Introduction
The meniscus has important roles in joint stability,
shock absorption and load distribution (1–3).
Injuries to the meniscus are a common source for knee dysfunction
and disability due to the limited self-repair capacity of the
meniscus (4–6). For symptomatic meniscus injury, a total
or partial meniscectomy is often performed. However, loss of
meniscal tissue often leads to long-term degenerative joint
changes, articular cartilage degeneration and eventually,
osteoarthritis (OA) (7,8). A novel strategy for meniscus repair is
required, and the less invasive cell-based therapy for meniscus
regeneration is a promising possibility.
The availability of large quantities of mesenchymal
stem cells (MSCs), which may be obtained from the majority of adult
tissues, and their multipotency, specifically their chondrogenic
differentiation properties, make MSCs the most suitable candidate
progenitor cell source for cell-based therapy. Fraser et al
(9) reported that the frequency of
MSCs in adipose tissue is ~500-fold more than that in bone marrow.
Adipose-derived mesenchymal stem cells (ASCs) are therefore a
promising candidate cell source for meniscus regeneration through
the less invasive technique of intra-articular injection.
The ability to monitor the in vivo behavior
of implanted MSCs and understand their fate is important for
developing successful cell therapies; an effective, non-invasive
and non-toxic technique for cell tracking is required for this
purpose (10). Superparamagnetic
iron oxide (SPIO) is an ideal tracer for MSCs, which may be labeled
with SPIO at >90% efficiency without the use of a transfection
agent (11,12). The SPIO-labeled MSCs may then be
visualized by magnetic resonance imaging (MRI). The success of cell
therapies depends on the ability to deliver the cells to the site
of injury and targeted magnetic cell delivery is a promising
emerging technique for localized cell transplantation therapy.
In the present study, SPIO was used to label ASCs. A
permanent magnet close to the joint was used to localize the
implanted ASCs. The effect and outcome of the targeted
intra-articular injection of SPIO-labeled ASCs in a rabbit model of
massive meniscal defect were then determined. These results enabled
investigation of meniscal regeneration, OA prevention and the
destination of the ASCs.
Materials and methods
Cell isolation and culture
The ASCs were isolated from the subcutaneous fat of
the nape of the neck of rabbits using methods described previously
(13). The experimental procedures
used were approved by the Experimental Animal Ethics Committee of
Zhejiang University (Hangzhou, China). The isolated tissues were
homogenized in Dulbecco's modified Eagle's medium (DMEM) and
digested in 0.1% type I collagenase (Sigma-Aldrich, St. Louis, MO,
USA) at 37°C for 1 h with intermittent shaking. Cells were
collected following centrifugation at 1,200 × g for 10 min,
cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/ml
penicillin and 100 mg/ml streptomycin (Thermo Fisher Scientific,
Inc., Waltham, MA, USA), and incubated at 37°C in 5%
CO2. The first medium change was performed 24 h after
plating and once every 2–3 days routinely. The cells were harvested
or subcultured as they reached 80–90% confluency. Cells were used
for the following experiments at the third passage.
SPIO labeling and Prussian blue
staining
For incubation with SPIO, Ferucarbotran (Bayer
Schering Pharma AG, Berlin, Germany) was added to the culture
medium at concentrations of 0, 1, 10 and 100 µg Fe/ml in accordance
with previous direct labeling studies (14,15).
After 24 h, the ASCs treated with 100 µg Fe/ml Ferucarbotran were
stained with Prussian blue. The ASCs were treated with 10%
potassium ferrocyanide and 20% hydrochloric acid for 20 min. ASCs
were then washed twice with double-distilled water and visualized
under an IX71 light microscope (Olympus Corporation, Tokyo,
Japan).
Cell proliferation
SPIO-treated ASCs at concentrations of 1, 10 and 100
µg Fe/ml, and unlabeled cells, were grown in 96-well plates at
1×104 cells/well for 24 h. The proliferation was
evaluated by
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-terazolium bromide
(MTT) assay (Sigma-Aldrich). This assay is based on the ability of
mitochondrial dehydrogenases to oxidize MTT, a tetrazolium salt,
into an insoluble blue formazan product. The optical density of the
plates was then read using a Model 550 microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using test and reference
wavelengths of 570 nm. This test was repeated 3 times.
