Introduction
Mesenchymal stem cells (MSCs) are multipotent cells
which can proliferate and differentiate into numerous cell types,
including adipocytes, hepatocytes, epithelial cells and islet cells
(1). With rapid cell proliferation,
low immunogenicity and the potential for straightforward
transfection of foreign genes, MSCs have been extensively used as
seed cells for tissue engineering (2). Previously, studies have demonstrated
that MSCs can be transplanted to repair damaged tissues, with the
potential for more valuable therapeutic research and clinical
treatments to be conducted through investigating the biological
characteristics of MSCs (3).
In the current study, the expression of a number of
genes and proteins, including vimentin, fibronectin, CD73, CD71,
CD44, CD34, CD29, CD45, Pax2 and CD166, were investigated in
Beijing duck MMSCs. CD29 is an integrin unit associated with late
antigen receptors, which can form a heterodimer with surface and
extracellular proteins to mediate cell-cell and cell-matrix
interactions (4). CD44 is a cell
surface glycoprotein, known as an adhesion molecule. It can bind to
collagen type I and fibronectin, and provide growth-anchoring sites
for MSCs. CD71 is a member of the transferrin receptor family that
is required for the import of iron into cells and is regulated in
response to intracellular iron concentrations. In the event of low
cellular iron concentration, the levels of transferrin receptors
increase, thus causing an increase in the uptake of iron by the
cells. Therefore, the transferrin receptor maintains cellular iron
homeostasis (5).
Although metanephric mesenchymal stem cells (MMSCs)
from humans, rats and livestock have been obtained and
characterized, there are few reports of harvesting duck MMSCs
(6). The Beijing duck is a
domesticated Aves Anseriformes Anatidae (from the mallard duck
species, Anas platyrhynchos), with a stable hereditary
character and excellent fertility. The Beijing duck embryo
metanephron can be obtained in a convenient, economic way without
ethical or histocompatibility problems, or immune rejection
(7). MMSCs have broad preclinical
application prospects, such as cell transplant or tissue
engineering (8).
In the present study, the self-renewal and
differentiation capabilities and gene expression patterns in
Beijing duck MMSCs were analyzed through in vitro cell
culture for the first time, to the best of our knowledge.
Materials and methods
Experimental animals
All animal procedures were approved by the
Institutional Animal Care and Use Committee of The Chinese Academy
of Agricultural Sciences (Beijing, China). In total, 300 Beijing
duck embryos (20 day-old) were provided by the Animal Husbandry
Experimental Base Institute of Animal Sciences, Chinese Academy of
Agricultural Sciences.
Isolation and culture of MMSCs
Enzymatic digestion was used as a stable method to
harvest MMSCs from metanephric tissues. Initially, metanephros
cells were collected from 20-day-old Beijing duck embryos. The duck
metanephros were exposed and ureteric buds were removed subsequent
to washing with phosphate-buffered saline (PBS; Sigma-Aldrich,
Santa Clara, CA, USA). Tissue blocks were cut into 1-mm3
pieces and digested with 0.1% collagenase type IV (Sigma-Aldrich)
for 25 min at 37°C, then neutralized with equal DMEM/F-12
containing 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher
Scientific, Inc., New York, NY, USA). The cell suspension was
filtered through a 300 mesh stainless steel sieve and centrifuged
at 250 × g for 8 min, then added to complete medium [DMEM/F-12, 10%
FBS, 10 ng/ml leukemia inhibitory factor (LIF; Peprotech, Rocky
Hill, NJ, USA)] and seeded into plates, incubated at 37°C with 5%
CO2 (9). The non-adherent
cells and fragments were removed with PBS 24 h post-seeding. When
cells reached 80% confluence, 0.125% trypsin and 0.02% EDTA
(Sigma-Aldrich) were added for subculturing. Purified MMSCs were
obtained after 3 passages (10).
MTS cell viability assay
P5 generation cells were inoculated into 96-well
plates at a cell density of 1.0×104 cells/ml. Following
the treatment period, the cytotoxicity assay was performed using
MTS reagent [3-(4,
5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium,
inner salt] according to the manufacturer's protocol (Promega
Corp., Beijing, China). Cell absorbance was spectrophotometrically
measured using an ELx800 absorbance microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA) at 490 nm (11). A growth curve was produced using the
average cell count data for each day of the 7-day study (12).
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
RNA was extracted from cells using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
cDNA was synthesized using an RNA PCR kit (Takara Biotechnology
Co., Ltd., Dalian, China) (13). The
cDNA was amplified by PCR with specific primers (designed by Sangon
Biotech, Shanghai, China; Table I),
using a Platinum PCR SuperMix (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. PCR
was performed in a 20 µl solution containing 2.0 µl 10X RT buffer,
13.4 µl double-distilled H2O, 0.2 µl Ex-Taq (Takara Bio
Inc., Otsu, Japan), 1.0 µl each of forward and reverse primers, 1.0
µl template cDNA and 1.4 µl dNTP (2.5 mM). The reaction conditions
consisted of an initial denaturation step at 94°C for 5 min,
followed by 30 cycles at 94°C for 30 sec, 55–60°C for 30 sec and
72°C for 30 sec, and a final cycle at 72°C for 10 min. The PCR
products were visualized by 2.5% agarose gel electrophoresis
(Gibco; Thermo Fisher Scientific, Inc.) at 140 V for 30 min
(14).
 | Table I.Primer sequences for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-polymerase chain reaction.
| Gene name | Primer
sequence | Tm
(°C) | Product length
(bp) |
|---|
| Fibronectin | F:
5′-CCTGTGTTCTGCCTTTCACC-3′ | 58 | 251 |
|
| R:
5′-GTTGTCTCTCCGTCCCTCAG-3′ |
|
|
| CD71 | F:
5′-GCTCGTGTGAATCCTGAACC-3′ | 58 | 290 |
|
| R:
5′-TATTGGAGGGCTGCTGTTG-3′ |
|
|
| CD34 | F:
5′-CTCAACGAGTCCAACACCTG-3′ | 60 | 338 |
|
| R:
5′-CCAGAAGTGACCAAAGCAGTC-3′ |
|
|
| CD29 | F:
5′-CACTCCCGTGCTGTGAATC-3′ | 60 | 257 |
|
| R:
5′-ACGCTGCTCATTTCCAACTC-3′ |
|
|
| GAPDH | F:
5′-GCACTGAACGACCATTTCG-3′ | 58 | 256 |
|
| R:
5′-CAGGTGGAGGAAGAAGTTGG-3′ |
|
|
| PPAR-γ | F:
5′-GCAGGAGATCACAGAATTTGA-3′ | 58 | 356 |
|
| R:
5′-TTGGGCTCCATAAAGTCACA-3′ |
|
|
| LPL | F:
5′-AGCTCTGAGTCTGATTGCTG-3′ | 58 | 256 |
|
| R:
5′-AATGGCTGGTTGGTCTTGGT-3′ |
|
|
| AFP | F:
5′-AACGATTGCTTTCTCTCCCTTA-3′ | 58 | 283 |
|
| R:
5′-TCACTACCTTTGGTGCCTGTC-3′ |
|
|
| ALB | F:
5′-GGCAAGGAAACTGGCATAAG-3′ | 58 | 317 |
|
| R:
5′-TCCACAATGGGCTTCTCAC-3′ |
|
|
| E-cadherin | F:
5′-TGCCACCAGTCAAGAAAGTG-3′ | 60 | 252 |
|
| R:
5′-ACCATTATCAACAGCCACGA-3′ |
|
|
| CK19 | F:
5′-ATCCTTGCTGCCACTATCG-3′ | 58 | 251 |
|
| R:
5′-GCACTCATTTCCTCCTCGTG-3′ |
|
|
| CD45 | F:
5′-CTCACCACACGCACTCTCAC-3′ | 60 | 350 |
|
| R:
5′-CTCTTCCCATCTTCCAGCAG-3′ |
|
|
| Pax2 | F:
5′-ATCTGCGACAACGACACG-3′ | 60 | 262 |
|
| R:
5′-CCTCGGACACATCTTCATCA-3′ |
|
|
| CD166 | F:
5′-AAGAAGACCTGCGTAACTGGA-3′ | 60 | 295 |
|
| R:
5′-CCTGCTAATGCCACGAGAGT-3′ |
|
|
| PDX-1 | F:
5′-GCTAATGAATACCGCAGACAGA-3′ | 58 | 314 |
|
| R:
5′-GAAGCAAAGGTTGGAATAGGC-3′ |
|
|
| Insulin | F:
5′-TGGAAGTGCGAAAGACACAC-3′ | 58 | 303 |
|
| R:
5′-GGTGAAAGGCAGAACACAGG-3′ |
|
|
Immunofluorescence analysis of MMSC
surface antigens
Cells were fixed with 4% paraformaldehyde
(Sigma-Aldrich) for 20 min at room temperature and washed three
times (every 5 min), permeabilized by 0.25% Triton X-100
(Sigma-Aldrich, St. Louis, MO, USA) for 10 min, which was diluted
with PBS (1:10), blocked with goat serum (OriGene Technologies,
Beijing, China) for 60 min (15).
