Introduction
Rikkunshi-to, a Japanese herbal medicine, is known
to have a beneficial effect on the gastric mucosa (1). By the development of novel antiemetic
agents such as aprepitant, a high control rate of
chemotherapy-induced nausea and vomiting was achieved. However,
chemotherapy-induced appetite loss has been one of the uncontrolled
adverse events and any promising drugs targeting such appetite loss
have not yet been developed. Recently, attention has been focused
on the appetite-stimulating effects of rikkunshi-to (2), and the mechanisms through which
rikkunshi-to improves appetite have been clarified in previous
studies (3–7). However, to the best of our knowledge,
no previous study has investigated the potential clinical role of
rikkunshi-to in improving the food intake of patients with lung
cancer undergoing chemotherapy treatment. Therefore, the aim of the
present study was to investigate the effect of rikkunshi-to on
post-chemotherapeutic appetite loss. The effects on food intake and
body weight were examined in patients with lung cancer who were
undergoing treatment with chemotherapy. The serum albumin levels
and total protein levels were also assessed in order to evaluate
the effect of rikkunshi-to on the nourishment state of the
patients.
Patients and methods
Patients
This prospective observational study was conducted
at the Mito Medical Center (Mito, Japan) between October 2012 and
April 2014. Patients with unresectable lung cancer, who were
admitted to the 2-WEST Ward of the hospital and treated with
cisplatin (CDDP)-containing, carboplatin (CBDCA)-containing and
non-platinum chemotherapies, were recruited for participation in
the study. Written informed patient consent was obtained from the
patient. During each course of chemotherapy, the patients were
randomly divided into two groups. Patients received rikkunshi-to
treatment for some chemotherapeutic cycles and not for others. One
group received a rikkunshi-to (TJ-43; Tsumura & Co., Tokyo,
Japan) prescription, which was administered at 7.5 g/day, t.i.d.
(three doses at 2.5 g per day.), prior to meals for seven days,
while the other group did not receive rikkunshi-to treatment. This
study was approved by the Ethics Committee of the Mito Medical
Center, University of Tsukuba, Mito, Japan.
Assessment of experimental
parameters
Food intake was evaluated in the patients with or
without a prescription of rikkunshi-to. Documentation of the total
food intake for each meal was performed by the nursing staff of the
2-WEST Ward, and the percentage of food intake was subsequently
calculated. An estimation of the daily dietary intake of staple
food and the main dish of every three meals in hospital was
recorded by nurses and was summed up to rate the dietary intake
(range 0 to 100). In addition, the levels of serum albumin and
total serum protein, and the body weight of the patients, were
measured prior to and at day 7 following the initiation of
chemotherapy. The experimental protocols of the current study were
approved by the Ethics Committee of the Mito Kyodo General
Hospital, and informed consent was obtained from all patients prior
to their participation in the study.
Statistical analysis
The Mann-Whitney U test was used to compare the
daily food intake between the two groups of patients (with or
without rikkunshi-to therapy) and the software SPSS 10.1 for
Windows (SPSS, Chicago, IL, USA). In addition, a non-parametric
test was used to compare the change in the levels of serum albumin
and total serum protein, and the body weight of the patients.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
During the study period, 140 courses of chemotherapy
in 48 patients were analyzed. All the courses were performed in an
inpatient setting. The patient characteristics are shown in
Tables I and II. The median age of the 48 patients was
64 years (range, 41–88 years). Of the 48 patients, 25 were male and
23 were female; 29 cases were diagnosed as lung adenocarcinoma,
while 15 cases were diagnosed as small cell lung cancer. Amongst
the other 4 patients, 2 cases had large cell carcinomas, 1 case had
squamous cell carcinoma and 1 case had adenosquamous cell
carcinoma. In total, 91 courses were CBDCA-containing chemotherapy
(64 with rikkunshi-to and 27 without rikkunshi-to), 21 courses were
CDDP-containing chemotherapy (10 with rikkunshi-to and 11 without
rikkunshi-to) and 28 course were non-platinum chemotherapy (16 with
rikkunshi-to and 12 without rikkunshi-to). In order to evaluate the
effect of rikkunshi-to efficiently, patients with CBDCA-containing
chemotherapy were allocated into two groups in a ratio of 2:1. In
patients with CDDP-containing chemotherapy and those with
non-platinum chemotherapy, patients were subdivided in a ratio of
1:1 with and without rikkunshi-to, respectively. With regard to the
rikkunshi-to treatment, there were no severe complications observed
(>grade 2 in the Common Terminology Criteria for Adverse Events
(8).
