Introduction
Oxalic acid (OA), otherwise known as ethane diacid,
is the most simple binary acid. OA exerts marked corrosive and
toxic effects, and is commonly used in industry for metal
polishing, cleaning and bleaching. OA can cause burns and
poisoning, and although industrial cases are extremely rare, OA
poisoning is being recognized as an emerging epidemic in certain
rural communities as it is a component of widely produced household
laundry detergents (1). The toxic
effects of OA are primarily described in associated with ethylene
glycol poisoning (2), as OA is a
metabolite of ethylene glycol. As a final metabolic product, OA is
ubiquitously present in plants, fungi and animals. Previously
reported cases of isolated OA poisoning involve the consumption of
food, medications and plants that contain the compound, such as
star fruit and ascorbic acid (3). As
OA is the primary component of certain domestic cleaning products,
oral OA poisoning cases are not uncommon. Direct intoxication with
OA is a relatively frequent occurrence, due to OA being a primary
component of some household laundry detergents, and reports of the
toxicological effects of OA poisoning in humans are not uncommon,
including gastrointestinal effects, hypocalcemia secondary to
calcium oxalate crystal formation and renal toxicity (4–6).
However, it is relatively uncommon for exfoliation of the
esophageal mucosa to be caused by OA poisoning. The present study
reports a case of oral OA poisoning in a woman that developed
large-area esophageal mucosa injury and acute kidney injury
following selfingestion of OA. The study was approved by the ethics
committee of Qilu Hospital of Shandong University (Jinan, China),
and informed consent was obtained from the patient.
Case report
A 44-year-old woman oral consumed ~500 ml 70% OA
aqueous solution in an attempt at suicide following a domestic
dispute. After consuming the OA, the patient immediately
experienced severe abdominal pain and vomited repeatedly. The
patient was transferred to a local hospital by her family 1 h
later. Following a gastric lavage, the establishment of venous
access and conventional glucose and saline infusion treatment, the
patient was transferred to the regional hospital. On arrival, 5 h
after ingestion, the patient underwent hemodialysis to remove
remaining toxic compounds and to prevent acute renal failure
provoked by calcium oxalate crystal formation and renal toxicity,
with a pulse rate of 98 bpm and a blood pressure (BP) of 116/77
mmHg. The peripheral capillary oxygen saturation (SpO2)
of the patient was 97%, her body temperature was 36.5°C and
respiratory rate was 23 breaths/min. Immediate volume resuscitation
was conducted, and sucralfate and montmorillonite were administered
to provide preventive and therapeutic activity against the acute
esophageal mucosal injury induced by OA. The patient continued to
vomit violently for the remainder of the day, with blood observed
in the vomit on a number of occasions. Over the following days
basic normal urine output was maintained. On day 9 following the
injury, the patient's condition was significantly exacerbated, the
entire body exhibited edema and the urine volume decreased
significantly. A computed tomography (CT) scan of the lung
indicated an inflammatory response in the right inferior lobar
bronchus, in addition to bilateral hydrothorax. The patient
continued to experience nausea and vomiting. Eventually, the vomit
contained blood clots and a long narrow strip of necrotic tissue
was expelled (Fig. 1), which was
subsequently identified as the avulsed mucosa of the esophagus. The
patient was transferred to Qilu Hospital for further clinical
treatment on day 29 following poisoning. During the physical
examination conducted during patient admission, the basic vital
signs of the patient were as follows: Temperature, 37.9°C; pulse,
98 bpm; respiratory rate, 22 breaths/min; BP, 116/77 mmHg; and
SpO2, 98%. The patient experienced nausea, mild
shortness of breath, obvious generalized edema and crepitations
were audible during double-lung auscultation. The levels of alanine
transaminase and aspartate transaminase were 172.6 and 52.4 U/l,
respectively. There was a gradual elevation in the levels of serum
creatinine (SCr, 269 µmol/l) and blood urea nitrogen (BUN, 9.9
mmol/l) levels, with a serum potassium level of 4.36 mmol/l, an
erythrocyte sedimentation rate of 66 mm/h and a white blood cell
(WBC) count of 11.47×109/l. Furthermore, the patient was
positive for urinary protein, hematuria and urine casts.