The following antibodies were added: Rabbit anti-chicken antibodies
against fibronectin, CD71 and CD73 (dilution, 1:100; cat. nos.
bs-4859R, bs-1782R and bs-4834R, respectively; Beijing Biosynthesis
Biotechnology Co. Ltd., Beijing, China), and mouse anti-chicken
antibodies against CD29 and CD45 (dilution, 1:100; cat. nos.
ab26841 and ab24909, respectively; Abcam, Cambridge, CA, USA). The
samples were incubated with the antibodies overnight at 4°C. The
primary antibody was discarded and washed, then the fluorescein
isothiocyanate (FITC)-conjugated goat anti-rabbit and goat
anti-mouse secondary antibodies (dilution, 1:100; cat. no. ZF-0311;
OriGene Technologies) were added. The cells were then placed in the
dark for 1 h at room temperature (16). The plates were washed and nuclear
staining was performed with 1 µg/ml DAPI (Sigma-Aldrich) for 30 min
(17). Immunofluorescence images
were acquired using a laser-scanning confocal microscope (FV1000;
Olympus Corp., Tokyo, Japan). Non-overlapping fields were observed
and images were captured.
Flow cytometry analysis of MMSCs
The flow cytometry protocol used was similar to the
immunofluorescence protocol described earlier, in terms of the cell
preparation and treatment. However, the selected polyclonal
antibodies used were as follows: Anti-vimentin, anti-CD44 and
anti-CD71 antibodies (all rabbit anti-chicken antibodies; dilution,
1:100; cat. nos. bs-0756R, bs-2507R and bs-1782R, respectively;
Beijing Biosynthesis Biotechnology Co. Ltd.). Flow cytometric
analysis was performed on the mononuclear cell suspension, and
5×104 cells were incubated with 10 µl FITC-conjugated
antibodies (18). Cells were
acquired using a FACSCalibur flow cytometer and CytExpert Cell
Quest software, and then analyzed with Paint-A-Gate software (BD
Biosciences, San Jose, CA, USA).
Frozen storage and recovery
For freeze storage, 50% DMEM/F-12, 10% dimethyl
sulfoxide and 40% FBS were used, and for long-term storage a liquid
nitrogen tank (19) was used to
preserve the genetic resources of the Beijing duck.
Differentiation of MMSCs
MMSCs with high reproductive ability were targeted
for differentiation. When cell proliferation attained 50%
confluence, cells were randomly divided into induced and control
groups. The complete medium was used for the control group.
The induction medium for adipocyte differentiation
consisted DMEM/F-12, 10% FBS, 10-7M dexamethasone, 8 µg/ml insulin,
70 µM indomethacin (Sigma-Aldrich), 0.5 mM 3-1-methyl
isobutyl-xanthine (IBMX) and 1% glutamine supplement (Gibco; Thermo
Fisher Scientific, Inc.) The induction was conducted for 6, 9 and
12 days. Accumulated oil droplets were detected by oil red O
staining (Peprotech) (20).
Hepatocyte differentiation was induced using the
following media: DMEM/F-12 containing 5% FBS, 40 nmol/ml
dexamethasone, 20 ng/ml fibroblast growth factor (FGF)-4, 20 ng/ml
hepatocyte growth factor (HGF), 10 ng/ml epidermal growth factor
(EGF) (PeproTech), 1% insulin-transferrin-selenium-ethanolamine
(ITS-X) and 1% 200 mM L-glutamine (Gibco; Thermo Fisher Scientific,
Inc.) for 8, 12 and 15 days (21).
Periodic acid-Schiff staining was conducted for hepatic glycogen,
following the manufacturer's instructions.
For epithelial cell differentiation, cells were
treated with DMEM/F-12 containing 10% FBS, 20 ng/ml EGF, 20 ng/ml
bone morphogenetic protein (BMP)-7, 15 ng/ml insulin-like growth
factor (IGF), 10 ng/ml LIF for 8, 10 and 12 days.
Immunofluorescence analysis of polyclonal anti-CK18 and anti-CK19
antibodies (rabbit anti-chicken; dilution, 1:100; cat. nos.
bs-1339R and bs-2190R, respectively; Beijing Biosynthesis
Biotechnology Co. Ltd.) was performed (22).
The following pre-induction medium was used for
islet differentiation: DMEM/F-12 containing 10 ng/ml bFGF, 10 ng/ml
EGF, 2% B27 (Sigma-Aldrich) for 3 days, the terminal induction
medium used was DMEM/F-12 containing 15 ng/ml HGF, 20 ng/ml activeA
(Sigma-Aldrich), 1 mM β-mercaptoethanol (Sigma-Aldrich), 15 mM
niacinamide (Gibco; Thermo Fisher Scientific, Inc.) and 2% B27.
Dithizone staining (Peprotech) was performed to identify islet-like
cells (23).
Following induction of the MMSCs, RNA extraction and
RT-PCR identification were performed simultaneously, with specific
marker genes used for different cell types. Gene-specific primer
pairs are listed in Table I. GAPDH
was used as the loading control.
