beneficial to flap
survival.
In conclusion, the results of the present study
demonstrated that a flow-through flap graft can survive with only
one remaining trunk artery, and a greater number of anastomosed
veins does not increase the survival of the flaps.
Acknowledgements
The present study was supported by Hubei Province's
Outstanding Medical Academic Leader Programme.
References
|
1
|
Mao H, Shi Z, Yin W, Dong W and Wapner KL:
Reconstruction of great toe soft-tissue defect with the
retrograde-flow medial pedis island flap. Plast Reconstr Surg.
134:120e–127e. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nikkhah D and Jones M: The bilobed flap as
a lifeboat flap for a forearm soft tissue defect. J Plast Reconstr
Aesthet Surg. 68:1153–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen C, Tang P and Zhang X: Treatment of
soft-tissue loss with nerve defect in the finger using the
boomerang nerve flap. Plast Reconstr Surg. 131:44e–54e. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Melton LJ III: Does high-trauma fracture
increase the risk of subsequent osteoporotic fracture? Nat Clin
Pract Endocrinol Metab. 4:316–317. 2008.PubMed/NCBI
|
|
5
|
Soutar DS, Scheker LR, Tanner NS and
McGregor IA: The radial forearm flap: A versatile method for
intra-oral reconstruction. Br J Plast Surg. 36:1–8. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Foucher G, van Genechten F, Merle N and
Michon J: A compound radial artery forearm flap in hand surgery: An
original modification of the Chinese forearm flap. Br J Plast Surg.
37:139–148. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsiao YC, Yang JY, Chang CJ, Lin CH, Chang
SY and Chuang SS: Flow-through anterolateral thigh flap for
reconstruction in electrical burns of the severely damaged upper
extremity. Burns. 39:515–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyamoto S, Kayano S, Fujiki M, Kamizono
K, Fukunaga Y and Sakuraba M: Flow-through divided latissimus dorsi
musculocutaneous flap for large extremity defects. Ann Plast Surg.
74:199–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parr JM, Adams BM and Wagels M:
Flow-through flap for salvage of fibula osseocutaneous vascular
variations: a surgical approach and proposed modification of its
classification. J Oral Maxillofac Surg. 72:1197–1202. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones-Bolin S: Guidelines for the care and
use of laboratory animals in biomedical research. Curr Protoc
Pharmacol Appendix. 4:4B2012.
|
|
11
|
Pitulescu ME and Adams RH: Regulation of
signaling interactions and receptor endocytosis in growing blood
vessels. Cell Adh Migr. 8:366–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bao P, Kodra A, Tomic-Canic M, Golinko MS,
Ehrlich HP and Brem H: The role of vascular endothelial growth
factor in wound healing. J Surg Res. 153:347–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Keswani SG, Balaji S, Le LD, Leung A,
Parvadia JK, Frischer J, Yamano S, Taichman N and Crombleholme TM:
Role of salivary vascular endothelial growth factor (VEGF) in
palatal mucosal wound healing. Wound Repair Regen. 21:554–562.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang JL, Zhang GL and Zhao LX: Delayed of
reverse sural nerve flap to repair large soft tissue defect on
foot: A case report. Zhongguo Gu Shang. 26:906–907. 2013.(In
Chinese). PubMed/NCBI
|
|
15
|
Lu S and Chai Y: Clinical application of
sural fasciomyocutaneous perforator flap in repair of soft tissue
defect in weight-bearing area of foot. Zhongguo Xiu Fu Chong Jian
Wai Ke Za Zhi. 28:1494–1497. 2014.(In Chinese). PubMed/NCBI
|
|
16
|
Persichetti P, Brunetti B, Aveta A and
Segreto F: Reverse-flow medial sural artery perforator flap:
Pedicle extension for distal lower limb defects. Ann Plast Surg.
70:246–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shahzad MN, Ahmed N and Qureshi KH:
Reverse flow posterior interosseous flap: Experience with 53 flaps
at Nishtar Hospital, Multan. J Pak Med Assoc. 62:950–954.
2012.PubMed/NCBI
|
|
18
|
Koshima I, Kawada S, Etoh H, Kawamura S,
Moriguchi T and Sonoh H: Flow-through anterior thigh flaps for
one-stage reconstruction of soft-tissue defects and
revascularization of ischemic extremities. Plast Reconstr Surg.
