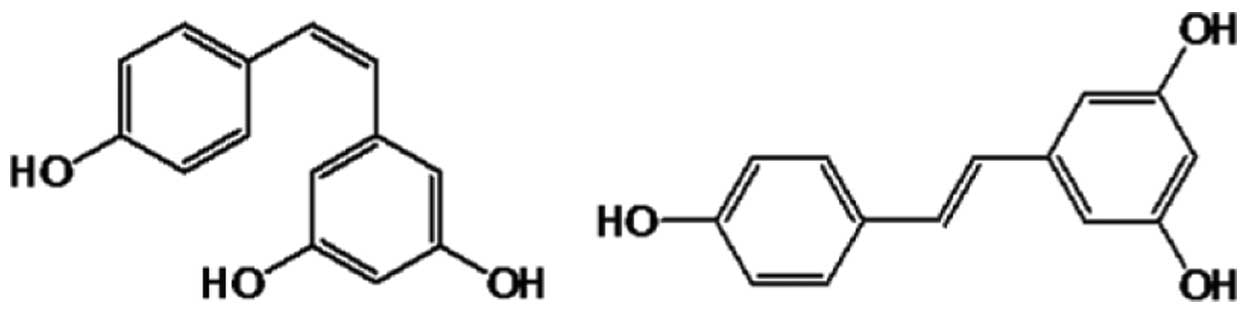
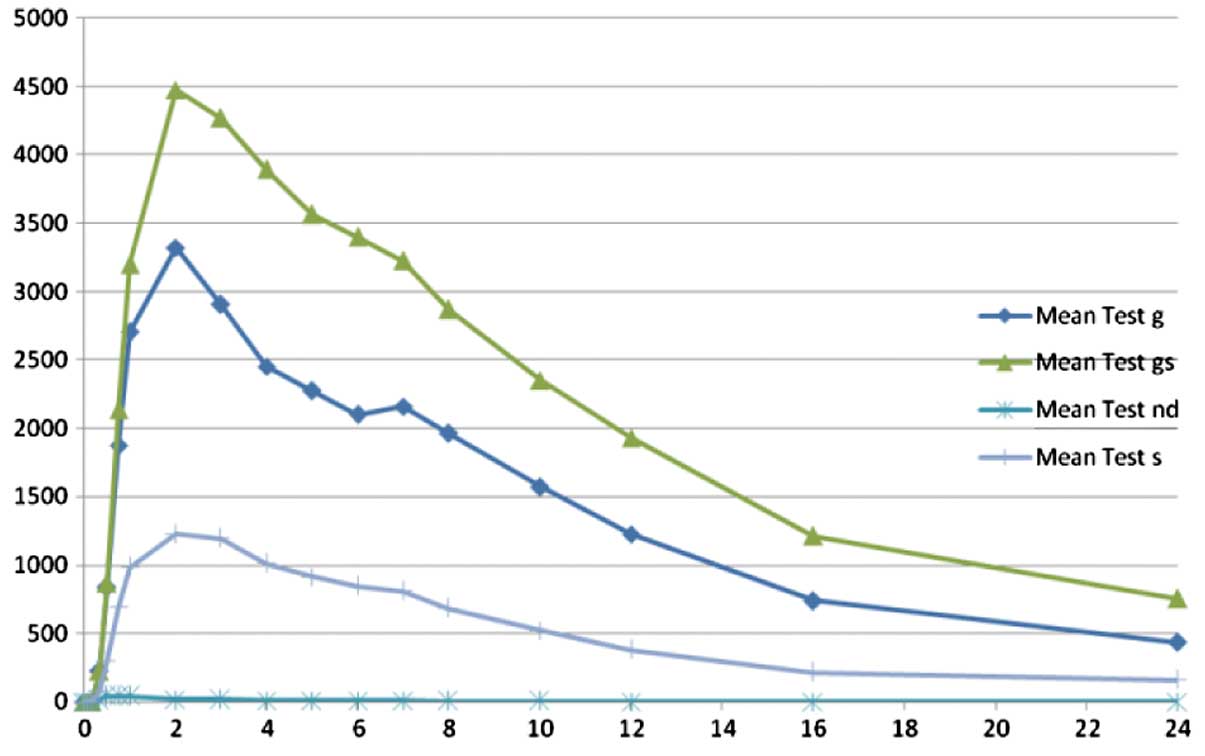
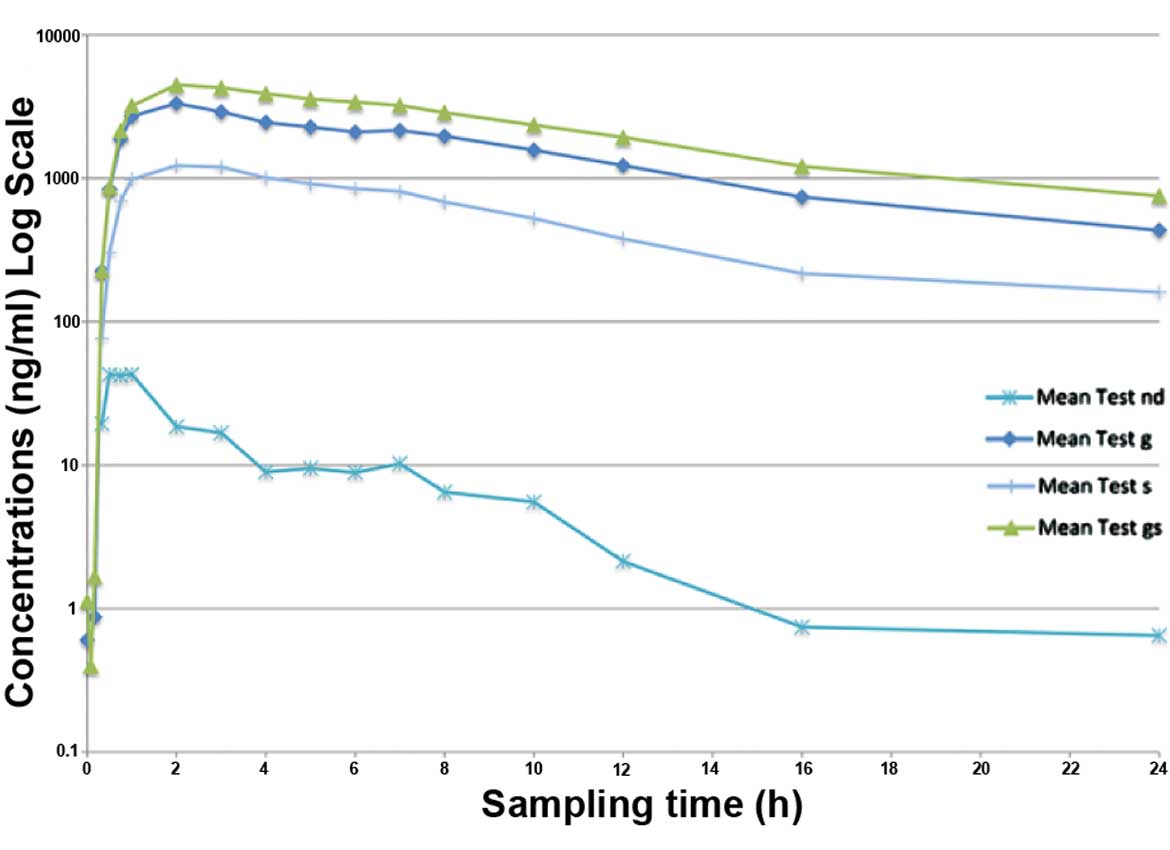
|
1
|
Arichi H, Kimura Y, Okuda H, Baba K,
Kozawa M and Arichi S: Effects of stilbene components of the roots
of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem
Pharm Bull (Tokyo). 30:1766–1770. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gambini J, López-Grueso R, Olaso-González
G, Inglés M, Abdelazid K, El Alam IM, Bonet-Costa V, Borrás C and
Viña J: Resveratrol: Distribution, properties and perspectives. Rev
Esp Geriatr Gerontol. 48:79–88. 2013.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bertell AA and Das DK: Grapes, wines,
resveratrol, and heart health. J Cardiovasc Pharmacol. 54:468–476.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bishayee A, Darvesh AS, Politis T and
McGory R: Resveratrol and liver disease: From bench to bedside and
community. Liver Int. 30:1103–1114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aires V, Limagne E, Cotte AK, Latruffe N,
Ghiringhelli F and Delmas D: Resveratrol metabolites inhibit human
metastatic colon cancer cells progression and synergize with
chemotherapeutic drugs to induce cell death. Mol Nutr Food Res.
57:1170–1181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aziz MH, Kumar R and Ahmad N: Cancer
chemoprevention by resveratrol: In vitro and in vivo studies and
