Introduction
The quantity, size and function changes of
erythrocytes have been demonstrated to be independent risk factors
for the development of cardiovascular diseases (1). Subsequent to general anesthesia,
patients presented a reduction in erythrocyte counts in their
peripheral blood, while the morphology of erythrocytes is also
altered (2,3). Erythrocytes are the main component of
the blood. The structure of mature erythrocytes is simple, without
a nucleus and other subcellular organelles (4). Notably, erythrocytes are the only
carrier of oxygen in the blood circulation and supply cells with
oxygen through deformation, adhesion and aggregation, while they
also regulate the body blood flow and affect the immune function
(5,6). Common pathological changes of
erythrocytes include decay and necrosis. Erythrocyte death and
phagocytosis by macrophages shorten the lifespan of erythrocytes
and cause thrombus, which may result in severe anemia (7,8), such as
uremia (9) or septicemia (10). The necrotic erythrocytes can release
free hemoglobin (Hb) in order to reduce the bioavailability of
nitric oxide (NO), causing changes in the biochemical metabolism,
function and morphology of erythrocytes and affecting the survival
and function of erythrocytes. Hydrogen peroxide
(H2O2), the primary active oxygen molecule in
the body, can easily penetrate the cell membrane under normal
physiological conditions (11,12), is
involved in signal transduction and has antimicrobial and
anti-inflammatory properties; however, an excess of
H2O2 induce hydroxyl radical oxidation and
erythrocyte damage due to severe hemolysis necrosis. A large number
of reactive oxygen species induce free radical damage to tissues
and organs, as well as vascular system dysfunction, which is
harmful to tissues and organs (13).
Sevoflurane, an agent used in anesthesia, has been
demonstrated to reduce the antioxidant capacity of erythrocytes and
release free radicals that damage erythrocytes (14). Whether sevoflurane contributes to the
H2O2-induced reduction of Hb in postoperative
patients has been seldom reported. When erythrocytes are treated
with H2O2 in vitro, the distribution
of phospholipids in the lipid bilayer of cell membrane is altered
(15). Thus,
H2O2 is usually used to imitate in
vivo oxidation-induced cell aging and pathological damages
(15). Thus, the present study used
this model to investigate the oxidative damage on erythrocytes,
which was induced by a low dose of H2O2 (200
µM) in vitro. The aim of the present study was to observe
the effects of sevoflurane on the antioxidant capacity, NO
metabolism and lifespan of erythrocytes, in the presence or absence
of H2O2.
Materials and methods
Materials
Fresh blood (12 ml) was collected from one healthy
34-year-old male volunteer at the Air Force General Hospital
(Beijing, China). Following centrifugation at 256 × g for 5 min at
4°C, the plasma was removed and the erythrocytes were obtained.
Ringer's solution containing 1% glucose (Fuzhou Maixin
Biotechnology Development Co., Ltd., Fuzhou, China) was added to
the erythrocytes, then the 2% erythrocyte suspension in Ringer's
solution was obtained. Next, the erythrocyte suspension was divided
into eight groups as follows: Group A, without any treatment (only
ventilation of air for 30 min; group S1, treated with 1%
sevoflurane (Jiangsu Hengrui Medicine Co., Ltd, Jiangsu, China);
group S3, treated with 3% sevoflurane; group S5, treated with 5%
sevoflurane; group A+H, treated with air +
H2O2 (no. 110618; Beijing Haiderun Pharmacy
Co., Ltd., Beijing, China); group S1+H, treated with 1% sevoflurane
+ H2O2; group S3+H, 3% sevoflurane +
H2O2; and group S5+H, 5% sevoflurane +
H2O2. In addition, a negative control group
(with distilled water replacing glucose and CaCl2) and
positive control group (with Ringer's solution replacing glucose
and CaCl2) were established for calculating the
hemolysis rate. The final concentration of
H2O2 in each corresponding group was 200
µmol/l. Each group was incubated at 37°C for 15 h, then Ringer's
solution was added, followed by incubation at 37°C for 3 h.