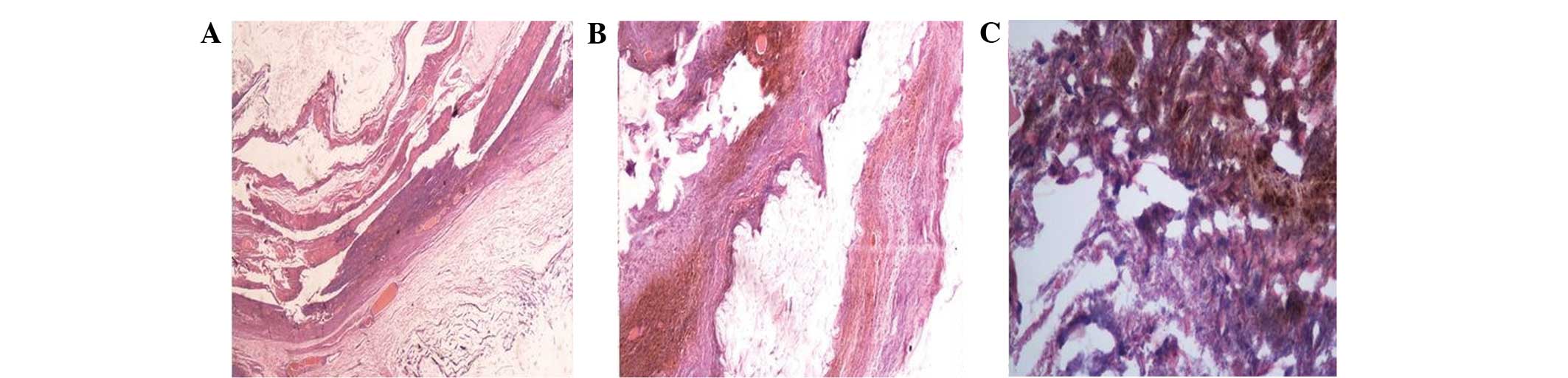
Histopathological examination of the necrotic tissue strip was
performed on day 30, and revealed fibrinoid necrosis, significant
inflammatory cellular infiltration and hemangiectasis (Fig. 2). The patient underwent pulse
methylprednisolone therapy and was treated with potassium-sparing
diuretics, hepatic protectants, antioxidants and anticoagulants on
the following days, and demonstrated symptomatic improvement. Urine
output increased and the SCr level returned to normal. The patient
was prescribed antacids and gastric mucosa protectant agents and
the upper gastrointestinal symptoms were attenuated. However, the
patient's temperature continued to increase and the patient
experienced pain in the right ear, right eye and head after day 29.