 | Table I.Overall characteristics of the
patients included in the study. |
Table I.
Overall characteristics of the
patients included in the study.
| Characteristics | Study population |
|---|
| Median age (range),
years | 67 (41–88) |
| Gender (male/female),
n | 25/23 |
| Pathology |
|
|
Adenocarcinoma | 29 patients, 81
courses |
| Small
cell lung cancer | 15 patients, 53
courses |
|
Other |
4 patients, 6
courses |
 | Table II.Characteristics of the patients
treated with or without rikkunshi-to. |
Table II.
Characteristics of the patients
treated with or without rikkunshi-to.
| Chemotherapy
treatment | With
rikkunshi-to | Without
rikkunshi-to | P-value |
|---|
| CBDCA-containing |
|
|
|
| Median
age (range), years | 74 (41–82) | 69 (43–82) | 0.6221 |
| Gender
(male/female), n | 27/37 | 14/13 | 0.4905 |
| Pathology
ratio AD:SCLC:other, n | 30:29:5 | 13:13:1 | 0.3010 |
| CDDP-containing |
|
|
|
| Median
age (range), years | 51 (49–67) | 51 (50–68) | 0.9999 |
| Gender
(male/female), n | 4/6 | 7/4 | 0.3949 |
| Pathology
ratio AD:SCLC:other, n | 10:0:0 | 9:2:0 | 0.4762 |
| Non-platinum |
|
|
|
| Median
age (range), years | 79 (43–88) | 67 (61–86) | 0.1839 |
| Gender
(male/female), n | 6/10 | 8/4 | 0.2519 |
| Pathology
ratio AD:SCLC:other, n | 12:4:0 | 7:5:0 | 0.4319 |