Subsequent to treatment, 2 ml erythrocyte suspension from each
group was sent to the laboratory of the Beijing Huaying
Biotechnology Institute (Beijing, China) in order to determine the
content of catalase (CAT) and endothelial NO synthase (eNOS). The
remaining erythrocyte suspension in each group was used to
determine the hemolysis rate using a spectrophotometer (RT-6000;
Shenzhen Leidu Electronics Co., Ltd., Shenzhen, China). The
hemolysis rate was calculated using the following formula:
Hemolysis rate (%) = (absorbance in the experimental group -
absorbance in the negative control group) / (absorbance in the
positive control group - absorbance in the negative absorbance)
×100. This study was conducted in accordance with the Declaration
of Helsinki and with approval from the Ethics Committee of the Air
Force General Hospital. Written informed consent was obtained from
all participants.
Flow cytometric analysis
Flow cytometric analysis was performed to
investigate the phosphatidylserine (PS) presentation and forward
scatter (FSC) of erythrocytes. Fluorescence-activated cell sorting
tubes (BD Biosciences, Franklin Lakes, NJ, USA) containing the
erythrocyte suspension were placed in a flow cytometer (BD
Biosciences), and each tube was marked by adding 2 ml binding
buffer (dilution, X10) (BD Biosciences). Subsequently, samples of
6×105 erythrocytes/tube were collected. Erythrocyte
suspension was labeled with fluorescein isothiocyanate (BD
Biosciences), and the labeled PS rate and FSC values were
determined using a flow cytometer (FACS420; BD Biosciences). The
results were analyzed using WinMDI version 2.9 (J. Trotter
1993–1998) software.
Statistical analysis
All data were presented as the mean ± standard
deviation and compared using the one-way analysis of variance
method. Experiments in each group were performed in four parallel
samples and each sample was analyzed in triplicate. All statistical
analyses were performed using SPSS version 13.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
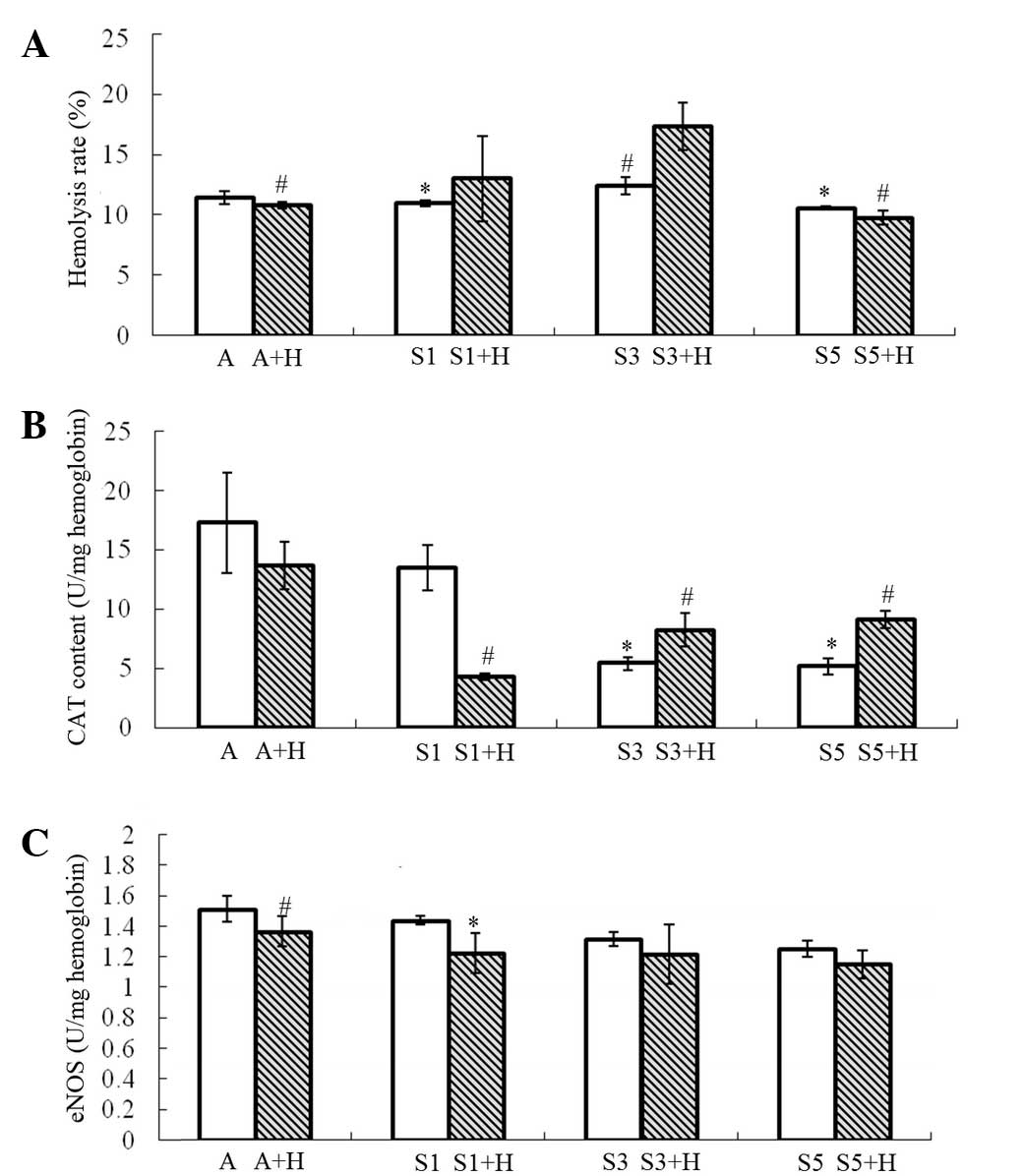
Hemolysis rate
Following treatment of the erythrocyte suspension
with different concentrations of sevoflurane, the hemolysis rates
in group S1, S3 and S5 (10.949±0.265, 12.417±0.716 and