In vitro cellular MRI
To determine the sensitivity of the MRI magnets for
the detection of labeled cells, labeled and unlabeled ASCs were
trypsinized, centrifuged at 800 × g for 5 min and placed in
Eppendorf tubes (5×105, 1×105 and
5×104 labeled cell precipitations in 2 ml culture
solutions). In vitro cellular imaging was performed with a
clinical 3.0-T MR imager using a T2*-weighted gradient-echo
(GRET2-WI). Sequence parameters were as follows: Repetition time
(TR), 600 msec; echo time (TE), 18 msec; field of view (FOV),
160×160; flip angle, 40°; matrix size, 512×512; slice thickness,
2.0 mm; and slice, 0.5 mm.
Meniscectomy and MSC injection
A total of 18 New Zealand 12 week-old rabbits,
weighing 2.5±3.0 kg, were used. The procedures were followed in
accordance with the standards described in the 8th edition of the
Guide for the Care and Use of Laboratory Animals. Following
anesthetization with 30 mg/kg sodium pentobarbital (3%; Sangon
Biotech Co., Ltd., Shanghai, China) via intravenous injection, a
straight incision was made on the anterior side of the bilateral
knee and the anteromedial side of the joint capsule, and the
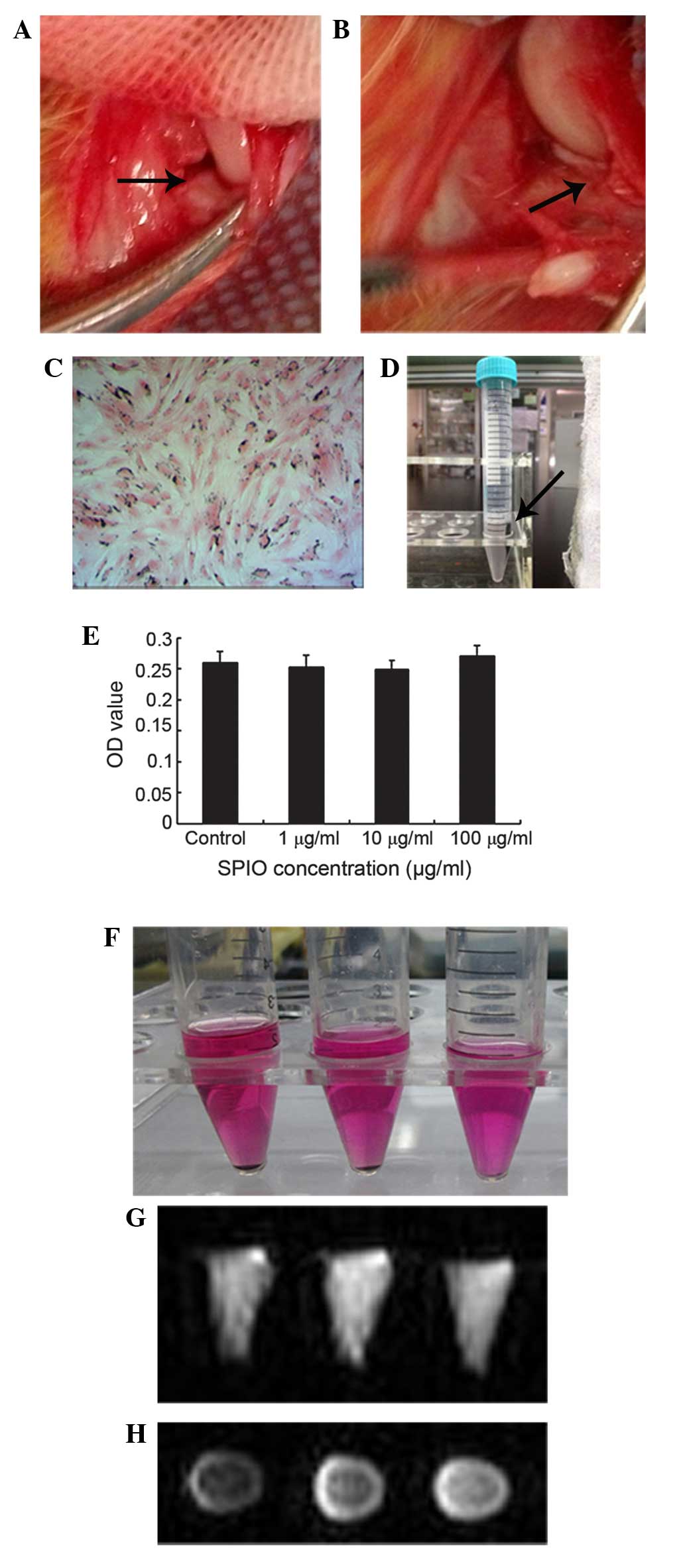
anterior horn of the medial meniscus was dislocated anteriorly with
forceps (Fig. 1A). The meniscus was
then incised vertically at the level of the medial collateral
ligament, and the anterior half of the medial meniscus was excised
(Fig. 1B). The dislocated meniscus
was removed and the wound was closed in layers. After 7 days,
2×106 ASCs (n=6) or 2×106 SPIO-labeled ASCs
(n=6) in 100 µl phosphate-buffered saline (PBS) were injected into
the knee joint via a medial approach with a 26-gauge needle. For
the control group, the same volume of PBS (only) was injected into
the knee (n=6). Following surgery, permanent magnets were fixed to
the outside of the treated joints for one day and the rabbits were
allowed to walk freely in the cage.
In vivo MRI
The rabbits were imaged under general anesthesia at
6 and 12 weeks post-injection, and MR images of the rabbit joints
were obtained on a clinical 3.0-T MR imager equipped with a coil.
Sagittal and axial images were obtained using a GRET2-WI sequence.
Imaging parameters were as follows: TR, 600 msec; TE, 18 msec; FOV,