CBDCA-containing chemotherapy
No statistically significant differences were
observed between the two groups of patients (with or without
rikkunshi-to treatment) in terms of age (P=0.6221), gender
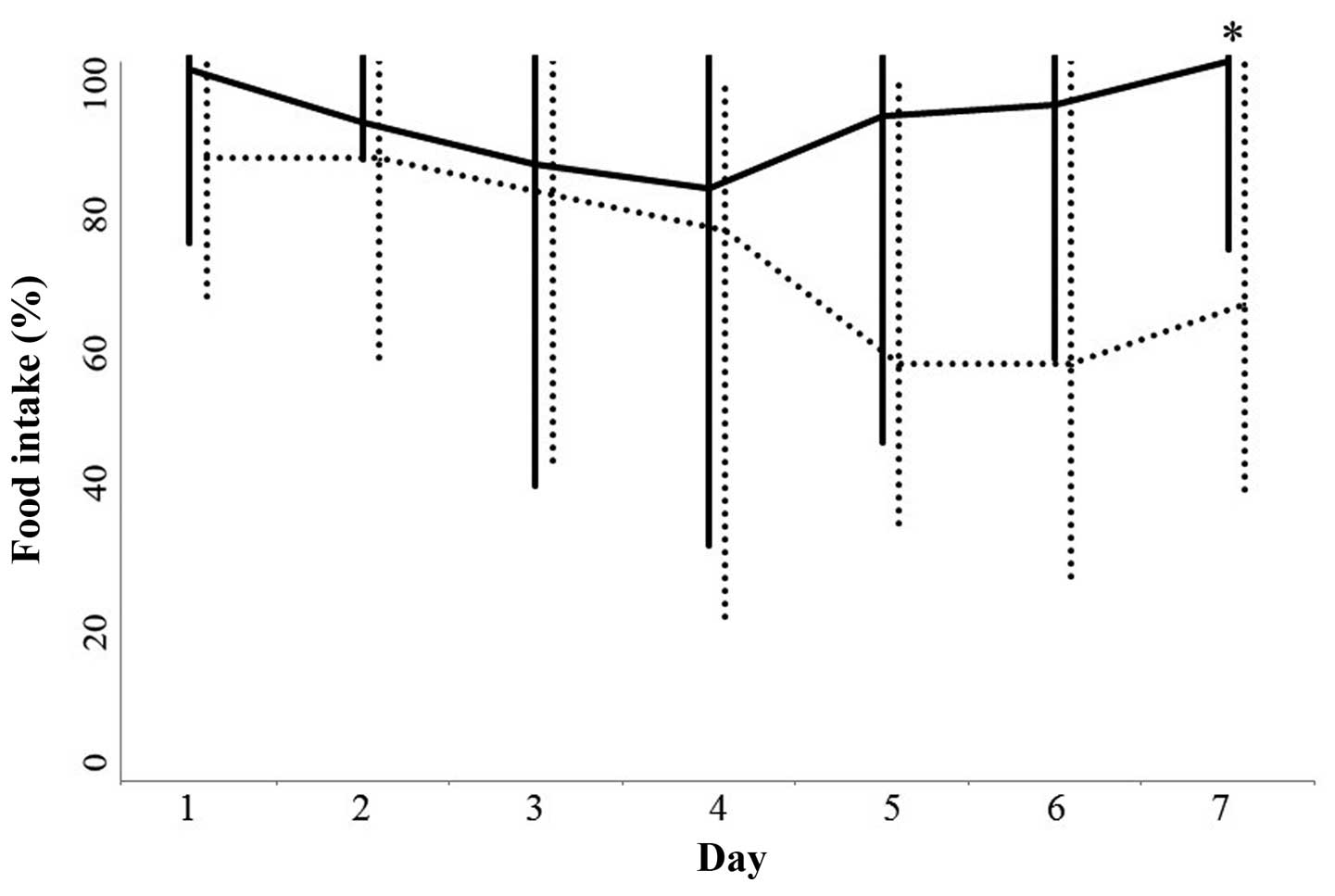
(P=0.4905) and histology (P=0.3010). Food intake between days 1 and
5 following the initiation of chemotherapy in the two groups was
not significantly different (Fig.
1). The food intake at day 6 in the rikkunshi-to treatment
group was higher compared with the untreated group; however, this
difference was not statistically significant (P=0.0626). By
contrast, the food intake on day 7 following the initiation of
chemotherapy was significantly higher in the rikkunshi-to treatment
group compared with the untreated group (P=0.0078; Fig. 1).
There was no statistically significant difference in
the change of the level of serum of albumin between pretreatment
and day 7 in the two groups (rikkunshi-to treatment group, median,
0 g/dl; range, −1.1–0.9 g/dl; untreated group, median, 0 g/dl;
range, −0.7–0.9 g/dl; P=0.3028). In addition, no statistically
significant differences in the total serum protein level (P=0.2604)
and body weight (P=0.6860) were observed between pretreatment and
day 7 in the patients with and without rikkunshi-to treatment (data
not shown).