Symptomatic treatment was administered, but produced no apparent
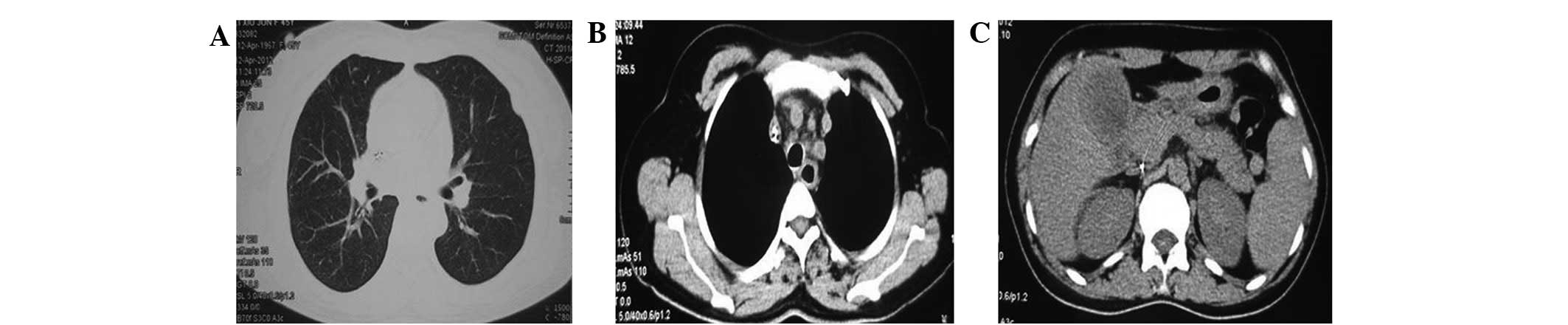
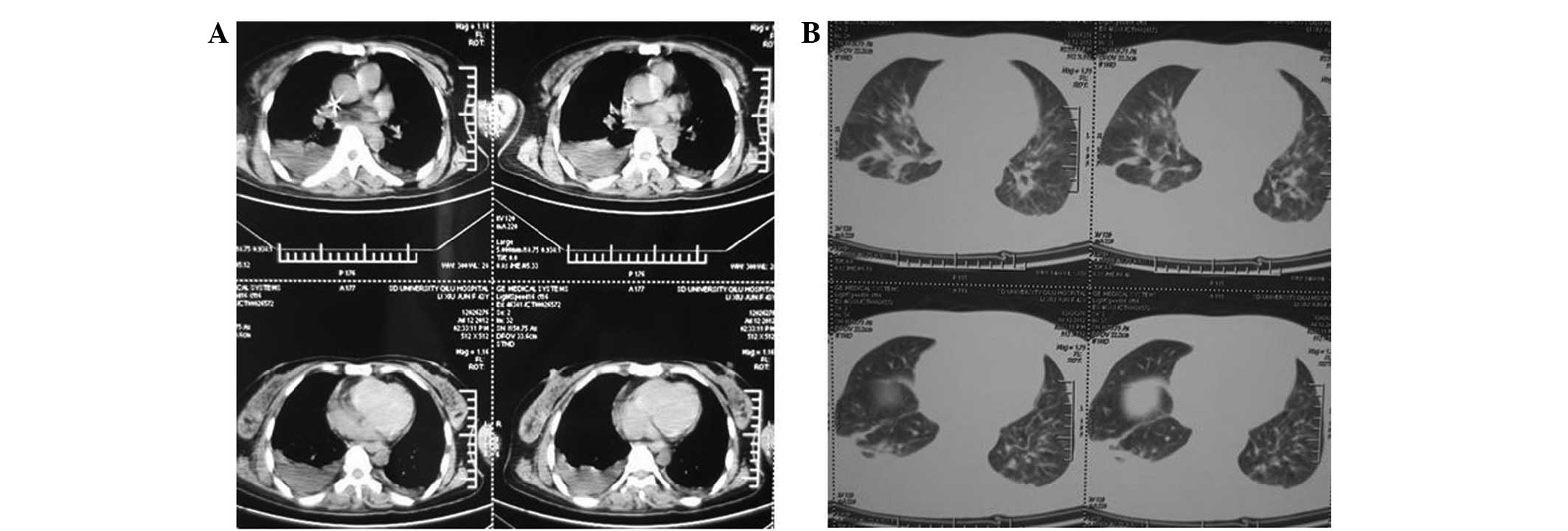
effect. CT scanning of the chest and abdomen revealed pneumonia,
left superior vena cava, twin pipe line-like high density shadow in
the precava and one pipe line-like high density shadow in the
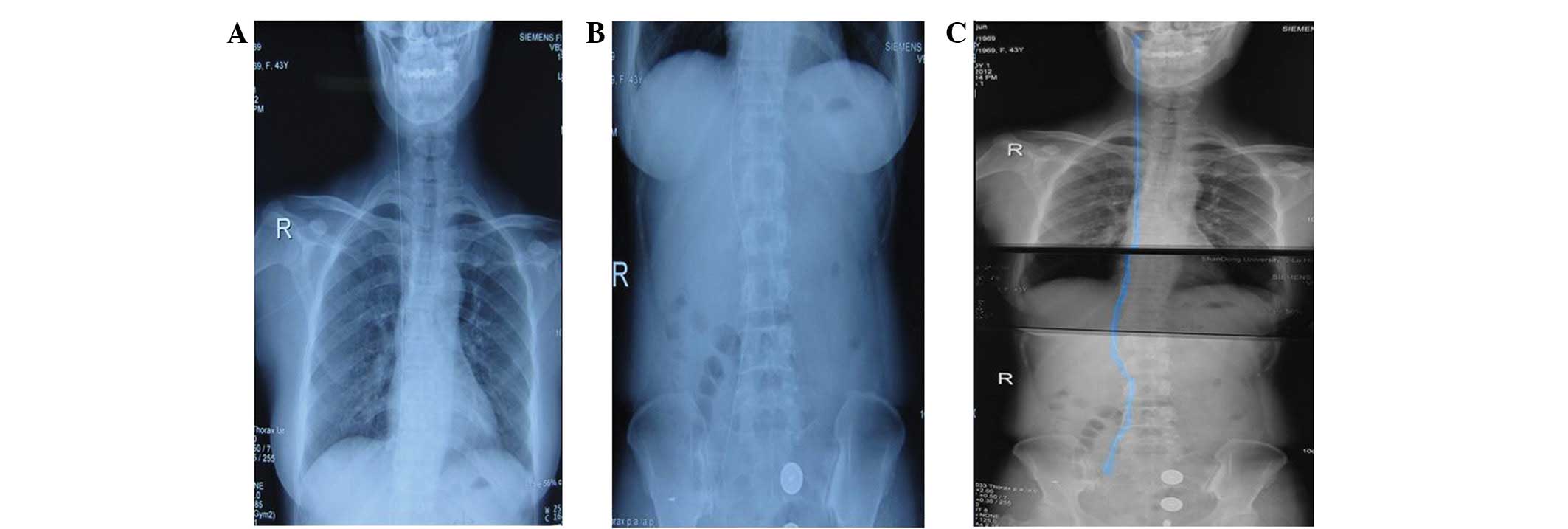
postcava, in addition to fatty liver and cholecystitis (Fig. 3). The abdomen and chest X-ray
revealed a thick-lined high-density shadow running through the
precava and the postcava, the upper end of which extended into the
retromandibular vein and its inferior extremity was in the right
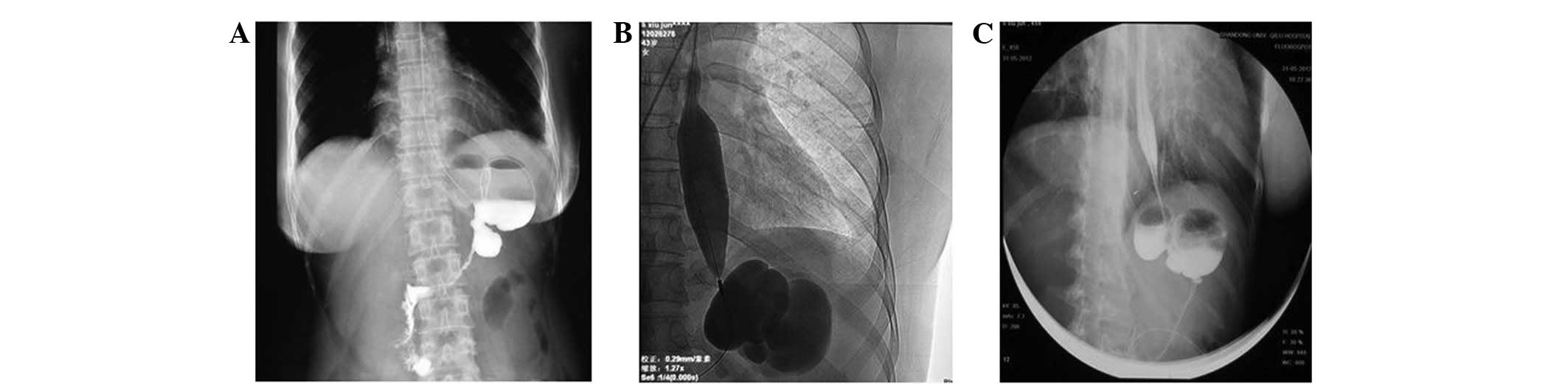
common iliac vein (Fig. 4). On day