10.561±0.128%, respectively) exhibited no significant differences
compared with group A (11.637±0.624%). However, the hemolysis rate
in group S3 was significantly higher compared with that in group S1
(P=0.038) and group S5 (P=0.017) (Fig.
1A). By contrast, upon addition of H2O2,
the hemolysis rate in group S3+H increased markedly
(17.384±1.976%), and was prominently higher compared with that in
group A+H (10.848±0.274%; P=0.007) and group S5+H (9.777±0.576%;
P=0.007, Fig. 1A). Furthermore, the
hemolysis rate in group S3+H was significantly higher compared with
that in group S3 (P=0.027; Fig.
1A).
CAT content
The CAT content of erythrocytes was found to be
reduced with increasing sevoflurane concentration. The CAT content
of erythrocytes in groups S3 and S5 (5.431±0.531 and 5.175±0.658
U/mg hemoglobin, respectively) decreased sharply, as compared with
that in group A (17.301±4.222 U/mg hemoglobin; P=0.009; Fig. 1B). Similarly, the CAT content of
erythrocytes in group S1 (13.534±1.906 U/mg hemoglobin) decreased
as well, but no statistically significant difference was observed
compared with that in group A (P=0.217). Upon addition of
H2O2, 1% sevoflurane was able to markedly
reduce the CAT content of erythrocytes (4.319±0.235 U/mg
hemoglobin), as compared with that in group A+H (13.689±2.003 U/mg
hemoglobin; P=0.002; Fig. 1B).
However, the CAT content of erythrocytes increased with increasing
concentration of sevoflurane (group S3+H, 8.257±1.389 U/mg
hemoglobin, and group S5+H, 9.156±0.742 U/mg hemoglobin); however,
the CAT content in groups S3+H and S5+H remained significantly
lower compared with that in group A+H (P<0.05; Fig. 1B). Furthermore, the CAT content in
group S1+H was markedly lower compared with that in group S1
(P<0.001), whereas it was notably higher in groups S3+H and S5+H
compared with that in groups S3 and S5, respectively (P<0.05;
Fig. 1B).
eNOS content
The eNOS content of erythrocytes was not evidently
affected by sevoflurane treatment alone. However, in the
H2O2 groups, it was significantly reduced by
1% sevoflurane (group S1+H; P=0.002), but not by 3 or 5%
sevoflurane (groups S3+H and S5+H; Fig.
1C). In the air-treated groups, H2O2
treatment markedly increased the eNOS content of erythrocytes
(P<0.001; Fig. 1C).
