Introduction
Non-Hodgkin lymphoma (NHL) is a common
lymphoproliferative disease. Liver involvement, which defines an
advanced classification of the disease, occurs in 10% of patients
with NHL (1).
Although the liver contains lymphoid tissue, host
factors may render the liver inhospitable for the development of a
malignant lymphoma (2). Accordingly,
primary hepatic lymphoma (PHL) is a very rare type of malignancy,
accounting for ~0.016% of all NHL cases (3). The most common symptoms of PHL at
presentation are abdominal pain and general malaise. Additional
presentation symptoms may include B symptoms, which encompass
low-grade fever, fatigue, night sweats and weight loss. In
addition, a mass with or without jaundice can occur, and in
exceptional cases, fulminant hepatic failure has been observed
(4–7). The majority of cases of PHL derive from
B-cell lymphoma (88.6%) and histologically diffuse large B-cell
lymphoma (52.5%), whereas other histological types account for
<5% of PHL cases (4,8,9). The
pathogenesis of PHL is yet to be fully established, although
several possible factors, including viral infection, cirrhosis and
immunosuppressive drugs, have been implicated (2,3). Due to
the infrequency of PHL occurrence, the clinical and radiological
features of PHL have not been fully identified. Accordingly, PHL is
very difficult to diagnose accurately and is often misdiagnosed as
a hepatocellular carcinoma (HCC), metastatic tumor or liver
abscess. Therefore, the aim of the current study was to report the
clinical, radiological and histopathological analysis observations
of four PHL cases, and briefly review the literature.
Materials and methods
Patient selection and data
collection
A search of the medical records of patients
diagnosed with histopathologically confirmed PHL between October
2007 and May 2013 was conducted. Ultimately, four cases with
radiological evidence and a pathological diagnosis of PHL were
included in the study. All patient information concerning the
demographic data, clinical and laboratory presentations, underlying
diseases, imaging manifestations and pathological results were
recorded. The study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of Zhongnan Hospital of Wuhan University (Wuhan, China). Written
informed consent was obtained from all the participants.
Imaging technique
With the patient in the supine position, plain and
two-phase (arterial and portal vein phases) iodinated
contrast-enhanced computed tomography (CT) scans were obtained in a
craniocaudal direction using either of two scanners, namely the
Sensation 16 CT or Somatom Definition dual-source CT (Siemens
Medical Solutions, Erlangen, Germany). Routine scanning was
conducted at an 8-mm section thickness and a 5-mm scan increment;
scans were reconstructed with a 2-mm thickness using an appropriate
algorithm. A dual-syringe injector system (Medrad Medical Equipment
Trading Co., Ltd., Beijing, China) was used to intravenously
administer 100 ml non-ionic contrast media (Ultravist; 370 mgI/ml;
Bayer AG, Leverkusen, Germany) at a rate of 3 ml/sec, followed by a
20–30-ml saline chaser bolus. Magnetic resonance imaging (MRI)
scans were acquired using a Magnetom Trio 3.0T scanner (Siemens
Medical Solutions). Routine scanning of transverse sections was
performed with T2-weighted fast spin-echo sequences,
two-dimensional gradient echo in the axial plane, and T2-weighted
half-Fourier acquisition single-shot turbo spin echo without fat
saturation. A three-dimensional gradient echo sequence (VIBE) with
fat saturation was performed prior to and following the intravenous
bolus administration of gadopentetate dimeglumine (Magnevist;
Schering, Berlin, Germany) at a dose of 0.1 mmol/kg.
Retrospective imaging review
Two radiologists with >10 years experience
evaluated the imaging features, including the location, shape,
margination, homogeneity/heterogeneity, density, signal intensity
and enhancement pattern.
Histopathological review
One patient underwent a surgical resection and three
patients underwent an ultrasound-guided biopsy. All liver samples
were fixed in 10% neutral-buffered formalin and processed routinely
for paraffin embedding, followed by sectioning (4 µm) and staining
with hematoxylin and eosin. An immunohistochemical study was
performed in three patients.