the underlying mechanisms (review). Int J Oncol. 23:17–28.
2003.PubMed/NCBI
|
|
8
|
Asensi M, Medina I, Ortega A, Carretero J,
Baño MC, Obrador E and Estrela JM: Inhibition of cancer growth by
resveratrol is related to its low bioavailability. Free Radic Biol
Med. 33:387–398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frémont L: Biological effects of
resveratrol. Life Sci. 66:663–673. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hung LM, Chen JK, Huang SS, Lee RS and Su
MJ: Cardioprotective effect of resveratrol, a natural antioxidant
derived from grapes. Cardiovasc Res. 47:549–555. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhat KPL, Kosmeder JW II and Pezzuto JM:
Biological effects of resveratrol. Antioxid Redox Signal.
3:1041–1064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de la Lastra CA and Villegas I:
Resveratrol as an anti-inflammatory and anti-aging agent:
Mechanisms and clinical implications. Mol Nutr Food Res.
49:405–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ulrich S, Wolter F and Stein JM: Molecular
mechanisms of the chemopreventive effects of resveratrol and its
analogs in carcinogenesis. Mol Nutr Food Res. 49:452–461. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jan-Kan C and Li-man H: Therapeutic use of
resveratrol for hyperglycemia. US patent 2006/0034763 A1. Filed.