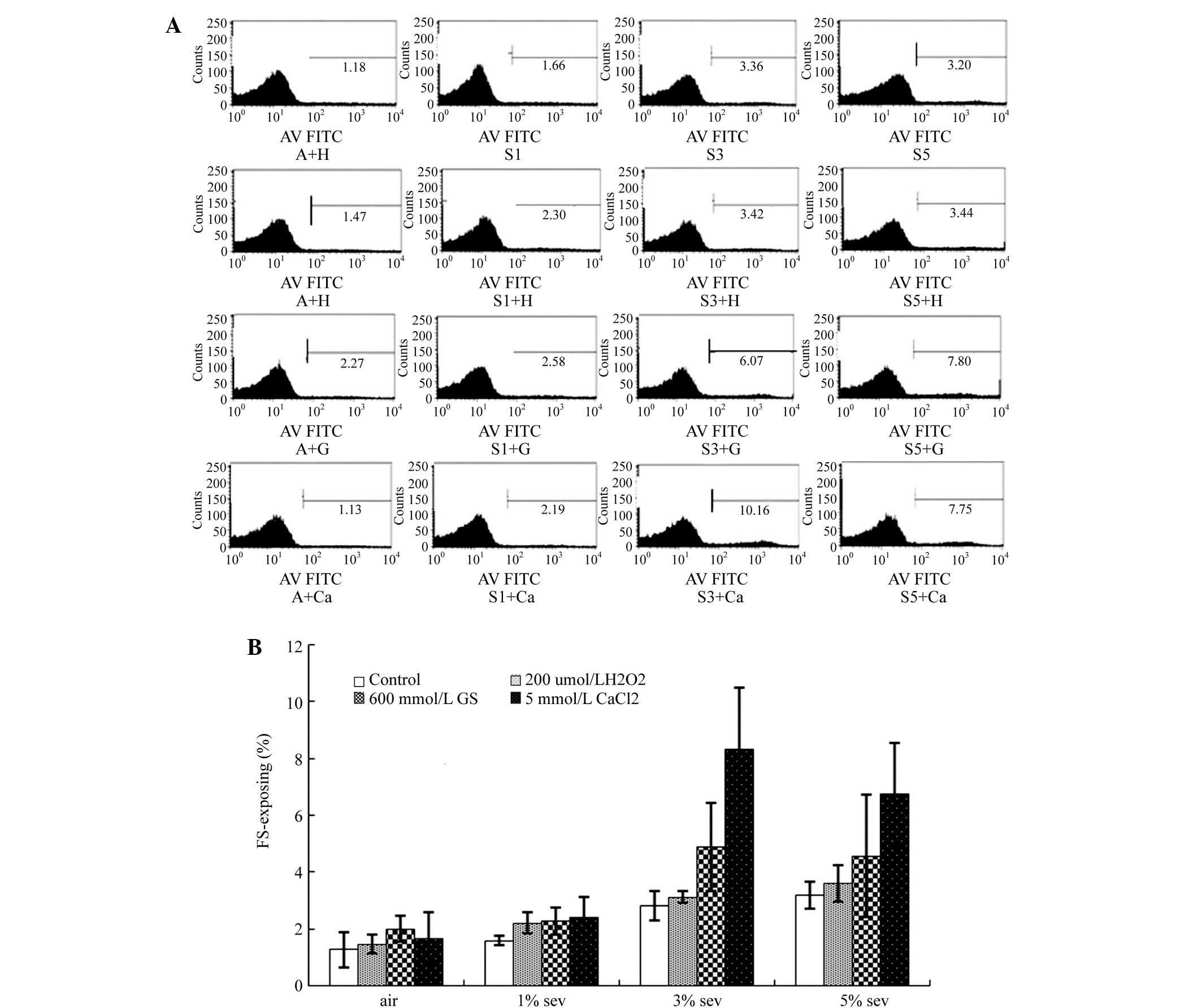
PS exposure
As compared with group A, treatment with 200 µmol/l
H2O2, 600 mmol/l glucose or 5 mmol/l
CaCl2 did not prominently increase the labeled PS rate.
By contrast, sevoflurane was found to increase the labeled PS rate
in a concentration-dependent manner (Fig. 2A and B). In the groups treated with
H2O2 or glucose, sevoflurane increased the
labeled PS rate, which reached a peak value upon treatment with 3%
sevoflurane (P<0.01), but then decreased. However, in the groups
treated with air or CaCl2, the labeled PS rate increased
gradually with increasing concentration of sevoflurane (Fig. 2A and B).