160×160; flip angle, 40°; matrix size, 512×512; slice thickness,
2.0 mm; and slice, 0.5 mm.
Gross observation
The rabbits were sacrificed at 6 and 12 weeks after
surgery. Macroscopic observations of the meniscus, tibial plateau
and surrounding joint tissues were performed, in accordance with
the International Cartilage Repair Society (ICRS) cartilage repair
assessment system (16,17). Using this system, the assessment was
recorded using macroscopic examination on the surface of the
cartilage. Overall repair assessment was scored and later graded,
from normal (grade I) to severely abnormal (grade IV).
Histological observation
Following gross examination, the samples were fixed
in 4% formalin, embedded in paraffin and sliced into 7-µm sections.
The sections were stained with hematoxylin and eosin for
morphological evaluations and with Safranin for glycosaminoglycan
distribution, and examined under an IX71 light microscope.
Tracking of SPIO-labeled ASCs
The sections of tissue from the SPIO-labeled ASC
group were stained with Prussian blue to detect whether implanted
ASCs were involved in meniscal regeneration. Surrounding joint
tissues were also fixed in 4% formalin, embedded in paraffin and
sliced into 7-µm sections, which were stained with Prussian blue to
track the mobilization of implanted ASCs.
Statistical analysis
Results were analyzed by one-way analysis of
variance using SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA).
Tukey's test was used for multiple comparisons, and the level of
significance was set at P<0.05.
Results
Cell labeling
ASCs may be directly labeled with SPIO. After 24 h,
nearly 100% of the ASCs were labeled with SPIO, as evidenced by
Prussian blue staining (Fig. 1C);
SPIO particles stained as blue granules. The blue granules were
only observed in the cytoplasm and were predominantly arranged
around the nucleus. However, there was no morphological change
following SPIO labeling.
Cell proliferation
The labeled ASCs may be orientated in the direction
of the magnet (Fig. 1D). No
statistically significant differences in cell viability were
detected between the SPIO-treated and untreated ASCs, as determined
by MTT assay (Fig. 1E).