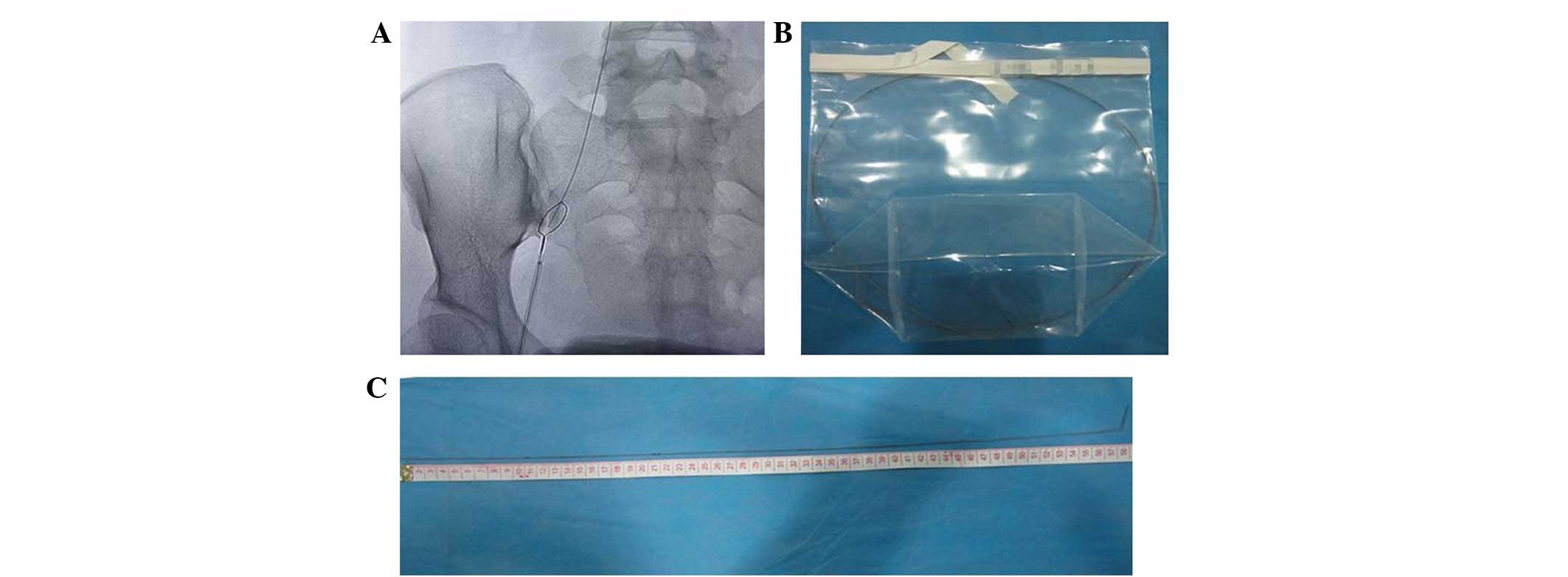
32, the patient accepted intravascular foreign body extraction and
a guide wire of ~59 cm was removed from her vein (Fig. 5). Analysis of the abdomen and chest
X-ray affirmed that the guide wire, which had remained in the
patient's vein since the process of catheter insertion in the
regional hospital, was removed. The painful sensations in the
patient's right eye and right ear gradually decreased. On day 40,
the blood test showed mild normochromic normocytic anemia
(hemoglobin, 96 g/l) with hypoproteinemia (serum albumin, 29 g/l),
and normal levels of WBCs and platelets. However, the patient
continued to exhibit dysphagia, and chemical burns were observed in