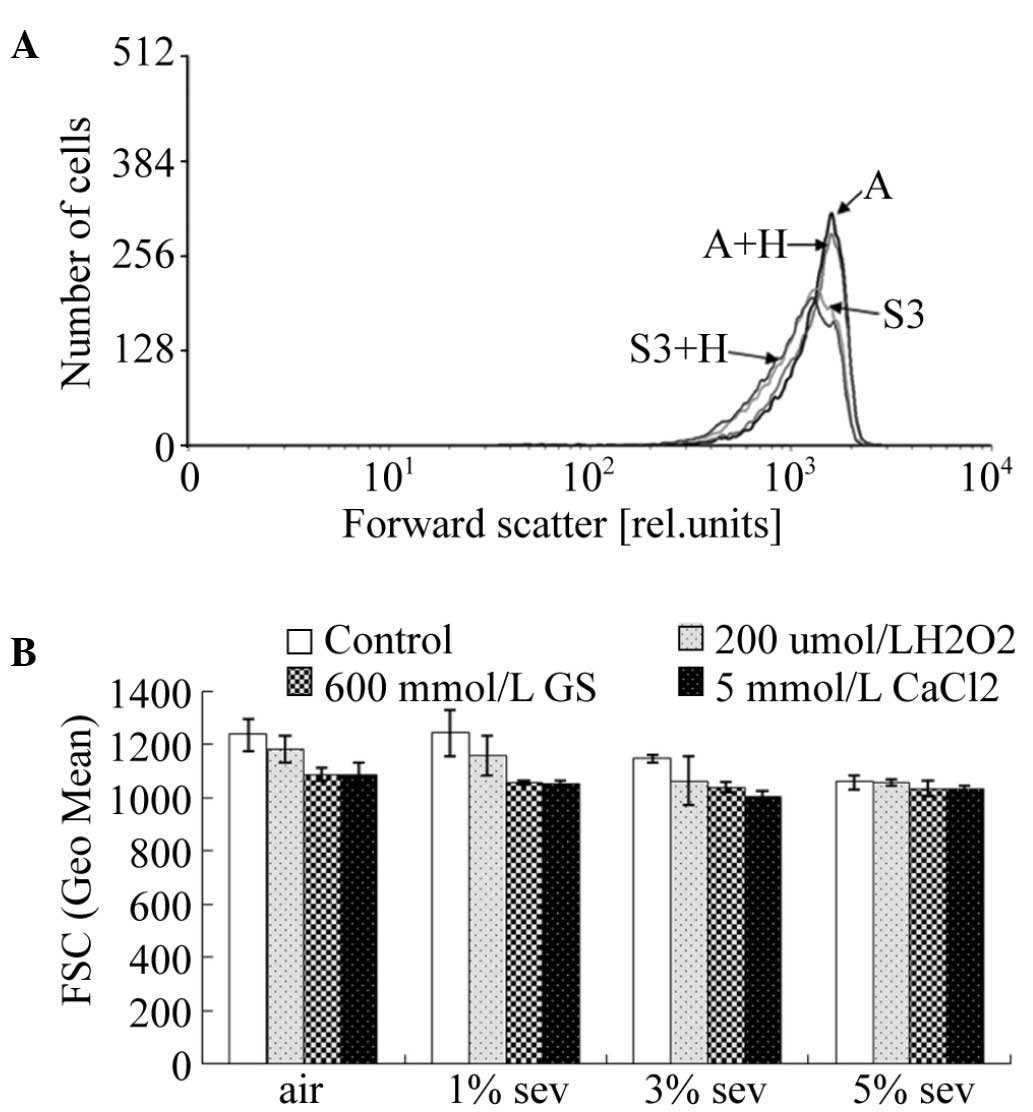
FSC
In order to investigate the effects of sevoflurane
on the volume of erythrocytes, the FSC of erythrocytes was also
determined by flow cytometry (Fig.
3A). Upon treatment of the erythrocyte suspension with air or
200 µmol/l H2O2, sevoflurane was able to
gradually reduce the FSC of erythrocytes (P<0.05). However, in
the groups treated with 600 mmol/l glucose or 5 mmol/l
CaCl2, sevoflurane exerted no significant effect on the
volume of erythrocytes (P>0.05; Fig.
3B).