95:252–260. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kasten SJ, Chung KC and Tong L:
Simultaneous revascularization and soft tissue coverage in the
traumatized upper extremity with a flow-through radial forearm free
flap. J Trauma. 47:416–419. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nişanci M, Selçuk I and Duman H:
Flow-through use of the osteomusculocutaneous free fibular flap.
Ann Plast Surg. 48:435–438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyamoto S, Okazaki M, Ohura N, Shiraishi
T, Takushima A and Harii K: Comparative study of different
combinations of microvascular anastomoses in a rat model:
End-to-end, end-to-side and flow-through anastomosis. Plast
Reconstr Surg. 122:449–455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyamoto S, Kayano S, Fujiki M, Chuman H,
Kawai A and Sakuraba M: Early mobilization after free-flap transfer
to the lower extremities: Preferential use of flow-through
anastomosis. Plast Reconstr Surg Glob Open. 2:e1272014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karacalar A and Ozcan M: Free arterialized
venous flap for the reconstruction of defects of the hand: New
modifications. J Reconstr Microsurg. 10:243–248. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan H, Fan C, Gao W, Chen Z, Li Z and Chi
Z: Finger pulp reconstruction with free flaps from the upper
extremity. Microsurgery. 32:406–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakayama Y, Soeda S and Kasai Y: Flaps
nourished by arterial inflow through the venous system: An
experimental investigation. Plast Reconstr Surg. 67:328–334. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baek SM, Weinberg H, Song Y, Park CG and
Biller HF: Experimental studies in the survival of venous island
flaps without arterial inflow. Plast Reconstr Surg. 75:88–95. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chavoin JP, Rouge D, Vachaud M, Boccalon H
and Costagliola M: Island flaps with an exclusively venous pedicle.
A report of eleven cases and a preliminary haemodynamic study. Br J
Plast Surg. 40:149–154. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsushita K, Firrell JC, Ogden L and Tsai
TM: Blood flow and tissue survival in the rabbit venous flap. Plast
Reconstr Surg. 91:127–137. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kells AF, Broyles JM, Simoa AF, Lewis VO
and Sacks JM: Anterolateral thigh flow-through flap in hand
salvage. Eplasty. 13:e192013.PubMed/NCBI
|
|
30
|
Yokota K, Sunagawa T, Suzuki O, Nakanishi
M and Ochi M: Short interposed pedicle of flow-through
anterolateral thigh flap for reliable reconstruction of damaged
upper extremity. J Reconstr Microsurg. 27:109–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kroll SS, Schusterman MA, Reece GP, Miller
MJ, Evans GR, Robb GL and Baldwin BJ: Timing of pedicle thrombosis
and flap loss after free-tissue transfer. Plast Reconstr Surg.
98:1230–1233. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen KT, Mardini S, Chuang DC, Lin CH,
Cheng MH, Lin YT, Huang WC, Tsao CK and Wei FC: Timing of
presentation of the first signs of vascular compromise dictates the
salvage outcome of free flap transfers. Plast Reconstr Surg.
120:187–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanasono MM, Kocak E, Ogunleye O, Hartley
CJ and Miller MJ: One versus two venous anastomoses in
microvascular free flap surgery. Plast Reconstr Surg.
126:1548–1557. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ross GL, Ang ES, Lannon D, Addison P,
Golger A, Novak CB, Lipa JE, Gullane PJ and Neligan PC: Ten-year
experience of free flaps in head and neck surgery. How necessary is
a second venous anastomosis? Head Neck. 30:1086–1089.
2008.PubMed/NCBI
|
|
35
|
Blondeel PN, Arnstein M, Verstraete K,
Depuydt K, Van Landuyt KH, Monstrey SJ and Kroll SS: Venous
congestion and blood flow in free transverse rectus abdominis
myocutaneous and deep inferior epigastric perforator flaps. Plast
Reconstr Surg. 106:1295–1299. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwon SS, Chang H, Minn KW and Lee TJ:
Venous drainage system of the transverse rectus abdominis
musculocutaneous flap. Scand J Plast Reconstr Surg Hand Surg.
43:312–314. 2009. View Article : Google Scholar : PubMed/NCBI
|