her pharynx and esophagus via TV fibrolaryngoscope. Furthermore,
upper gastrointestinal opacification and gastroscopy confirmed
scarring from the chemical burn. Pathological damage included
hyperemia of the esophageal mucous membrane, edema, increased
susceptibility to hemorrhage and ulcers, and contracture that
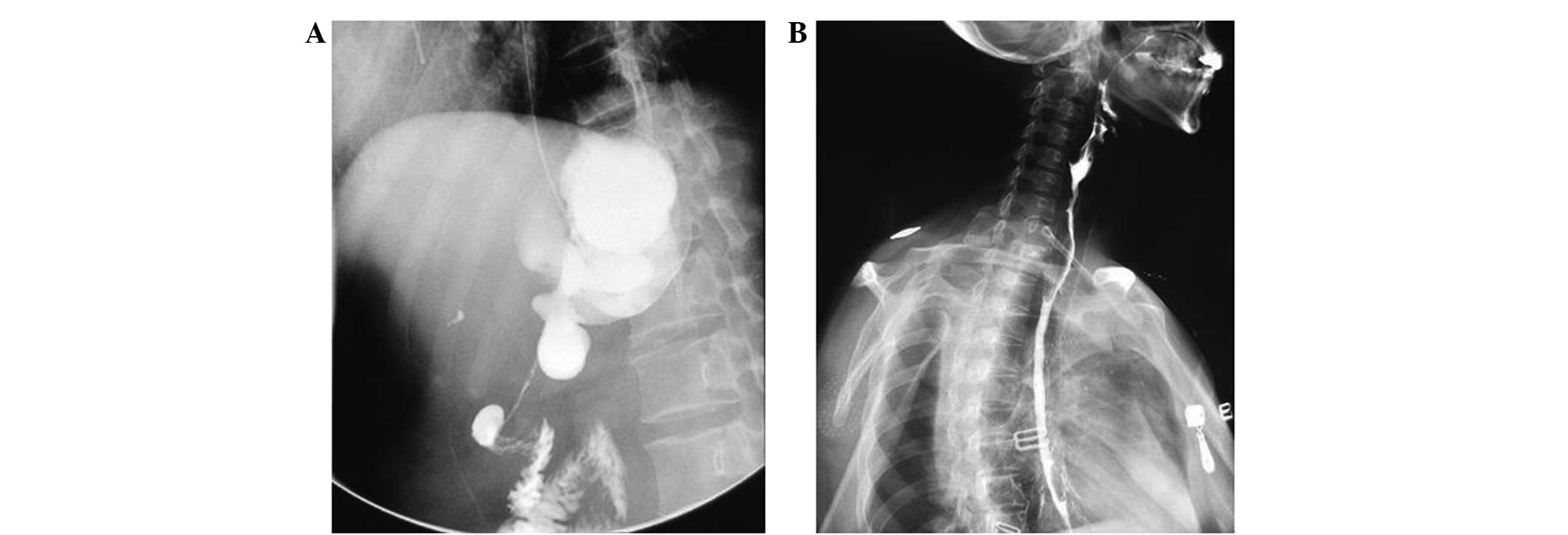
resulted in stricture of the esophagus. The patient underwent a
balloon distention operation in the upper digestive tract, using
X-ray location, a dumb-bell bladder and an interventional wire
(Fig. 6). This procedure did not
improve the dysphagia caused by atrophy of the gastric mucous
membrane, as had been expected. The patient underwent a second
procedure to stretch the esophagus, via endoscopic dilation, 4
months later. During this procedure the stimuli of dilation
provoked convulsions with opisthotonos and sudden lapses of
consciousness. The procedure was stopped immediately and effective
emergency measures were required. No evident abnormalities were
detected in the brain by CT scan, and a brain magnetic resonance
imaging (MRI) scan showed that the normal signal of the left
parietal lobe of the frontal cortex had been replaced by abnormally
high signal intensity, which indicated embolization. Following the
administration of dehydrants, cranial hypertension-reducing
treatment, neuro-nutritional drugs and parenteral nutrition, the
patient regained consciousness and her vital signs stabilized.