Discussion
The primary source of reactive oxygen species in
erythrocytes is Hb. The underlying mechanism involves polarization
of the Fe-O bond (between heme iron and oxygen), after which Hb is
oxidized spontaneously and produces peroxide (16). CAT is a type of conjugase that uses
iron porphyrin as its prosthetic group and has a strong radical
scavenging function, which can protect the tissues from oxidative
damage (17). With the action of
CAT, H2O2 transforms into water and
O2, preventing H2O2 from reacting
with O2− and producing OH− in the
presence of iron chelating agents (16). When CAT inactivates
H2O2, its consumption increases and thereby
causes the deterioration of its activity. In endothelial cells, 200
µmol/l H2O2 can induce erythrocyte decay and
death in vitro, characterized by PS exposure and cell size
reduction. Its underlying mechanism mainly includes peroxidation
damage on erythrocytes caused by free radicals, which is induced by
H2O2 (18).
The present study identified that with the increase of the
sevoflurane concentration, the hemolysis rate of erythrocytes
increased initially and then showed a downward trend. The results
indicated that the CAT content of erythrocytes was significantly
reduced following sevoflurane treatment when compared with the air
group, and the reduction was positively correlated with the
concentration of sevoflurane; these findings have also been
confirmed in humans in a previous study (19). In terms of the hemolysis rate of
erythrocytes, there was no statistically significant difference in
the air group treated with or without H2O2.
In the presence of H2O2, sevoflurane had a
more significant effect on the hemolysis rate of erythrocytes, with
its effect reaching a peak at the concentration of 3% and then
reducing at a higher sevoflurane concentration. A previous study
reported that sevoflurane can also cause liver and kidney function
damage through the damage of red blood cells (20). Compared with intravenous anesthesia,
sevoflurane reduces the antioxidant capacity of erythrocytes
(21) and improves lipid
peroxidation by inhibiting the content of CAT and other antioxidant
enzymes, and thereby causing cell hemolysis and necrosis. However,
the effects of sevoflurane on animal and humans are not similar
(22), and thus, the results of
in vitro experiments should be generalized with caution.
As a relatively stable gas free radical, NO exerts a
dual biological function in humans. Under normal physiological
conditions, NO can adjust the normal physiological function of the
human body (23); however, NO is
harmful to the body when present at extremely high or low
concentrations in vivo (24).
NO can induce the production of CAT, strengthening the cell's
resistance against H2O2 (25). The classic pathway for the production
of NO depends on the activity of NOS, which gradually oxidizes
L-arginine into L-guanidine amino acid and produces NO. In
addition, NO is able to dilate blood vessels, relax the vascular
smooth muscle and inhibit the proliferation of endothelial cells
(26). Previous studies on
sevoflurane revealed its direct role in the inhibition of
endothelial cells, releasing NO (27). In the present study, the eNOS content
in group S1+H was lower compared with that in group A+H, indicating
that in the presence of H2O2, sevoflurane is
able to inhibit the activity of eNOS and thereby reduce the NO
content in red blood cells.
As detected by flow cytometry, the labeled rate of
PS represents the decay and death rate of erythrocytes (28), while changes in the FSC value
represent changes in the volume of erythrocytes, with a decreased
FSC value indicating a reduced cell size (29). In the present study, the labeled rate
of PS was found to increase with increasing concentration of
sevoflurane, while the FSC was found to be reduced. Furthermore, in
the presence of H2O2, the effect of
sevoflurane in reducing the erythrocyte antioxidative capacity was
improved. As a result, a high concentration of inhaled sevoflurane
is able to induce red blood cell decay and death, and reduce the
antioxidant capacity of erythrocytes.
H2O2, a metabolite of cells in
an aerobic environment, is a type of primary active oxygen molecule
with crucial biological functions, including its function as a
signaling molecule and the regulation of cell division,
differentiation, migration, aging or death (30). The oxidative stress or pathological
conditions inducing more H2O2, as well as the
defected or decreased anti-oxidation system of red blood cells, may
cause H2O2 to easily react with divalent
metal ions (such as Fe2+ and Cu2+) and form
hydroxyl free radicals (also known as the Fenton reaction) with
stronger oxidation capabilities, leading to oxidative damage on
cells (12). The degree of
H2O2-induced oxidative damage on cells mainly
depends on the strength of the oxidative stress factors. The
present study identified that a low dose of
H2O2 can stimulate red blood cells exposing
PS and result in cell size reduction, inducing red blood cell decay
and death in vitro.