CDDP-containing chemotherapy
No statistically significant differences were
observed in the patients with or without rikkunshi-to therapy with
regard to age (P=0.9999), gender (P=0.3949) and histology
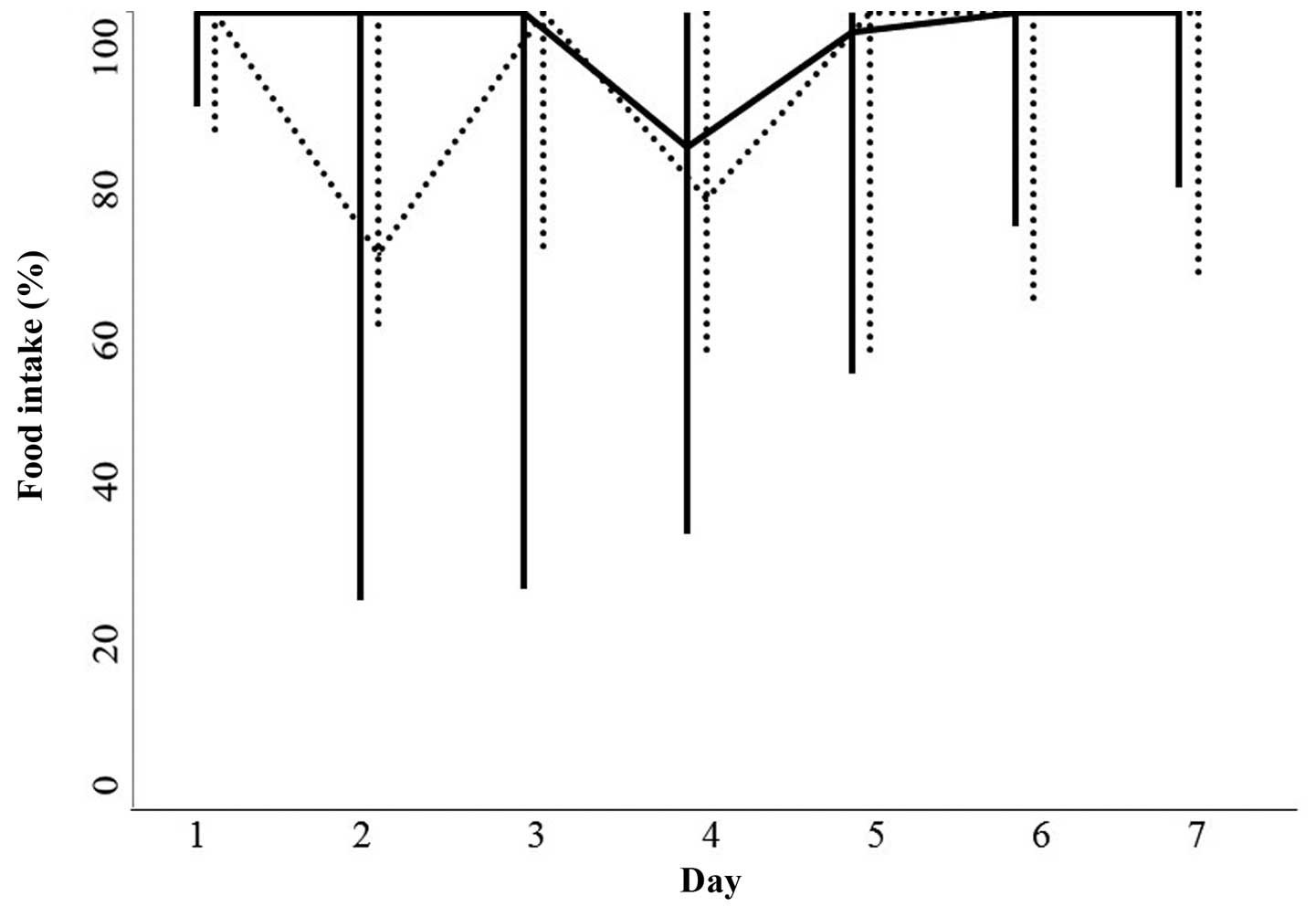
(P=0.4762). Food intake between days 1 and 7 after the initiation
of chemotherapy in the patients with or without rikkunshi-to
therapy was not significantly different (Fig. 2). In addition, no statistically
significant differences were observed in the serum albumin level
(P=0.5698), total serum protein level (P=0.6764) and body weight
(P=0.7243) between pretreatment and day 7 in the two groups (data
not shown).