Subsequently, the patient's cough worsened, and a chest CT scan
revealed double pneumonia, pulmonary parenchymal exudation and
consolidation in the inferior lobe of the right lung (Fig. 7). Abdominal CT scanning showed no
evident abnormalities. The patient was administered
anti-inflammatory, anticoagulant and thrombolytic drugs, and her
cough gradually decreased. However, the digestive symptoms
continued and the repeated upper gastrointestinal barium
examinations indicated numerous severe stenoses in her esophagus,
gastric body and sinuses ventriculi, in addition to severe stomach
contracture (Fig. 8). Metallic stent
implantation is considered an effective treatment for patients with
such symptoms; however, in the present case the dilating catheter
was not able to pass through the stenotic area of the esophagus.
Finally, the patient received a percutaneous endoscopic jejunostomy
for the management of delayed gastric emptying. A 14 F nutrition
tube was inserted between the jejunum and the abdominal wall, which
was required to prevent the patient from succumbing to her
injuries. Postoperative enteral nutrition later effectively
improved the patient's nutritional status, and her general
condition improved. The patient was readmitted to the local
hospital and subsequently discharged a number of days later.
Discussion
Suicide by the consumption of poison is not uncommon
in certain rural areas of China. Numerous cases of poisoning
involve the ingestion of diazepam, paraquat and organophosphorus
pesticides (7). The prevalence of
self-poisoning cases is 315–364 per 100,000 individuals per year
(8). In recent years, easily
obtained household cleaning products have been increasingly used by
individuals attempting to commit suicide. A number of these
cleaning products contain oxalic acid (OA) as a primary component.
Oral OA is not readily absorbed, and has a bioavailability of 2–5%.
OA is excreted in an unmodified form via urine. The lethal dose of
oral OA poisoning for an adult human is 15–30 g, although in a
previously reported case the ingestion of 5 g OA was sufficient to
result in patient mortality (9). OA
can cause corrosive damage to the eyes, oral mucosa and
gastrointestinal tract. However, following absorption, OA and
calcium ions may reaction to form insoluble calcium oxalate
crystals. Calcium oxalate precipitation in the renal proximal
tubule may cause renal tubular epithelial cell necrosis and kidney
failure (10). The pathophysiology
of oxalate renal tubular damage includes energy depletion at the
cellular level, cell swelling, the inflow of calcium, intracellular
acidosis and enzyme activation (11). In addition, oxalate crystals may
block the renal tubule, resulting in further renal damage. The
clinical response to acute renal failure caused by oxalate
poisoning is typically comprehensive symptomatic treatment, with a
minority of reported cases requiring dialysis. The renal biopsy
results of patients with OA poisoning suggest the occurrence of
acute tubulointerstitial nephritis (1). Chronic hyperoxaluria cases exhibit
pathological changes associated with interstitial nephritis
(12,13). In such cases, the results may be
attributable to oxalate itself, although the patients were
typically treated with omeprazole, a proton pump inhibitor,
simultaneously due to gastrointestinal symptoms. Omeprazole is
known to cause interstitial nephritis (14). Absorbed OA can form calcium oxalate,
which may cause hypocalcemia directly. However, in the present case
there was no biochemical or electrocardiographic evidence of
hypocalcemia and the serum calcium level of this patient was
normal. However, the recorded state of shock with bradycardia at
presentation at the local hospital may have been a manifestation of
hypocalcemia. In cases of ethylene glycol poisoning, ethylene
glycol is metabolized via aldehyde metabolites to generate OA,
which is generally assumed to be the cause of the acute renal
failure caused by ethylene glycol poisoning (15). The damage to the central nervous
system and heart may be caused by aldehyde metabolites. In the
present case, OA caused acute renal failure; however, no
significant injury to the central nervous system or cardiovascular
system was detected.