Results
Clinical characteristics
All four patients were male, with ages ranging
between 38 and 64 years (average age, 56 years). Their basic
information and clinical characteristics are summarized in Table I. All four patients complained of
right upper abdominal pain and bloating, belching, discomfort,
fatigue and weight loss, among other symptoms, and all exhibited
elevated serum lactate dehydrogenase (LDH) levels. Two patients had
abnormal liver function test results, and one patient exhibited an
elevated β2-microglobulin level. The serum levels of
α-fetoprotein (AFP), carcinoembryonic antigen (CEA) and other tumor
markers were normal. In addition, the serology results were
negative for human immunodeficiency virus (HIV) and the hepatitis B
and C viruses (HBV and HCV; Table
I). All patients underwent plain CT, enhanced CT and
T1-weighted imaging (Figs.
1–4), and three patients
underwent immunohistochemistry.
 | Table I.Clinical characteristics and imaging
observations of the four patients. |
Table I.
Clinical characteristics and imaging
observations of the four patients.
| Case | Gender/age
(years) | Complaint | Laboratory results
(positive) | Imaging methods | Location | General shape | Margination | Numbers |
Homogeneity/heterogeneity | CT density | MRI signal
features | Enhancement | Histological
features |
|---|
| 1 | M/63 | Right upper abdominal
pain, abdominal bloating, belching | ALT, 54 U/l;
β2-MG, 5866.5 µg/l; LDH, 312 UI/ml | CT, MRI | Right lobe | Round | Distinct | Solitary | Homogeneous | Low | T1, hypointense T2,
hyperintense | Mild, vessels were
seen in the tumor | B-cell CD20 (+) |
| 2 | M/59 | Right upper abdominal
pain, fatigue, weight loss | ALT, 175 U/l; AST,
222 U/l; LDH, 441 UI/ml | CT, MRI | Quadrate lobe | Oval | Distinct | Solitary | Homogeneous | Low | T1, hypointense T2,
hyperintense | Moderate,
ring-like | T-cell CD3, CD5,
TIA.1, mum.1 (+) B-cell |
| 3 | M/38 | Right upper abdominal
pain and bloating | LDH, 297 UI/ml | CT | Right and left
lobe | Round | Distinct | Multifocal | Heterogeneous | Low |
| Mild | B-cell |
| 4 | M/64 | Right upper abdominal
discomfort | LDH, 423 UI/ml | CT | Right and left