Non-platinum containing
chemotherapy
No statistically significant differences were
identified between the patients with or without rikkunshi-to
therapy in terms of age (P=0.1839), gender (P=0.2519) and histology
(P=0.4319).
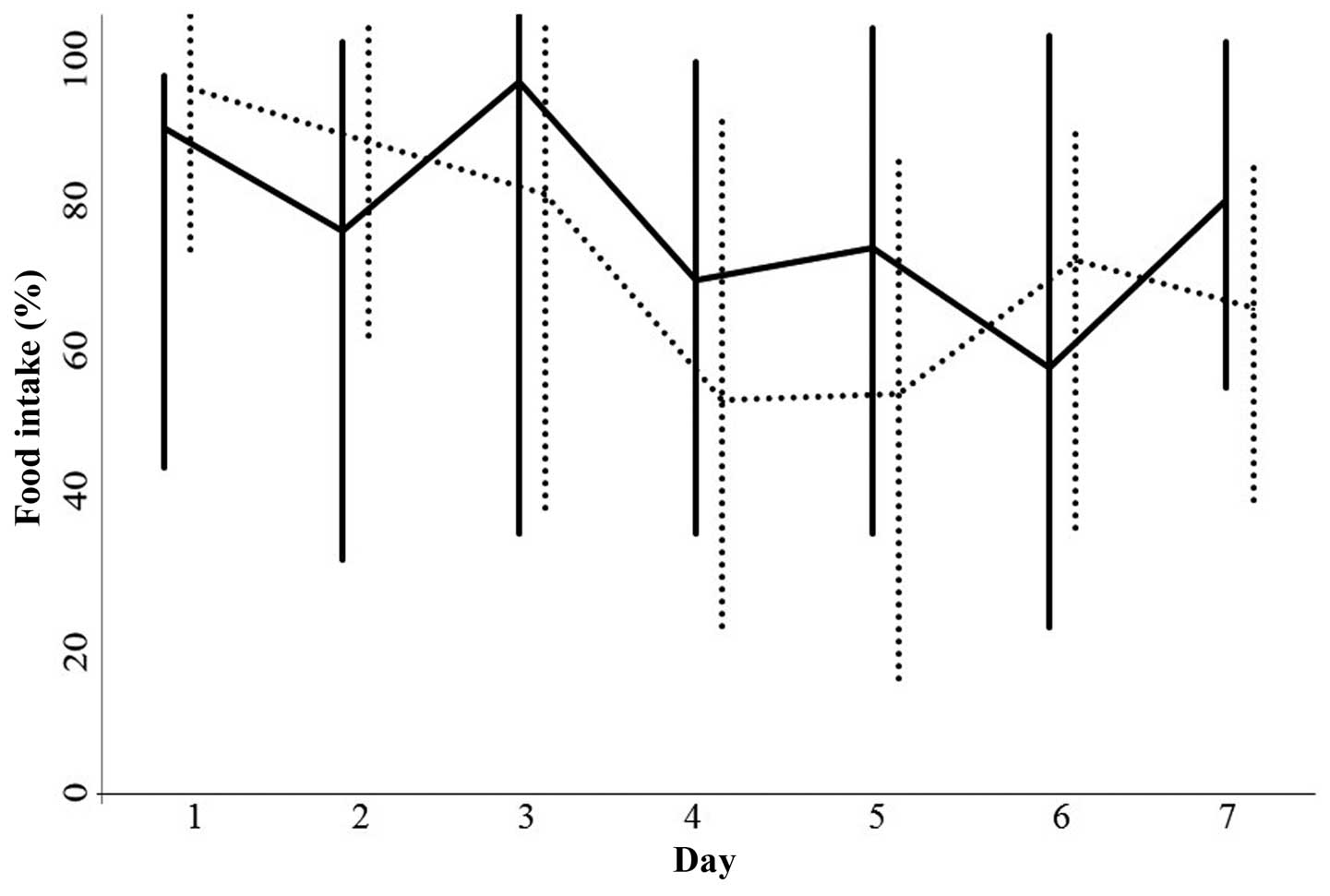
Food intake between days 1 and 7 following the
initiation of chemotherapy in the two groups was not significantly
different (Fig. 3). Furthermore, no
statistically significant differences were observed in the serum
albumin level (P=0.5569), total serum protein level (P=0.9290) and
body weight (P=0.4153) between pretreatment and day 7 in the
patients with or without rikkunshi-to treatment (data not
shown).