Results
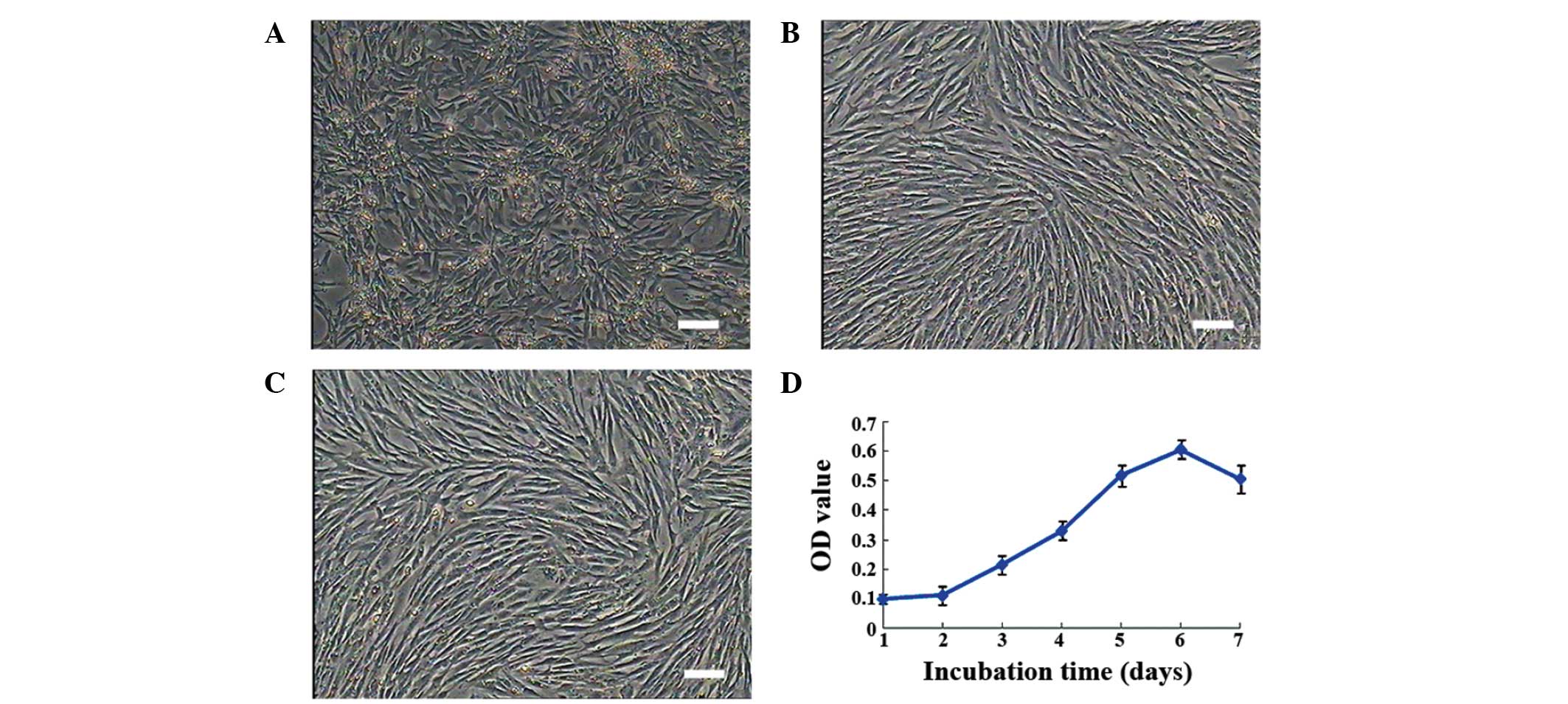
Morphological characterization of duck
MMSCs
Primary cells began to adhere to plates at 16 h and
proliferate 48 h later. After 3 passages, treatment with trypsin
for a set period of time resulted in a purer population of MMSCs.
Cells presented a fibroblast-like morphology, with long polygonal
shapes, expanding rapidly until passage 15. Subsequent to this,
cells presented signs of senescence (Fig. 1A–C).
Growth curve
After 2 days of incubation, cellular growth follows
an exponential pattern. Subsequent to day 6, growth begins to
decline. Duck MMSCs follow the typical logistic growth pattern
after 3 generations (Fig. 1D).