August 11–2004.issued February 16, 2006.
|
|
15
|
Boocock DJ, Faust GE, Patel KR, Schinas
AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher
AJ, et al: Phase I dose escalation pharmacokinetic study in healthy
volunteers of resveratrol, a potential cancer chemopreventive
agent. Cancer Epidemiol Biomarkers Prev. 16:1246–1252. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agarwal B, Campen MJ, Channell MM, Wherry
SJ, Varamini B, Davis JG, Baur JA and Smoliga JM: Resveratrol for
primary prevention of atherosclerosis: Clinical trial evidence for
improved gene in vascular endothelium. Int J Cardiol. 166:246–248.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu X, Creasy L, Kester A and Zeece M:
Capillary electrophoretic determination of resveratrol in wines. J
Agric Food Chem. 47:3223–3227. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Almeida L, Vaz-da-Silva M, Falcão A,
Soares E, Costa R, Loureiro AL, Fernandes-Lopes C, Rocha JF, Nunes
T, Wright L and Soares-da-Silva P: Pharmacokinetic and safety
profile of trans-resveratrol in a rising multiple-dose study
in healthy volunteers. Mol Nutr Food Res. 53(Suppl 1): S7–S15.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cottart CH, Nivet-Antoine V,
Laguillier-Morizot C and Beaudeux JL: Resveratrol bioavailability
and toxicity in humans. Mol Nutr Food Res. 54:7–16. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuhnle G, Spencer JP, Chowrimootoo G,
Schroeter H, Debnam ES, Srai SK, Rice-Evans C and Hahn U:
Resveratrol is absorbed in the small intestine as resveratrol
glucuronide. Biochem Biophys Res Commun. 272:212–217. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rotches-Ribalta M, Andres-Lacueva C,
Estruch R, Escribano E and Urpi-Sarda M: Pharmacokinetics of
resveratrol metabolic profile in healthy humans after moderate
consumption of red wine and grape extract tablets. Pharmacol Res.
66:375–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brown A, Patel KR, Viskaduraki M, Crowell
JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli
G, et al: Repeat dose study of the cancer chemopreventive agent
resveratrol in healthy volunteers: Safety, pharmacokinetics, and
effect on the insulin-like growth factor axis. Cancer Res.
70:9003–9011. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang D, Hang T, Wu C and Liu W:
Identification of the major metabolites of resveratrol in rat urine
by HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci.
829:97–106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walle T: Bioavailability of resveratrol.
Ann N Y Acad Sci. 1215:9–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu DL, Ding DJ, Yan WJ, Li RR, Dai F, Wang
Q, Yu SS, Li Y, Jin XL and Zhou B: Influence of glucuronidation and
reduction modifications of resveratrol on its biological
activities. Chembiochem. 14:1094–1104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sergides C and Pittas A: Resveratrol
compositions for use as dietary supplements. Patent EP2403479 A1.
Filed. March 3–2010.issued January 11, 2012.
|
|
27
|
Liu X, Teng Z, Zhang Y, Huan M and Zhou S:
High performance liquid chromatographytandem mass spectrometric
determination of resveratrol and its metabolites in rat tissues.
Anal Lett. 43:557–569. 2010. View Article : Google Scholar
|
|
28
|
Muzzio M, Huang Z, Hu SC, Johnson WD,
McCormick DL and Kapetanovic IM: Determination of resveratrol and
its sulfate and glucuronide metabolites in plasma by LC-MS/MS and
their pharmacokinetics in dogs. J Pharm Biomed Anal. 59:201–208.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Biasutto L, Marotta E, Garbisa S, Zoratti
M and Paradisi C: Determination of quercetin and resveratrol in
whole blood-implications for bioavailability studies. Molecules.
15:6570–6579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel KR, Brown VA, Jones DJ, Britton RG,
Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowel JA, et
al: Clinical pharmacology of resveratrol and its metabolites in
colorectal cancer patients. Cancer Res. 70:7392–7399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tomé-Carneiro J, Larrosa M,
González-Sarrías A, Tomás-Barberán FA, García-Conesa MT and Espín
JC: Resveratrol and clinical trials: The crossroad from in vitro
studies to human evidence. Curr Pharm Des. 19:6064–6093. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brasnyó P, Molnár GA, Mohás M, Markó L,
Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R, et al:
Resveratrol improves insulin sensitivity, reduces oxidative stress
and activates the Akt pathway in type 2 diabetic patients. Br J
Nutr. 106:383–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhatt JK, Thomas S and Nanjan MJ:
Resveratrol supplementation improves glycemic control in type 2
diabetes mellitus. Nutr Res. 32:537–541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Witte AV, Kerti L, Margulies DS and Flöel
A: Effects of resveratrol on memory performance, hippocampal
functional connectivity, and glucose metabolism in healthy older
adults. J Neurosci. 34:7862–7870. 2014. View Article : Google Scholar : PubMed/NCBI
|