Excessive amounts of free radicals induce oxidative
stress reaction in red blood cells, causing membrane lipid
peroxidation damage and protein denaturation and degradation
(31). Oxidative stress itself is
able to selectively oxidize aminophospholipids, in particular PS,
and cause their translocation and exposure, as well as cause
aminophospholipid translocase deactivation. In addition, oxidative
stress can activate the Fas-caspase signaling pathway and lead to
PS exposure (32). Exposed PS then
activates the blood clotting system, leading to local thrombosis or
ischemia. Thus, high concentration of sevoflurane inhaled can
induce PS exposure and cell size reduction, causing red blood cell
decay and death, and can reduce the antioxidant capacity of
erythrocytes.
In conclusion, sevoflurane is able to reduce the
antioxidative activity of erythrocytes, decreasing their ability to
resist H2O2 damage and increase their
hemolysis rate. The underlying mechanism may be associated with the
inhibitory effect of sevoflurane on the CAT activity in
erythrocytes. Furthermore, sevoflurane is able to inhibit the
generation of NO in erythrocytes, and reduce the tolerance of
erythrocytes against oxidative stress damage induced by
H2O2. The mechanism may be associated with
its inhibition of eNOS activity in erythrocytes. However, the
present study was conducted using an in vitro model. The
function of sevoflurane requires further study in vivo and
in clinical settings in order to evaluate potential hazards
associated with its use.
Acknowledgements
This study was supported by Youth Project of the
Chinese People's Liberation Army (No. 14QNP065).
References
|
1
|
Tsuchiya M, Asada A, Kasahara E, Sato EF,
Shindo M and Inoue M: Antioxidant protection of propofol and its
recycling in erythrocyte membranes. Am J Respir Crit Care Med.
165:54–60. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang XM, Liu J, Ji J and Xie J: Effects of
dexmedetomidine on the deformability of erythrocytes in vitro and
in anesthesia. Exp Ther Med. 7:1631–1634. 2014.PubMed/NCBI
|
|
3
|
Yerer MB, Aydoğan S and Comu FM:
Gender-related alerations in erythrocyte mechanical activities
under desflurane or sevoflurane anesthesia. Clin Hemorheol
Microcirc. 39:423–427. 2008.PubMed/NCBI
|
|
4
|
Berg CP, Engels IH, Rothbart A, Lauber K,
Renz A, Schlosser SF, Schulze-Osthoff K and Wesselborg S: Human
mature red blood cells express caspase-3 and caspase-8, but are
devoid of mitochondrial regulators of apoptosis. Cell Death Differ.
8:1197–1206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Young IS and Woodside JV: Antioxidants in
health and disease. J Clin Pathol. 54:176–186. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gutteridge JM: Lipid peroxidation and
anti-oxidants as biomarkers of tissue damage. Clin Chem.
41:1819–1828. 1995.PubMed/NCBI
|
|
7
|
Lang KS, Lang PA, Bauer C, Duranton C,
Wieder T, Huber SM and Lang F: Mechanisms of suicidal erythrocyte
death. Cell Physiol Biochem. 15:195–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boas FE, Forman L and Beutler E:
Phosphatidylserine exposure and red cell viability in red cell
aging and in hemolytic anemia. Proc Natl Acad Sci USA.
95:3077–3081. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lang F, Gulbins E, Lerche H, Huber SM,
Kempe DS and Foller M: Eryptosis, a window to systemic disease.
Cell Physiol Biochem. 22:373–380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kempe DS, Akel A, Lang PA, Hermle T,
Biswas R, Muresanu J, Friedrich B, Dreischer P, Wolz C, Schumacher
U, et al: Suicidal erythrocyte death in sepsis. J Mol Med (Berl).
85:273–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Halliwell B, Clement MV and Long LH:
Hydrogen peroxide in the human body. FEBS Lett. 486:10–13. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Veal E and Day A: Hydrogen peroxide as a
signaling molecule. Antioxid Redox Signal. 15:S147–S151. 2011.
|
|
13
|
Windsant Vermeulen IC, Hanssen SJ, Buurman
WA and Jacobs MJ: Cardiovascular surgery and organ damage, Time to
reconsider the role of hemolysis. J Thorac Cardiova Surgy.
142:1–11. 2011. View Article : Google Scholar
|
|
14
|
Türkan H, Aydin A, Sayal A and Karahalil
B: The effect of sevoflurane and desflurane on markers of oxidative
status in erythrocyte. Toxicol Ind Health. 27:181–186. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson RM, Goyette GJ, Ravindranath Y and
Ho YS: Hemoglobin autoxidation and regulation of endogenous H2O2
levels in erythrocytes. Free Radic Biol Med. 39:1407–1417. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida KI and Okabe EO: Selective
impairment of endothelium-dependent relaxation by sevoflurane:
Oxygen free radicals participation. Anesthesioloy. 76:440–447.