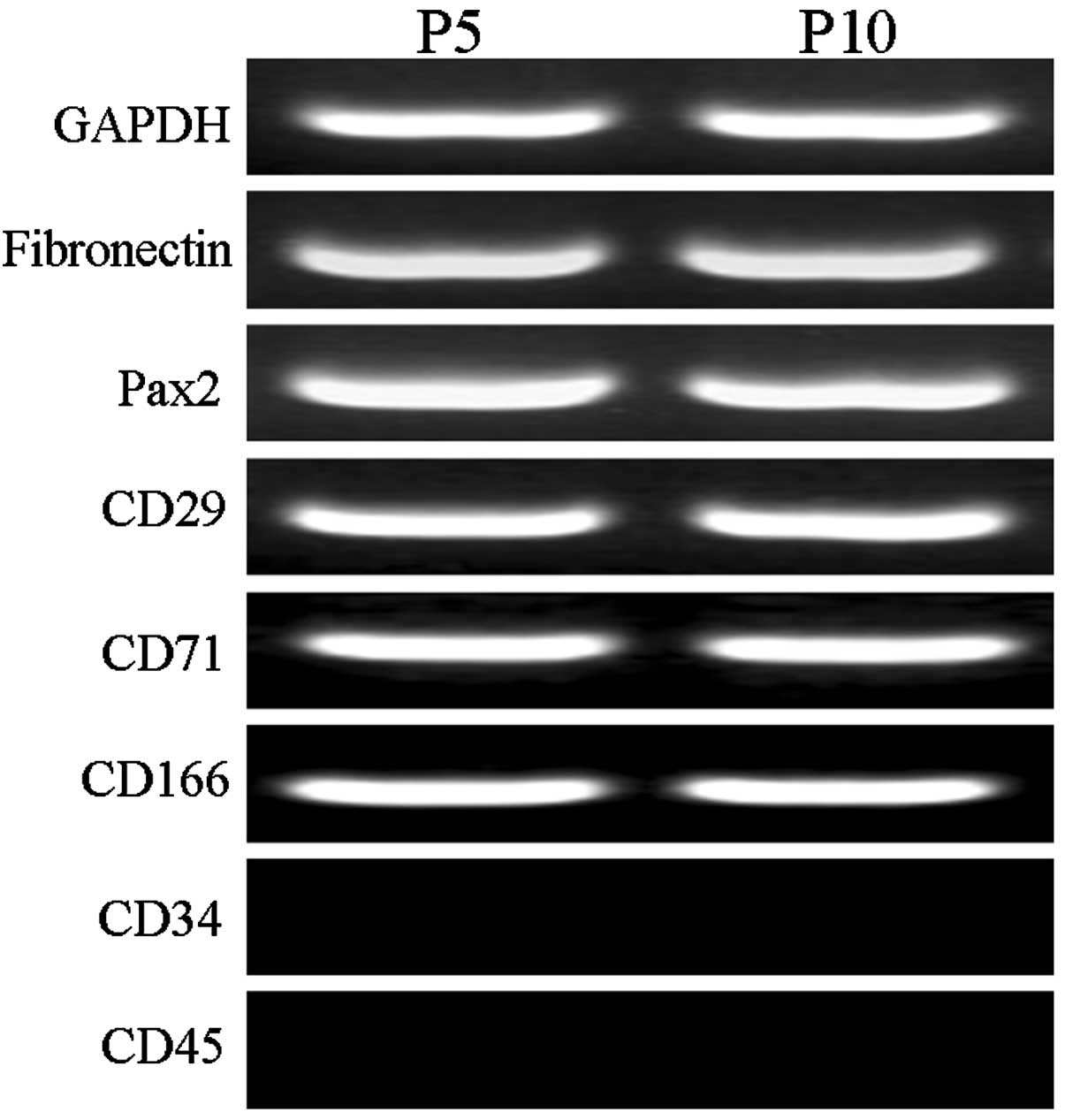
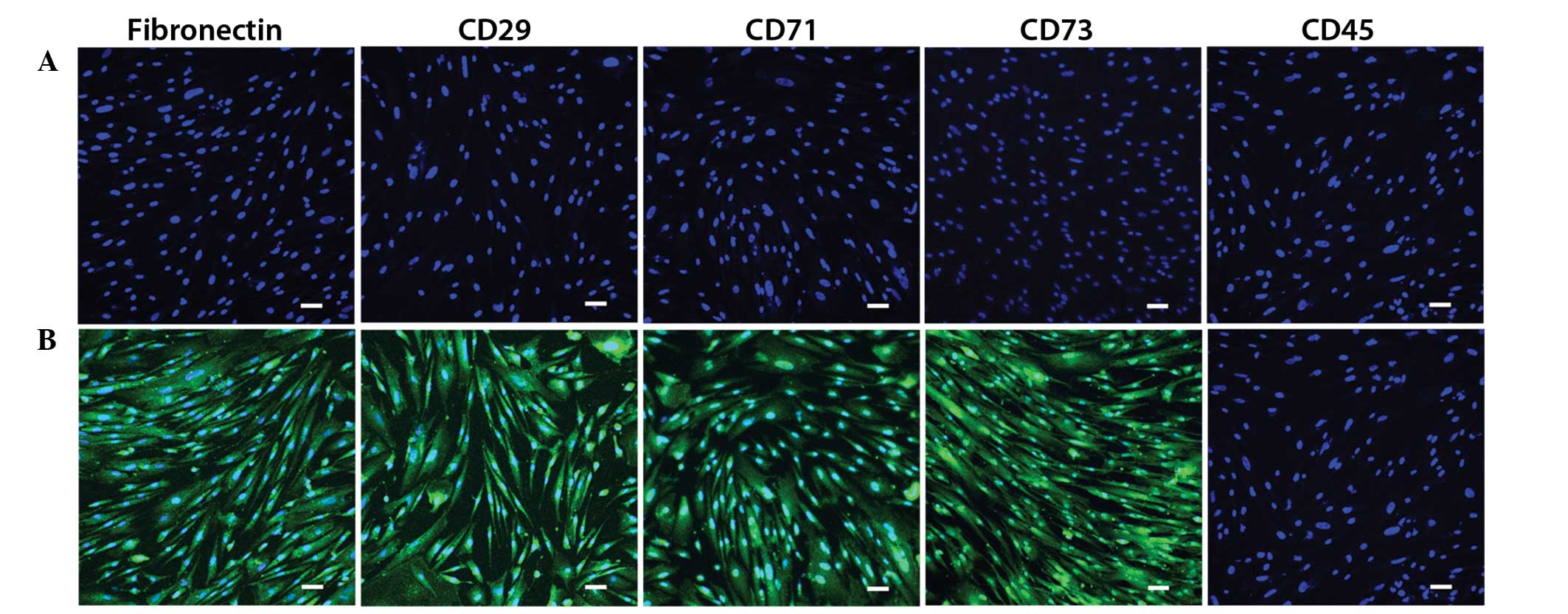
RT-PCR and immunofluorescence
detection in the MMSCs
Cells from passages 5 and 10 exhibited no variation
in the expression of the following markers: Fibronectin, Pax2,
CD29, CD71, CD166, CD34 and CD45. All markers were expressed, with
the exception of CD34 and CD45 (Fig.
2). As presented in Fig. 3, the
MMSC-specific antigen markers fibronectin, CD29, CD71 and CD73 were
positive, whilst the hematopoietic cell marker CD45 was
negative.
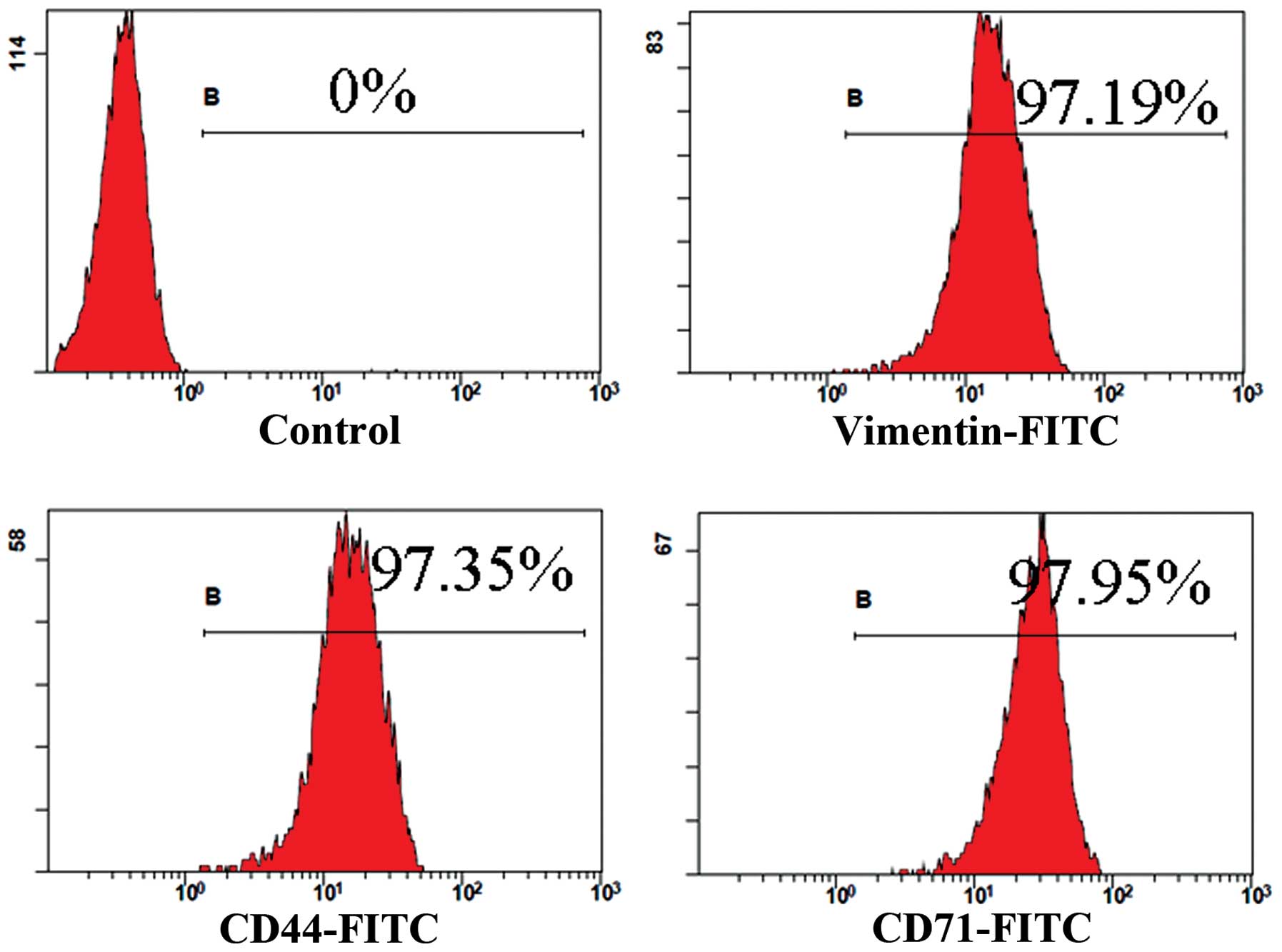
Flow cytometry analysis in the
MMSCs
The positive rate of specific surface markers was
97.19% for vimentin, 97.35% for CD44 and 97.95% for CD71 (Fig. 4).