lobe | Patchy | Vague | Multiple | Homogeneous | Low |
| Minimum | T-cell CD3, CD43,
vimentin (+) |
Imaging features
The four patients underwent plain and enhanced CT
evaluations. From the scans, one lesion was identified in the right
lobe of the liver, one lesion was located in the quadrate lobe,
while two lesions were located in the right and left lobes. One
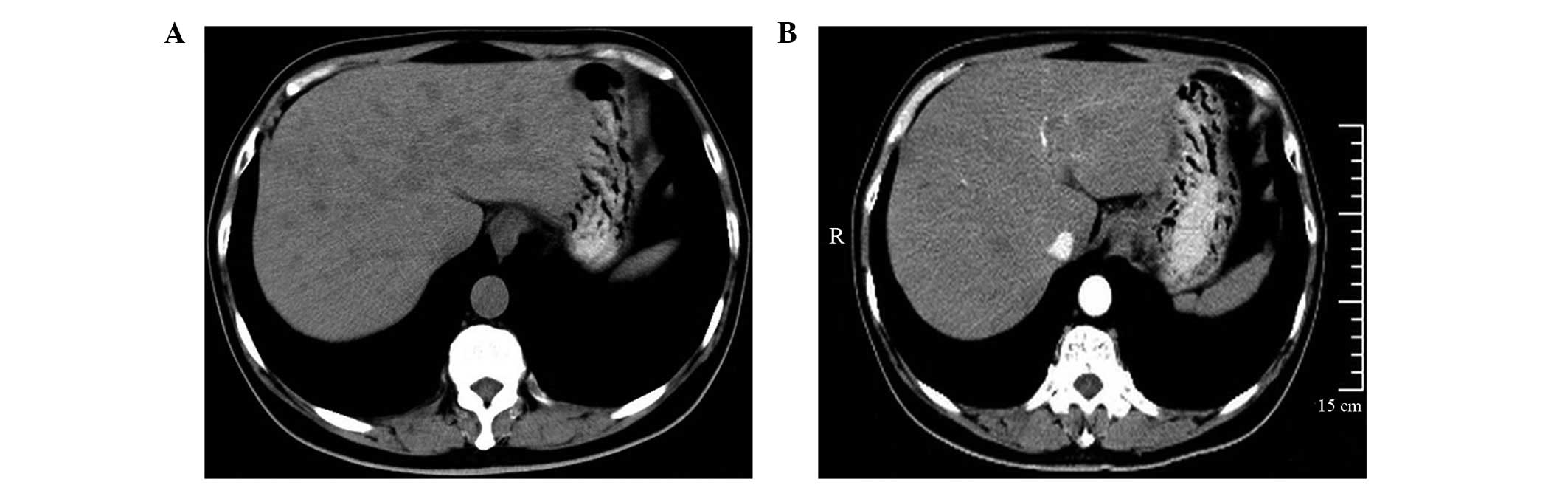
patient presented with diffuse, multi-speckled lesions (Fig. 1A), two patients presented with
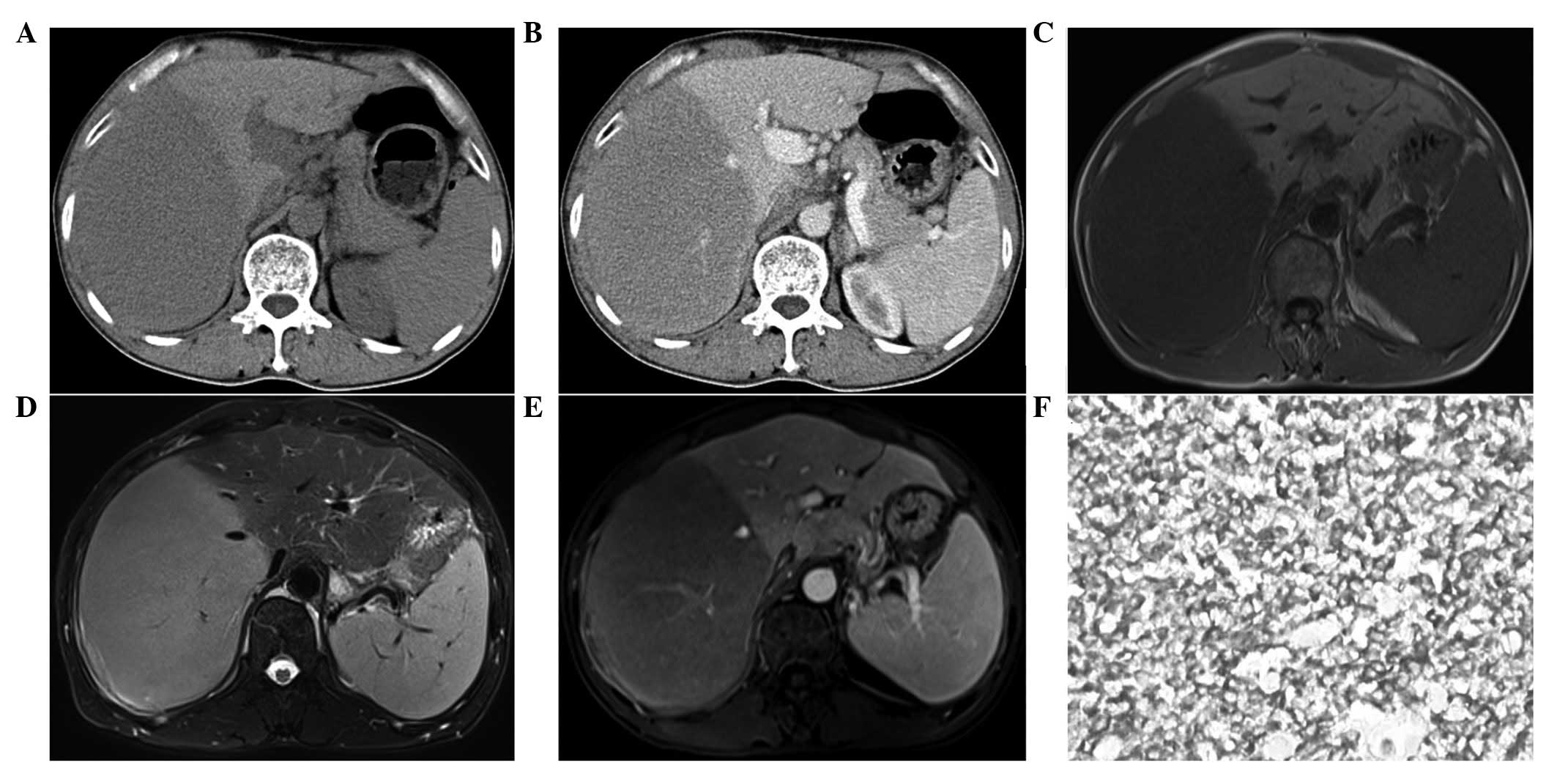
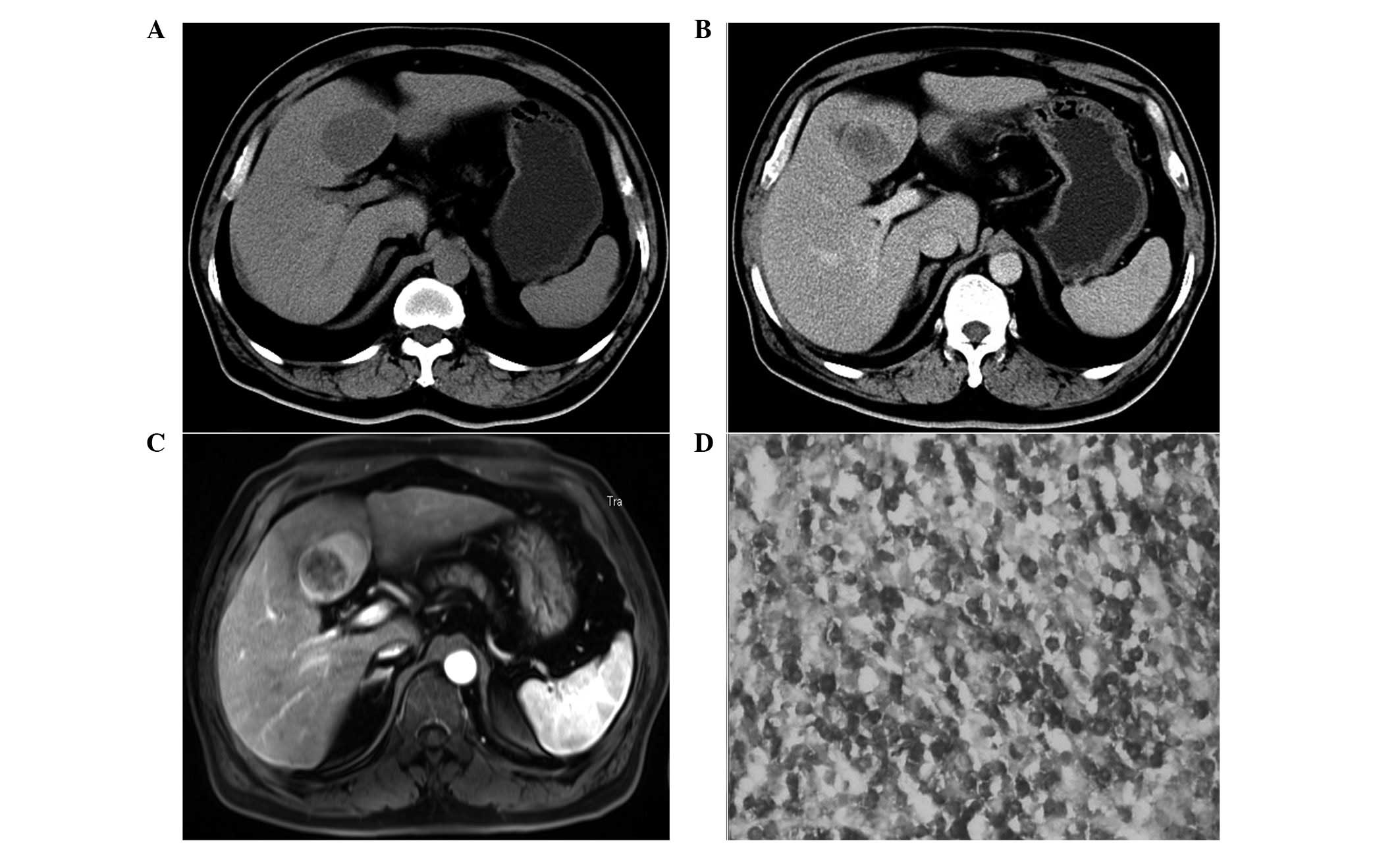
solitary masses (Figs. 2A and
3A), wheras the final patient
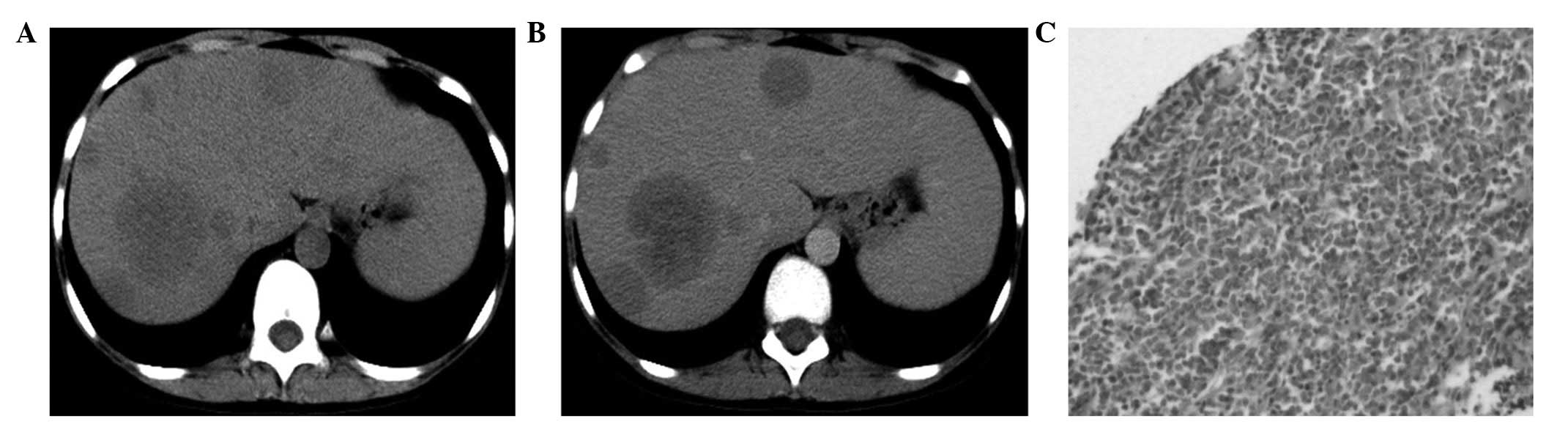
presented with multiple nodules and masses (Fig. 4A).
CT images were available for all four patients. All
the lesions appeared as low-density areas on the non-enhanced CT
scans. Three of the lesions were homogeneous and one lesion was
heterogeneous with a necrotic area in the center. On the
contrast-enhanced CT scans, two patients exhibited minimal or no
enhancement (Figs. 1B and 2B), one patient exhibited a moderate
ring-like reinforcement (Fig. 3B),
and one patient exhibited mild, uneven enhancement (Fig. 4B). In one lesion, normal blood
vessels were visible (Fig. 2B).
Two patients underwent MRI evaluations with
gadopentetate dimeglumine-based contrast enhancement. In these
images, two round masses with distinct edges were observed. On the
T1-weighted imaging (WI) scans, all the lesions appeared
hypointense. On the T2WI scans, all the lesions were moderately
hyperintense. Vessel signals were observed in one patient (Fig. 2C and D). On the post-contrast T1WI,
one lesion exhibited a mild and homogeneous pattern, in which the
enhanced liver vessels were visible (Fig. 2E), whereas another patient exhibited
a moderate and ring-like pattern of enhancement (Fig. 3C).
Pathological observations
Histopathological specimens were obtained from the
four patients via an ultrasound-guided biopsy or surgery. The
pathological sections showed diffuse lymphocytic infiltration in
the tissues (Fig. 4C). All the
patients were pathologically confirmed with a diagnosis of PHL.