1992. View Article : Google Scholar
|
|
17
|
Njuma OJ, Ndontsa EN and Goodwin DC:
Catalase in peroxidase clothing, Interdependent cooperation of two
cofactors in the catalytic versatility of KatG. Arch Biochem
Biophys. 544:27–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benfeitas R, Selvaggio G, Antunes F,
Coelho PM and Sakvador A: Hydrogen peroxide metabolism and sensing
in human erythrocytes, A validated kinetic model and reappraisal of
the role of peroxiredoxin II. Free Radic Biol Med. 74:35–49. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Budić I, Pavlović D, Cvetković T,
Djordjević N, Simić D, Milojević I and Stojanović M: The effects of
different anesthesia techniques on free radical production after
tourniquet-induced ischemia-reperfusion injury at children's age.
Vojnosanit Pregl. 67:659–664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Masin-Spasovska J, Dimitrovski K,
Stavridis S, Stankov O, Dohcev S, Saidi S, Jakovski K, Balkanov T,
Labacevski N, Stankov V, et al: Acute fulminant hepatatis in kidney
transplant recipient after repeated sevoflurane anesthesia-a case
report and literature review. Curr Drug Saf. 8:141–144. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Budic I, Pavlovic D, Kocic G, Cvetkovic T,
Simic D, Basic J and Zivanovic D: Biomarkers of oxidative stress
and endothelial dysfunction after tourniquet release in children.
Physiol Res. 60((Suppl 1)): S137–S145. 2011.PubMed/NCBI
|
|
22
|
Soares JH, Brosnan RJ, Fukushima FB,
Hodges J and Liu H: Solubility of haloether anesthetics in human
and animal blood. Anesthesiology. 117:48–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo W, Cheng ZY and Zhu YZ: Hydrogen
sulfide and translational medicine. Acta Pharmacol Sin.
34:1284–1291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lo Faro ML, Fox B, Whatmore JL, Winyard PG
and Whiteman M: Hydrogen sulfide and nitric oxide interactions in
inflammation. Nitric Oxide. 41:38–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshioka Y, Kitao T, Kishino T, Yamamuro A
and Maeda S: Nitric oxide protects macrophages from hydrogen
peroxide-induced apoptosis by inducing the formation of catalase. J
Immunol. 176:4675–4681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cortese-Krott MM and Kelm M: Endothelial
nitric oxide synthase in red blood cells: Key to a new erythrocrine
function? Redox Biol. 2:251–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanna T, Akata T, Izumi K, Nakashima M,
Yonemitsu Y, Hashizume M and Takahashi S: Sevoflurane and
bradykinin-induced calcium mobilization in pulmonary arterial
valvular endothelial cells in situ, Sevoflurane stimulates
plasmalemmal calcium influx into endothelial cells. J Cardiovasc
Pharmacol. 40:714–724. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lupescu A, Bissinger R, Jilani K and Lang
F: In vitro induction of erythrocyte phosphatidylserine
translocation by the natural naphthoquinone shikonin. Toxins
(Basel). 6:1559–1574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirsch J, Menzebach A, Welters ID,
Dietrich GV, Katz N and Hempelmann G: Indicators of erythrocyte
damage after microwave warming of packed red blood cells. Clin
Chem. 49:792–799. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weigert A and Brüne B: Nitric oxide
apoptosis and macro-phagepolarization during tumor progression.
Nitric Oxide. 19:95–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perrone S, Tataranno ML and Stazzoni G:
DelV ecchio A and Buonocore G: Oxidative injury in neonatal
erythrocytes. J Matern Fetal Neonatal Med. 25((Suppl 5)):
S104–S108. 2012. View Article : Google Scholar
|
|
32
|
Tyurina YY, Tyurin VA, Zhao Q, Djukic M,
Quinn PJ, Pitt BR and Kagan VE: Oxidation of phosphatidyl-serine: A
mechanism for plasma membrane phospholipid scrambling during
apoptosis? Biochem Biophys Res Commun. 324:1059–1064. 2004.
View Article : Google Scholar : PubMed/NCBI
|