Adipocyte differentiation of
MMSCs
The control cell group gradually increased in size,
with no significant change in cell morphology. Oil red O staining
was negative (Fig. 5A). The growth
of cells in the induced group was reduced on day 3, cells stretched
to a flat shape, with a small number of oil droplets. By day 9,
mast cells were more apparent, with shiny fat droplets (Fig. 5B). Cells were stained with 0.5% oil
red O and fat droplets became more visible (Fig. 5C). On day 12, large fat droplets
became evident, indicating that the cells had successfully
differentiated into adipocytes. As expected, the induced group
expressed PPAR-γ and LPL at days 6, 9 and 12; this was not evident
in the control group (Fig. 5D).
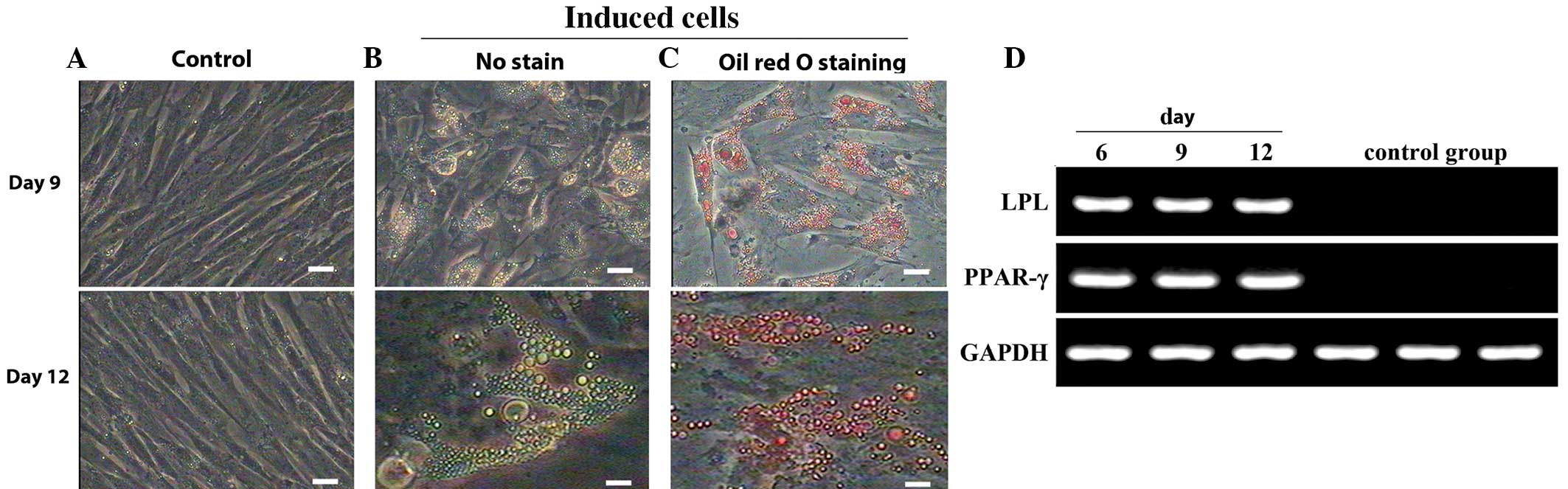 | Figure 5.Morphology and gene expression of
adipocytes following differentiation of MMSCs. (A) Negative control
cells cultured in complete medium presented no changes in
morphology and were negative for oil red O staining throughout the
experimental period. (B) Following induction for 9 days, MMSCs
became oblate and formed intracellular lipid droplets. At day 12,
the mast cells possessed more apparent shiny fat droplets. (C)
Lipid droplets, apparent at days 9 and 12, were stained with oil
red O. Scale bars, 100 µm. (D) Expression of adipocyte-specific
genes. LPL and PPAR-γ were detected in the induced group following
induction for 6, 9 or 12 days, but not expressed in the control
group. GAPDH served as the internal control. MMSCs, metanephric
mesenchymal stem cells; LPL, lipoprotein lipase; PPAR-γ, peroxisome
proliferator-activated receptor-γ. |
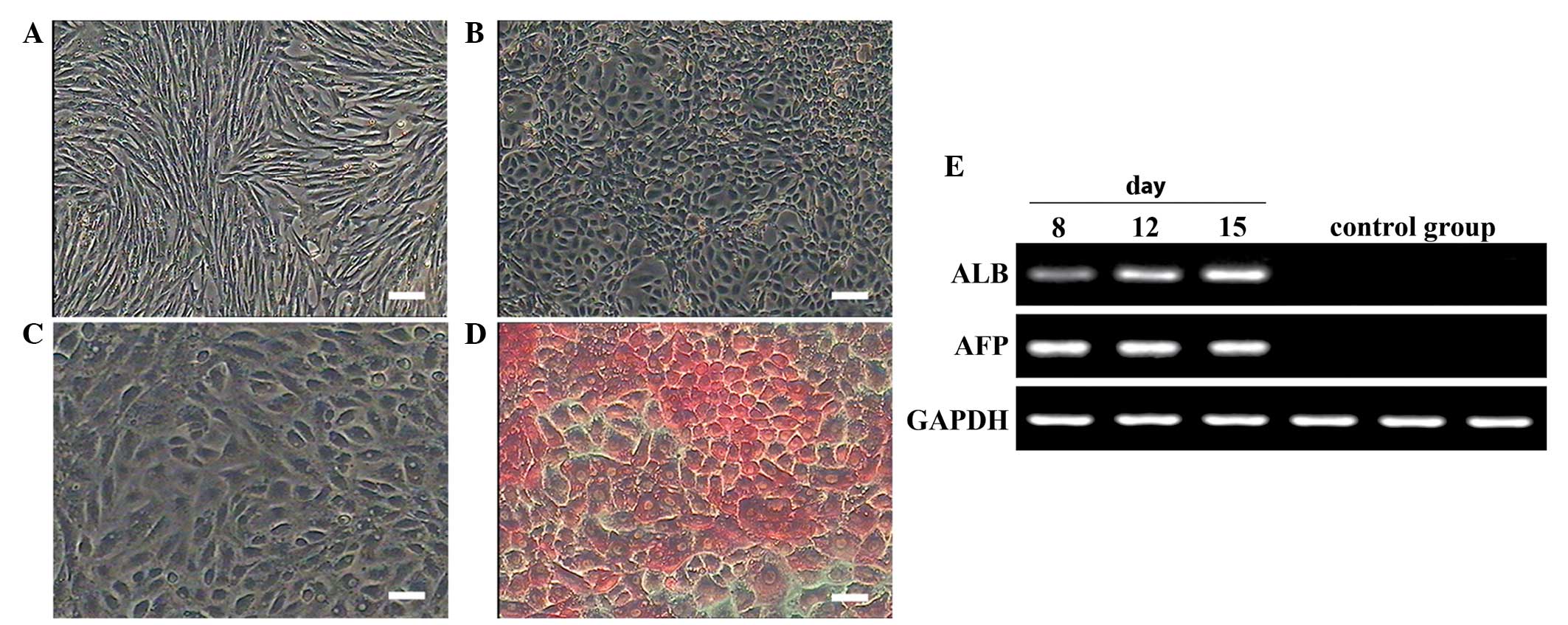
Hepatocyte differentiation of
MMSCs
The control group exhibited no changes in
morphology, and staining results were negative for the duration of
the experiment (Fig 6A). In the
induced group, cells with polygonal shape increased in numbers on
day 8 of the induction. On day 15 of the induction, the distinctive
cubic hepatocyte shape became apparent (Fig. 6B and C). Successful periodic
acid-Schiff staining confirmed the glycogen synthesis and storage
functions of the induced cells (Fig.