Furthermore, two patients were classified as having the B-cell
type, while the remaining two patients were classified with the
T-cell type. Three patients underwent immunohistochemical
examinations, revealing positivity for B-cell or T-cell markers,
such as CD20 (Fig. 2F), CD3
(Fig. 3D), CD43, CD5, T
cell-restricted intracellular antigen 1 and melanoma-associated
antigen (mutated)-1 (Table I).
Discussion
According to the criteria outlined by Caccamo et
al, PHL is defined as a lymphoma with only liver involvement at
presentation, while there is absence of spleen, lymph node,
peripheral blood, bone marrow or other tissue involvement for at
least 6 months post-diagnosis (5).
Although the liver contains lymphoid tissue, host factors can
render the liver inhospitable for the development of a malignant
lymphoma. Thus, PHL is a rare condition (2), accounting for ~0.016% of all NHL cases
(3).
The pathogenesis of PHL is yet to be fully
established, although several possible etiological factors have
been proposed. For example, there is evidence of an association
between HCV infection and PHL. Previous studies have reported HCV
infection rates ranging between 20 and 60% among patients with PHL,
and this frequent association indicates that HCV may play a role in
the pathogenesis of PHL (10,11).
However, a number of conflicting theories exist with regard to the
association between HBV infection and PHL. Aozasa et al
(12) reported a 20% HBV surface
antigen positivity rate in a series of 69 patients with PHL,
whereas Yang et al (3)
reported a 33.3% HBV positivity rate in a series of nine patients
with PHL. However, Noronha et al (4) concluded that any association between
PHL and HBV was coincidental. PHL has also been associated with
several other viral infections, including HIV and Epstein-Barr
virus (EBV), inflammatory diseases, such as liver and primary
biliary cirrhosis, and immunological disorders, including
autoimmune diseases and immunosuppressive therapy (13,14).
However, none of the patients in the present study were found to
have HCV infection or presented with signs of immunodeficiency, as
determined by the negative serology results for active infection
with HIV, HBV, HCV and EBV. Therefore, PHL is speculated to occur
in patients without any liver disease.
Although PHL can occur at any age, the disease is
more frequently observed in the fifth or sixth decade of life, with
a male/female incidence ratio of 2–3/1 (15). In the present study, the majority of
patients were elderly males, in accordance with the previously
reported trend. The limited experience during the study revealed
that PHL exhibited non-specific clinical manifestations. The most
common symptoms of PHL at presentation are abdominal pain (39–70%)
and general malaise (4), along with
additional symptoms, such as low-grade fever, night sweats and
weight loss (more commonly known as B symptoms), nausea, vomiting
and itching, as observed in the four patients of the current study.
Liver function test results are abnormal in up to 70% of cases,
with increased levels of LDH observed in 30–80% of cases, and
increased expression levels of β2-microglobulin, a
well-described prognostic marker, observed in 90% of cases
(4,10,16,17). A
previous study also reported that dynamic changes in the serum LDH
level may serve as a diagnostic marker (14). However, the value of LDH as a
diagnostic marker of PHL is limited due to the low level of
specificity. The tumor markers, AFP and CEA, are present at normal
levels in almost 100% of patients with PHL, which assists in the
differential diagnosis (10). In the
present study, all the patients had elevated serum LDH levels and
normal AFP and CEA levels, whereas the alanine and aspartate
aminotransferase levels were elevated in two patients and the
β2-microglobulin level was elevated in one patient.
The imaging features of hepatic lymphoma are
non-specific. PHL can appear as a solitary lesion (39–60%), as
multiple lesions (25–40%), or as a diffuse infiltration of the
liver (18,19). However, diffuse infiltration is rare
and indicates a poor prognosis. Based on the imaging examinations
of the four patients in the present study, two cases had solitary
lesions, one patient had multi-speckled damage, and one case
exhibited multiple nodular or massive lesions. On CT scans, PHL
lesions appear as hypoattenuating lesions; half of these cases show
no enhancement following intravenous administration of a contrast
agent, whereas ~25% of cases exhibit a ring of enhancement
(4,7,20).