Discussion
Despite the development of novel antiemetic agents,
such as aprepitant, a considerable proportion of patients receiving
chemotherapy continue to experience appetite loss and a decreased
food intake (9). In a previous
study, a high control rate of nausea and vomiting was achieved
through aprepitant administration; however, appetite loss was one
of the uncontrolled chemotherapy-induced symptoms (10), which is consistent with the results
of additional studies (11,12). At present, a promising drug targeting
chemotherapy-induced appetite loss has yet to be developed.
However, the ability of rikkunshi-to to increase food intake has
been increasingly studied (2).
Therefore, the current study investigated the effect of
rikkunshi-to administration on post-chemotherapeutic appetite
loss.
The mechanism underlying chemotherapy-induced
appetite loss has yet to be elucidated. In addition, the mechanism
of action of rikkunshi-to on appetite loss following chemotherapy
is unclear. However, a number of studies have investigated
chemotherapy-induced appetite loss (2,13,14).
Hattori et al demonstrated that CDDP-induced appetite loss
was, similarly to the onset of nausea and vomiting, caused by large
amounts of serotonin (5-HT) release, as a result of CDDP
administration on 5-HT receptors in the tissue (14). Among the 5-HT receptors, the
activation of 5-HT2b and 5-HTc receptors plays a major role in
CDDP-induced appetite loss (14).
Following activation of these two receptors, there is reduced
gastric and hypothalamic secretion of the appetite-stimulating
hormone, ghrelin (2). There is
substantial evidence demonstrating the efficiency of exogenous
ghrelin and synthetic ghrelin agonists in the clinical treatment of
appetite loss (13,14). The endogeneous ghrelin
signal-enhancer, rikkunshi-to, is also expected to play a
significant role in preventing appetite loss and improving food
intake following chemotherapy in patients with various types of
cancer (3,13,14).
The present study evaluated food intake in patients
undergoing three types of chemotherapeutic regimens. In the
patients treated with CBDCA-containing chemotherapy, an improvement
in food intake was observed at day 7, although improvements in food
intake were not observed during days 1–5. In the patients who did
not receive rikkunshi-to treatment, food intake decreased gradually
following the initiation of chemotherapy and was lowest at days
5–6. By contrast, in the patients who were administered
rikkunshi-to treatment, an improvement in food intake was observed
from day 4. However, no improvement in food intake was observed in
the patients treated with CDDP-containing chemotherapy. In the two
groups of patients, with or without rikkunshi-to administration,
food intake decreased at days 3–4, but improved on day 5. The
reason why a significant difference in food intake was not observed
between the two groups of patients may be associated with the
younger age of the patients treated with this chemotherapy regimen
compared with the other two regimens, although there were
significant differences in the characteristics of patients in both
groups (with or without rikkunshi-to). In all the patients
undergoing non-platinum chemotherapy, food intake on day 1 was
lower when compared with the patients in the other chemotherapy
groups. The food intake decreased gradually until day 5 in the
patients not receiving rikkunshi-to treatment. In the patients
treated with rikkunshi-to, food intake improved at day 3 and 7,
however, the difference between the two groups was not
statistically significant. In the non-platinum chemotherapy
regimen, the poorer clinical condition of the patients and that a
chemotherapy line performed second or later may have influenced the
poor control of food intake in both groups of patients. In all the
chemotherapy regimen groups, no improvements in the levels of serum
albumin and total serum protein, or patient body weight, were
observed.