6D). Gene expression of AFP and ALB confirmed the
differentiation of MMSCs into hepatocytes in the induced group,
whilst control cells did not express any hepatocyte-specific genes
(Fig. 6E).
Epithelial cell differentiation of
MMSCs
On day 10, the epithelioid cells were plate-shaped
and exhibited single-layer adherent growth, whilst the control
cells did not differentiate (Fig. 7A and
B). Immunofluorescence analysis demonstrated that the induced
cells were positive for CK18 and CK19 (Fig. 7C–F). Expression of E-cadherin and
CK19 was also detected, by PCR analysis (Fig. 7G).
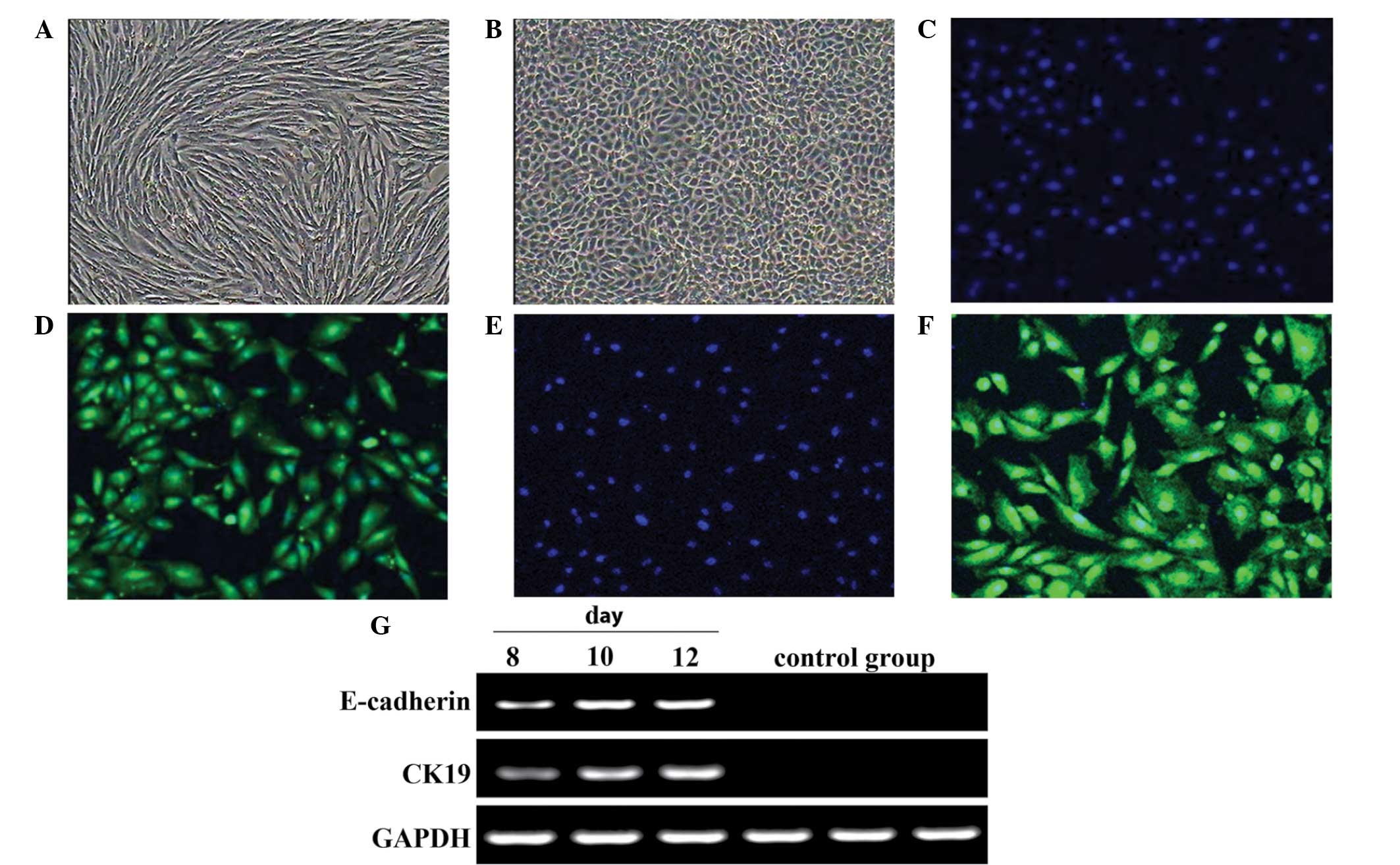 | Figure 7.Morphology and gene expression of
epithelial cells following differentiation of metanephric
mesenchymal stem cells. (A) The control group presented no clear
change in form. Scale bar, 100 µm. (B) In the induced group,
following 10 days, epithelioid cells were plates like pebbles or
paving stones, with single-layer adherent growth. Normal clear cell
boundary and refraction, stereoscopic, closely linked cells. Scale
bar, 100 µm. Immunofluorescence analysis: (C) DAPI/without
antibody; (D) CK18+/DAPI; (E) DAPI/without antibody; (F)
CK19+/DAPI. Scale bars, 50 µm. (G) Epithelial
cell-specific genes CK19 and E-cadherin were expressed in the
induced group following 8-, 10- and 12-day induction, but the
control group was negative. |
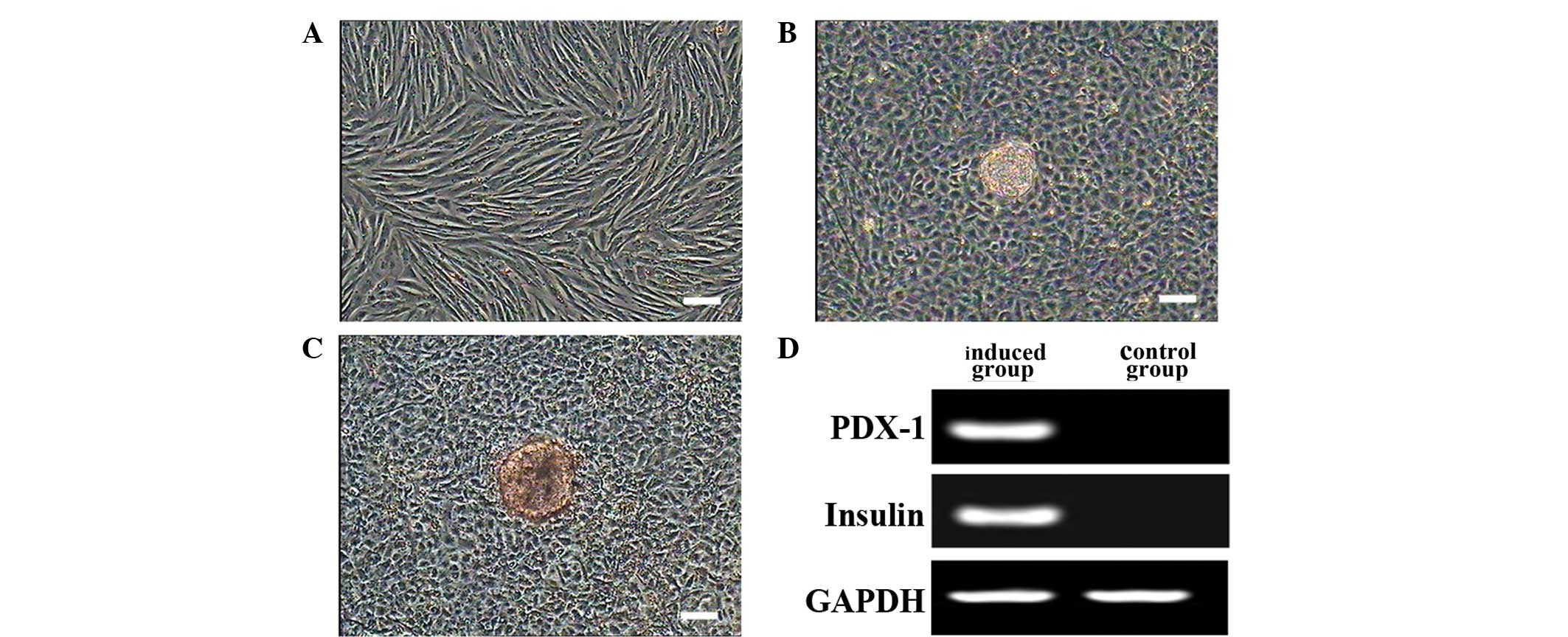
Islet cell differentiation of
MMSCs
Following pre-induction, cells expanded rapidly. The
addition of the terminal medium limited cell growth and caused the
formation of clusters. Control cells did not differentiate
morphologically (Fig. 8A). Cell
clusters were observed, and stained red by dithizone (Fig. 8B and C). PDX-1 and insulin were
expressed in differentiated cells but not in the control group
(Fig. 8D).
Discussion
In the current study, MMSCs were isolated from
20-day-old Beijing duck metanephros and assessed for their
self-renewal potential, biological characteristics and
differentiation abilities. The duck embryo is a classic model of
vertebrate developmental biology, which has been used extensively
(24). Enzymatic digestion was used
as a stable method to harvest MMSCs from metanephric tissues. Cell
lines exhibited typical logistic growth, with an S-shaped growth
curve, in accordance with previously observed in vitro cell
growth patterns (25). Due to
mechanical and chemical damage caused by external conditions in
subculture, cells require adaptation and recovery periods.
Increasing cell passages, and external environmental effects cause
cells to gradually age (26).
At present, specific surface markers of MMSCs are
unknown, and MMSCs are generally identified based on the expression
of certain markers that have been previously associated with MSCs.
The present study used immunofluorescence, RT-PCR and flow
cytometry to detect whether MMSCs possessed the surface
characteristics of MSCs. MMSCs were long and fusiform, with a small
number of polygonal cells. Furthermore, they exhibited growth
trends similar to MSCs (27). MMSCs
in the present study were indicated to have morphological
characteristics comparable to MSCs. CD29 (also known as integrin
β1) is a β-subunit protein and a member of the integrin family.
CD29 can form a heterodimer with surface and extracellular
proteins, such as CD49 and CD51, in MSCs in order to mediate the
cell-cell and cell-matrix interactions. In the present study, CD29,
CD71 and CD44 levels were detected as the surface markers of MMSCs
using immunofluorescence and RT-PCR, which were shown to have a
sensitive expression in different passages. Therefore, CD29, CD71
and CD44 may be considered as specific makers of MMSCs.
Depending on induction conditions, MMSCs are able to