Classically, the MRI findings of PHL have been described as
hypointense or isointense on T1WI and hyperintense on T2WI
(4,20). The imaging findings of the cases in
the present study were similar to those reported in previous
studies.
Considering the rarity of this disease entity, the
non-specific clinical presentation and the non-specific laboratory
and radiological features, confirming a diagnosis of PHL is very
difficult. PHL can be confused with focal nodular hyperplasia, a
primary hepatic tumor and hepatic metastases, among other
conditions. Radiological and laboratory findings are extremely
useful in aiding the differentiation between PHL and other
diseases. In 80% of cases, HCC develops in the cirrhotic liver;
these patients are HBV- or HCV-positive and often have elevated AFP
levels. In addition, HCC usually presents as a hypervascular tumor
with marked enhancement in the arterial phase and washout in the
delayed phase (21). Hepatic
metastases are generally detected in patients with a history of a
primary tumor. Furthermore, focal nodular hyperplasia usually
appears as a hypointense or isodense lesion relative to the
surrounding liver on CT images, and as an isointense lesion on MRI
scans. These lesions are fairly homogeneous unless a central scar
is present, which typically appears as a hypodense lesion on CT
scans and bright on T2WI. This central scar, when present, is
highly specific. In multiphasic CT or MRI studies, focal nodular
hyperplasia typically exhibits rapid homogeneous contrast uptake in
the early arterial phase and a rapid return to near-normal
enhancement in the portal venous and delayed phases; however, the
central scar may be slightly enhanced during the delayed phase due
to the uptake of the contrast material by the fibroconnective
tissue (22–25). By contrast, PHL appears as a
low-density lesion on CT scans and exhibits no enhancement or
minimally patchy or ring-like enhancement on contrast-enhanced CT.
In the present study, the patients exhibited normal levels of AFP
and other tumor markers, and exhibited hypointensity on T1WI scans
and hyperintensity on T2WI scans. This combination of clinical and
laboratory features allows a speculative diagnosis of PHL. However,
a definite diagnosis requires histological results obtained via a
liver biopsy or surgical resection, and the absence of
lymphoproliferative disease outside the liver. The patients in the
present study underwent a surgical resection or liver biopsy, and
immunohistochemical staining of the liver biopsy samples with
specific antibodies confirmed the diagnosis of PHL.
Limitations of the present study included the design
as a retrospective study of only four cases, which relied primarily
on a review of the medical records. Therefore, future studies
should include a larger sample size, which may aid the
comprehension of the radiological and clinical spectra of this
disease presentation.
In conclusion, PHL is a notably rare disease that
lacks specific imaging findings, clinical manifestations and
biochemical markers, subsequently rendering the diagnosis of the
condition very difficult. A biopsy or surgical resection should be
performed in such cases, since only histological examination can
ensure an accurate differential diagnosis. The presentation of
clinical lymphoma B symptoms, an elevated serum LDH level, or
minimal/rim enhancement on contrast CT or MRI scans, particularly
in the absence of vessels with architectural distortion in the
lesion, greatly supports a diagnosis of PHL.
Acknowledgements
The authors thank Zhi-gao Xu for her assistance in
obtaining and reviewing the pathological sections. The present
study was supported by the Natural Science Foundation of Hubei
Province (grant no. 2012FFB04422).
References
|
1
|
Agmon-Levin N, Berger I, Shtalrid M,
Schlanger H and Sthoeger ZM: Primary hepatic lymphoma, a case
report and review of the literature. Age Ageing. 33:637–640. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu YD, Kim DS, Byun GY, et al: Primary
hepatic marginal zone B cell lymphoma: A case report and review of
the literature. Indian J Surg. 75(Suppl 1): 331–336. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang XW, Tan WF, Yu WL, Shi S, Wang Y,
Zhang YL, Zhang YJ and Wu MC: Diagnosis and surgical treatment of
primary hepatic lymphoma. World J Gastroenterol. 16:6016–6019.
2010.PubMed/NCBI
|
|
4
|
Noronha V, Shafi NQ, Obando JA and Kummar
S: Primary non-Hodgkin's lymphoma of the liver. Crit Rev Oncol
Hematol. 53:199–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caccamo D, Pervez NK and Marchevsky A:
Primary lymphoma of the liver in the acquired immunodeficiency
syndrome. Arch Pathol Lab Med. 110:553–555. 1986.PubMed/NCBI
|
|
6
|
Cameron AM, Truty J, Truell J, et al:
Fulminant hepatic failure from primary hepatic lymphoma: successful
treatment with orthotopic liver transplantation and chemotherapy.