In conclusion, OA poisoning may result in acute
renal impairment, which may result in mortality. Therefore, the
safe and informed use of household cleaners should be encouraged,
and household cleaning products containing OA should include clear
identification and warnings regarding the potential harm associated
with OA poisoning.
Acknowledgements
This study was supported by Natural Science
Foundation of Shandong Province (no. Y2008C123); and the Taishan
Scholar Program of Shandong Province (no. ts20130911).
References
|
1
|
Dassanayake U and Gnanathasan A: Acute
renal failure following accidental potassium bromate poisoning: A
case report. J Occup Med Toxicol. 7:172012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leth PM and Gregersen M: Ethylene glycol
poisoning. Forensic Sci Int. 155:179–184. 2006. View Article : Google Scholar
|
|
3
|
Chen CL, Fang HC, Chou KJ, Wang JS and
Chung HM: Acute oxalate nephropathy after ingestion of star fruit.
Am J Kidney Dis. 37:418–422. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo C and McMartin KE: The cytotoxicity of
oxalate, metabolite of ethylene glycol, is due to calcium oxalate
monohydrate formation. Toxicology. 208:347–355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McMartin KE and Cenac TA: Toxicity of
ethylene glycol metabolites in normal human kidney cells. Ann NY
Acad Sci. 919:315–317. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gawarammana IB, Ariyananda PL,
Palangasinghe C, De Silva NG, Fernando K, Vidanapathirana M,
Kuruppuarachchi MA, Munasinghe MA and Dawson AH: Emerging epidemic
of fatal human self poisoning with a washing powder in Southern Sri
Lanka: A prospective observational study. Clin Toxicol. 47:407–411.
2009. View Article : Google Scholar
|
|
7
|
Zhang Z, Jian X, Zhang W, Wang J and Zhou
Q: Using bosentan to treat paraquat poisoning-induced acute lung
injury in rats. PLoS One. 8:e759432013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eddleston M, Sudarshan K, Senthilkumaran
M, Reginald K, Karalliedde L, Senarathna L, de Silva D, Rezvi
Sheriff MH, Buckley NA and Gunnell D: Research-patterns of hospital
transfer for self-poisoned patients in rural Sri Lanka:
Implications for estimating the incidence of self-poisoning in the
developing world. Bull World Health Organ. 84:276–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silberhorn EM: Oxalates. Encyclopedia of
Toxicology. Wexler P: (2nd). (New York, NY). Elsevier, Inc.
320–322. 2005. View Article : Google Scholar
|
|
10
|
Tsujihata M: Mechanism of calcium oxalate
renal stone formation and renal tubular cell injury. Int J Urol.
15:115–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brady HR, Brenner BM and Lieberthal W:
Acute renal failure. Brenner and Rector's The kidney. Brenner BM:
(5th). (Philadelphia, PA). W.B. Saunders Co. 1212–1222. 1996.
|
|
12
|
Rathi S, Kern W and Lau K: Vitamin
C-induced hyperoxaluria causing reversible tubulointerstitial
nephritis and chronic renal failure: A case report. J Med Case Rep.
1:1552007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allen A, Clutterbuck E, Maidment G,
Thompson E, Watts R and Pusey C: Enteric hyperoxaluria and renal
failure associated with lymphangiectasia. Nephrol Dial Transplant.
12:802–806. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torregrosa E, Rovira RE, Calvo C,
Hernández-Jaras J, Maduell F and García H: Acute interstitial
nephritis associated with omeprazole therapy. Nefrologia. 24:61–63.
2004.(In Spanish). PubMed/NCBI
|
|
15
|
McMartin K: Are calcium oxalate crystals
involved in the mechanism of renal toxicity in ethylene glycol
poisoning. Clin Toxicol. 47:859–869. 2009. View Article : Google Scholar
|