differentiate into various cell types. MMSCs originate from the
mesoderm and are pluripotent, meaning they can differentiate into
ectoderm, mesoderm and endoderm cells (28). In the present study, MMSCs were
induced into adipocytes, hepatocytes, epithelial cells and islet
cells. The induced cells illustrated typical staining and expressed
specific marker genes.
Oil red O staining is a method used for the
identification of lipid droplets in cells. IBMX increases levels of
cAMP to regulate the expression of PPAR-γ. In the present study,
PPAR-γ and LPL were expressed in the early stages of adipogenic
differentiation. This suggests that MMSCs may be induced to
differentiate into adipocytes under appropriate conditions and
induction times.
Liver cells (hepatocytes) can produce and store
glycogen, and the induced cells in the current study were
identified to contain glycogen by periodic acid-Schiff staining.
This indicated that these cells have a glycogen synthesis and
storage function.
HGF induces mitosis and prevents apoptosis in
hepatocytes, boosting glycogen formation, while FGF-4 is an
important factor for hepatocyte growth and development. The present
study identified positive expression of AFP and ALB, which may
indicate that hepatocyte differentiation is associated with gene
expression. AFP expression was higher in the early stages of
differentiation, indicating that AFP may serve a regulatory role in
liver-like cells during early induction processes. This raises the
question whether hepatocyte differentiation is associated with gene
expression to a certain degree.
EGF is able to promote mitosis and proliferation in
a variety of cells, stimulating the division of keratinocytes in
vitro and in vivo and promoting epithelial cell
regeneration. IGF, another common growth factor, possesses various
physiological functions, including the regulation of body growth
and the promotion of mitosis in cell cultures. Collectively, IGF,
EGF and BMP-7 can function to induce the differentiation of MMSCs
to epithelial cells, in addition to inhibiting fibroblast growth
and promoting epithelial cell growth and proliferation (29). Cytokeratins maintain the structural
integrity of keratinized epithelium, stratified squamous
epithelium, stratified epithelium, hyperplastic cutinized
epithelium and simple epithelium (30).
In the present study, pancreatic islet cells were
induced by adding HGF, active A and niacinamide. Active A activates
β-cells in the pancreatic islets, while niacinamide aids the
converging and expanding of islet cells (31). Dithizone staining revealed scarlet
stained cell clusters, indicating that the induced cell cytoplasm
possessed zinc ions, corresponding to islet cell characteristics
(32). The induced cells also
expressed PDX-1 and insulin. Further research should focus on
improving β-cell induction and promoting insulin secretion, which
may benefit patients with diabetes.
In conclusion, the Beijing duck embryo MMSC
cultivation system was established in the present study. MMSCs were
found to express MSC marker genes, which was determined using
RT-PCR, immunofluorescence and flow cytometry. In addition, MMSCs
were found to have a self-proliferation and multidirectional
differentiation potential. MMSCs may be applicable to kidney
transplantation for kidney disease treatment in the future, since
they are able to repair the damage of kidney tissue and help
restore the normal kidney function.
Acknowledgements
The current research was supported by the
Agricultural Science and Technology Innovation Program (ASTIP;
cxgc-ias-01), the National Natural Science Foundation of China
(grant no. 31472099), the National Infrastructure of Animal
Germplasm Resources (2014).