Transplantation. 80:993–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elsayes KM, Menias CO, Willatt JM, Pandya
A, Wiggins M and Platt J: Primary hepatic lymphoma, imaging
findings. J Med Imaging Radiat Oncol. 53:373–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gomyo H, Kagami Y, Kato H, et al: Primary
hepatic follicular lymphoma: a case report and discussion of
chemotherapy and favorable outcomes. J Clin Exp Hematop. 47:73–77.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeuchi N and Naba K: Primary hepatic
lymphoma is difficult to discriminate from a liver abscess. Case
Rep Gastrointest Med. 2014:9253072014.PubMed/NCBI
|
|
10
|
Page RD, Romaguera JE, Osborne B, et al:
Primary hepatic lymphoma: favorable outcome after combination
chemotherapy. Cancer. 92:2023–2029. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bronowicki JP, Bineau C, Feugier P, et al:
Primary lymphoma of the liver: clinical pathological features and
relationship with HCV infection in French patients. Hepatology.
37:781–787. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aozasa K, Mishima K and Ohsawa M: Primary
malignant lymphoma of the liver. Leuk Lymphoma. 10:353–357. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santos ES, Raez LE, Salvatierra J,
Morgensztern D, Shanmugan N and Neff GW: Primary hepatic
non-Hodgkin's lymphomas, case report and review of the literature.
Am J Gastroenterol. 98:2789–2793. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masood A, Kairouz S, Hudhud KH, Hegazi AZ,
Banu A and Gupta NC: Primary non-Hodgkin lymphoma of liver. Curr
Oncol. 16:74–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haider FS, Smith R, Khan S and Rahman O:
Primary hepatic lymphoma presenting as fulminant hepatic failure
with hyperferritinemia, a case report. J Med Case Reports.
2:2792008. View Article : Google Scholar
|
|
16
|
Lei KI: Primary non-Hodgkin's lymphoma of
the liver. Leuk Lymphoma. 29:293–299. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Avlonitis VS and Linos D: Primary hepatic
lymphoma: a review. Eur J Surg. 165:725–729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Levy AD: Malignant liver tumors. Clin
Liver Dis. 6:147–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cerban R, Gheorghe L, Becheanu G, Serban V
and Gheorghe C: Primary focal T-cell lymphoma of the liver, a case
report and review of the literature. J Gastrointestin Liver Dis.
21:213–216. 2012.PubMed/NCBI
|
|
20
|
Maher MM, McDermott SR, Fenlon HM, et al:
Imaging of primary non-Hodgkin's lymphoma of the liver. Clin
Radiol. 56:295–301. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hussain SM, Semelka RC and Mitchell DG: MR
imaging of hepatocellular carcinoma. Magn Reson Imaging Clin N Am.
10:31–52. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mortelé KJ, Praet M, Van Vlierberghe H,
Kunnen M and Ros PR: CT and MR imaging findings in focal nodular
hyperplasia of the liver, radiologic-pathologic correlation. AJR Am
J Roentgenol. 175:687–692. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Terkivatan T, van den Bos IC, Hussain SM,
Wielopolski PA, de Man RAI and Jzermans JN: Focal nodular
hyperplasia: lesion characteristics on state-of-the-art MRI
including dynamic gadolinium- enhanced and superparamagnetic
iron-oxide-uptake sequences in a prospective study. J Magn Reson
Imaging. 24:864–872. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hussain SM, Terkivatan T, Zondervan PE,
Lanjouw E, de Rave S, Ijzermans JN and de Man RA: Focal nodular,
hyperplasia, findings at state-of-the-art MR imaging, US, CT and
pathologic analysis. Radiographics. 24:3–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blachar A, Federle MP, Ferris JV, et al:
Radiologists' performance in the diagnosis of liver tumors with
central scars by using specific CT criteria. Radiology.
223:532–539. 2002. View Article : Google Scholar : PubMed/NCBI
|