Although the present study demonstrated an
improvement in food intake with rikkunshi-to administration at day
7 in the patients undergoing CBDCA-containing chemotherapy, whether
rikkunshi-to has sufficient power to improve food intake is unable
to be concluded, since the results may have been influenced by the
small sample size included in the present study. Thus, the results
of the current study require conformation in well-planned,
larger-scale prospective studies.
Appetite loss remains a problem for patients with
lung cancer undergoing chemotherapy, and the incidence rate may be
underestimated by medical staff. An improved assessment of the
incidence rate of chemotherapy-induced complications is essential
for achieving adequate control. In conclusion, the present study
indicated the possibility of using rikkunshi-to in clinical
practice to improve appetite loss in patients with lung cancer
undergoing chemotherapy.
Acknowledgements
The authors thank the nursing staff at the 2-WEST
Ward of Mito Kyodo General Hospital for their precise descriptions
of the food intake of each patient.
References
|
1
|
Goso Y, Ogata Y, Ishihara K and Hotta K:
Effects of traditional herbal medicine on gastric mucin against
ethanol-induced gastric injury in rats. Comp Biochem Physiol C
Pharmacol Toxicol Endocrinol. 113:17–21. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takeda H, Muto S, Nakagawa K, Ohnishi S
and Asaka M: Rikkunshito and ghrelin secretion. Curr Pharm Des.
18:4827–4838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeda H, Sadakane C, Hattori T, et al:
Rikkunshito, an herbal medicine, suppresses cisplatin-induced
anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology.
134:2004–2013. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asakawa A, Inui A, Kaga T, et al: Ghrelin
is an appetite stimulatory signal from stomach with structural
resemblance to motilin. Gastroenterology. 120:337–345. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakazato M, Murakami N, Date Y, et al: A
role for ghrelin in the central regulation of feeding. Nature.
409:194–198. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebihara K, Ogawa Y, Masuzaki H, et al:
Transgenic overexpression of leptin rescues insulin resistance and
diabetes in a mouse model of lipoatrophic diabetes. Diabetes.
50:1440–1448. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inui A: Ghrelin an orexigenic and
somatotrophic signal from the stomach. Nat Rev Neurosci. 2:551–560.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cancer Therapy Evaluation Program, Common
Toxicity Criteria for Adverse Events. Version 3.0, CTCAE Version
3.0. June 10–2003.http://ctep.cancer.govAccessed. May 15–2014
|
|
9
|
Navari RM: Management of
chemotherapy-induced nausea and vomiting: focus on newer agents and
new uses for older agents. Drugs. 73:249–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishikawa A, Ohara G, Nakazawa K, et al:
Chemotherapy-induced complications in patients with lung cancer: an
evaluation by pharmacists. Mol Clin Oncol. 1:65–68. 2013.PubMed/NCBI
|
|
11
|
Dando TM and Perry CM: Aprepitant: a
review of its use in the prevention of chemotherapy-induced nausea
and vomiting. Drugs. 64:777–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Cutsem E and Arends J: The causes and
consequences of cancer-associated malnutrition. Eur J Oncol Nurs.
9:S51–S63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Camilleri M, Papathanasopoulos A and
Odunsi ST: Actions and therapeutic pathways of ghrelin for
gastrointestinal disorders. Nat Rev Gastroenterol Hepatol.
6:343–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hattori T, Yakabi K and Takeda H:
Cisplatin-induced anorexia and ghrelin. Vitam Horm. 92:301–317.
2013. View Article : Google Scholar : PubMed/NCBI
|