References
|
1
|
Salgado AJ, Oliveira JT, Pedro AJ and Reis
RL: Adult stem cells in bone and cartilage tissue engineering. Curr
Stem Cell Res Ther. 1:345–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ding DC, Shyu WC and Lin SZ: Mesenchymal
stem cells. Cell Transplant. 20:5–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tocci A and Forte L: Mesenchymal stem
cell: Use and perspectives. Hematol J. 4:92–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cooper K and Viswanathan C: Establishment
of a mesenchymal stem cell bank. Stem Cells Int. 2011:9056212011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao Y, Bai C, Xiong H, Li Q, Shan Z, Huang
L, Ma Y and Guan W: Isolation and characterization of chicken
dermis-derived mesenchymal stem/progenitor cells. Biomed Res Int.
2013:6262582013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duffield JS, Park KM, Hsiao LL, Kelley VR,
Scadden DT, Ichimura T and Bonventre JV: Restoration of tubular
epithelial cells during repair of the postischemic kidney occurs
independently of bone marrow-derived stem cells. J Clin Invest.
115:1743–1755. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brown WR, Hubbard SJ, Tickle C and Wilson
SA: The chicken as a model for large-scale analysis of vertebrate
gene function. Nat Rev Genet. 4:87–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deans RJ and Moseley AB: Mesenchymal stem
cells: Biology and potential clinical uses. Exp Hematol.
28:875–884. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baddoo M, Hill K, Wilkinson R, Gaupp D,
Hughes C, Kopen GC and Phinney DG: Characterization of mesenchymal
stem cells isolated from murine bone marrow by negative selection.
J Cell Biochem. 89:1235–1249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang W, Zhang F, Shi H, Tan R, Han S, Ye
G, Pan S, Sun F and Liu X: Comparisons of rabbit bone marrow
mesenchymal stem cell isolation and culture methods in
vitro. PloS One. 9:e887942014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fong CY, Richards M, Manasi N, Biswas A
and Bongso A: Comparative growth behaviour and characterization of
stem cells from human Wharton's jelly. Reprod Biomed Online.
15:708–718. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colter DC, Sekiya I and Prockop DJ:
Identification of a subpopulation of rapidly self-renewing and
multipotential adult stem cells in colonies of human marrow stromal
cells. Proc Natl Acad Sci USA. 98:7841–7845. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bühring HJ, Battula VL, Treml S, Schewe B,
Kanz L and Vogel W: Novel markers for the prospective isolation of
human MSC. Ann N Y Acad Sci. 1106:262–271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu Y, Liao L, Wang Q, Ma L, Ma G, Jiang X
and Zhao RC: Isolation and identification of mesenchymal stem cells
from human fetal pancreas. J Lab Clin Med. 141:342–349. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X,
Gong W, Han ZB, Xu ZS, Lu YX, Liu D, et al: Isolation and
characterization of human umbilical cord mesenchymal stem cells
with hematopoiesis-supportive function and other potentials.
Haematologica. 91:1017–1026. 2006.PubMed/NCBI
|
|
16
|
Huss R, Lange C, Weissinger EM, Kolb HJ
and Thalmeier K: Evidence of peripheral blood-derived,
plastic-adherent CD34(−/low) hematopoietic stem cell clones with
mesenchymal stem cell characteristics. Stem Cells. 18:252–260.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uppalapati D, Ohta N, Zhang Y, Kawabata A,
Pyle MM, Becker KG, Troyer D and Tamura M: Identification and
characterization of unique tumoricidal genes in rat umbilical cord
matrix stem cells. Mol Pharm. 8:1549–1558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Păunescu V, Deak E, Herman D, Siska IR,
Tănasie G, Bunu C, Anghel S, Tatu CA, Oprea TI, Henschler R, et al:
In vitro differentiation of human mesenchymal stem cells to
epithelial lineage. J Cell Mol Med. 11:502–508. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thirumala S, Gimble JM and Devireddy RV:
Evaluation of methylcellulose and dimethyl sulfoxide as the
cryoprotectants in a serum-free freezing media for cryopreservation
of adipose-derived adult stem cells. Stem Cells Dev. 19:513–522.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poloni A, Maurizi G, Leoni P, Serrani F,
Mancini S, Frontini A, Zingaretti MC, Siquini W, Sarzani R and
Cinti S: Human dedifferentiated adipocytes show similar properties
to bone marrow-derived mesenchymal stem cells. Stem Cells Dev.
30:965–974. 2012. View Article : Google Scholar
|
|
21
|
Lysy PA, Smets F, Najimi M and Sokal EM:
Leukemia inhibitory factor contributes to hepatocyte-like
differentiation of human bone marrow mesenchymal stem cells.
Differentiation. 76:1057–1067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
DiRenzo J, Signoretti S, Nakamura N,
Rivera-Gonzalez R, Sellers W, Loda M and Brown M: Growth factor
requirements and basal phenotype of an immortalized mammary
epithelial cell line. Cancer Res. 62:89–98. 2002.PubMed/NCBI
|
|
23
|
Chao KC, Chao KF, Fu YS and Liu SH:
Islet-like clusters derived from mesenchymal stem cells in
Wharton's Jelly of the human umbilical cord for transplantation to
control type 1 diabetes. PloS One. 3:e14512008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rao MS and Mattson MP: Stem cells and
aging: Expanding the possibilities. Mech Ageing Dev. 122:713–734.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaufman DS, Hanson ET, Lewis RL, Auerbach
R and Thomson JA: Hematopoietic colony-forming cells derived from
human embryonic stem cells. Proc Natl Acad Sci USA. 98:10716–10721.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Wei X, Ling J, Huang Y, Gong Q and
Huo Y: Identification and characterization of side population cells
from adult human dental pulp after ischemic culture. J Endod.
38:1489–1497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lindenmair A, Hatlapatka T, Kollwig G,
Hennerbichler S, Gabriel C, Wolbank S, Redl H and Kasper C:
Mesenchymal stem or stromal cells from amnion and umbilical cord
tissue and their potential for clinical applications. Cells.
1:1061–1088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lorenz K, Sicker M, Schmelzer E, Rupf T,
Salvetter J, Schulz-Siegmund M and Bader A: Multilineage
differentiation potential of human dermal skin-derived fibroblasts.
Exp Dermatol. 17:925–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pera EM, Ikeda A, Eivers E and De Robertis
EM: Integration of IGF, FGF, and anti-BMP signals via Smad1
phosphorylation in neural induction. Genes Dev. 17:3023–3028. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alkhalidi HM and Alhumaidy AA: Cystic
panfolliculoma of the scalp: Report of a very rare case and brief
review. Indian J Pathol Microbiol. 56:437–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li HY, Chen YJ, Chen SJ, Kao CL, Tseng LM,
Lo WL, Chang CM, Yang DM, Ku HH, Twu NF, et al: Induction of
insulin-producing cells derived from endometrial mesenchymal
stem-like cells. J Pharmacol Exp Ther. 335:817–829. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zanini C, Bruno S, Mandili G, Baci D,
Cerutti F, Cenacchi G, Izzi L, Camussi G and Forni M:
Differentiation of mesenchymal stem cells derived from pancreatic
islets and bone marrow into islet-like cellphenotype. PLoS One.
6:e281752011. View Article : Google Scholar : PubMed/NCBI
|