Cellular MRI of SPIO
After 24 h of incubation with Ferucarbotran (100
µg/ml), the ASCs were centrifuged; 5×104,
1×105 and 5×104 SPIO-labeled cell
precipitations in 2 ml medium were placed in test tubes (Fig. 1F). Under T2-weighted MRI, the
centrifuged, labeled cell precipitations in the test tubes were
imaged (Fig. 1G and H). However, the
centrifuged 1×104 SPIO-labeled cell precipitation was
not detected by MRI (data not shown).
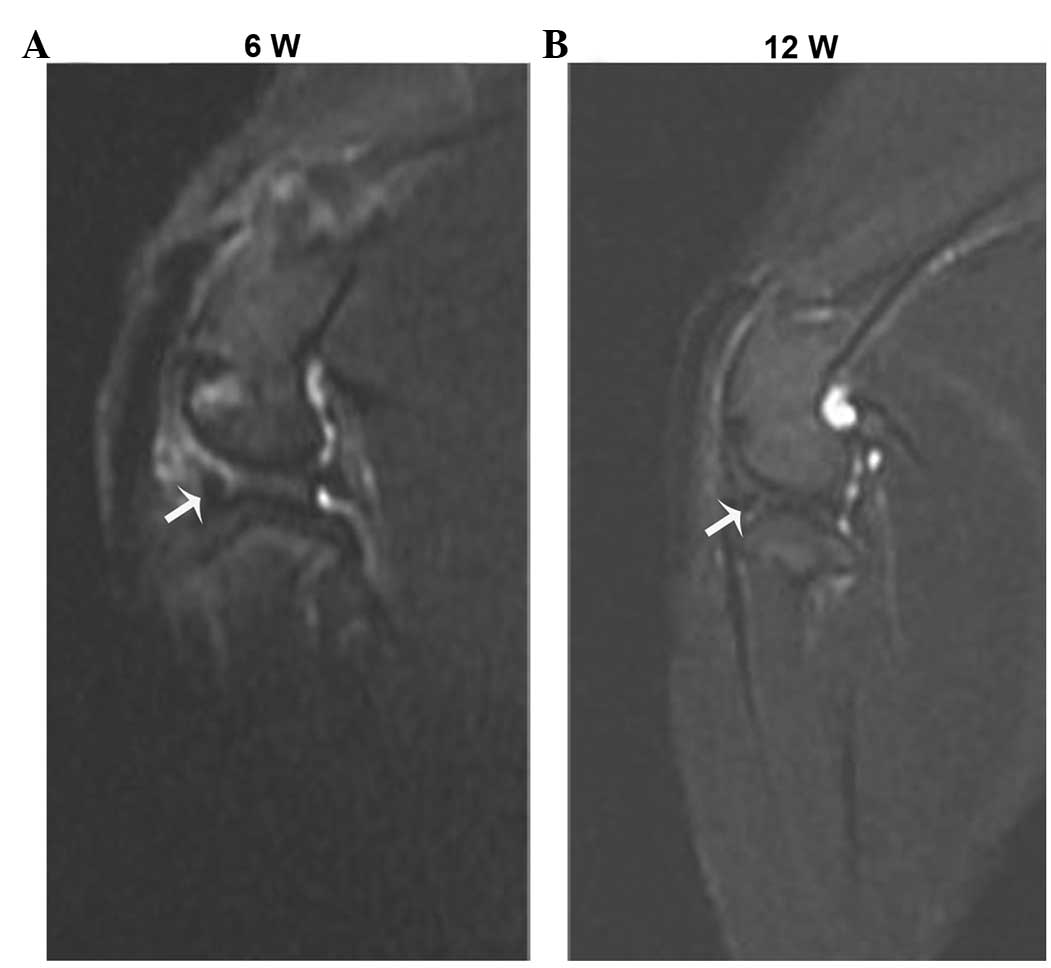
In vivo MR tracking of MSCs
At 6 and 12 weeks after implantation, clear
hypointense artifacts, caused by SPIO-positive cells in the
meniscus, were detected using MRI (Fig.
2A and B). However, the intensity of these hypointense signals
decreased between 6 and 12 weeks.
Macroscopic observation
All animals were mobile 1–2 h after surgery and
there were no instances of infection. The animals tolerated the
cell injection well, and there was no evidence of local
inflammation, immobilization or unloading of the joint.
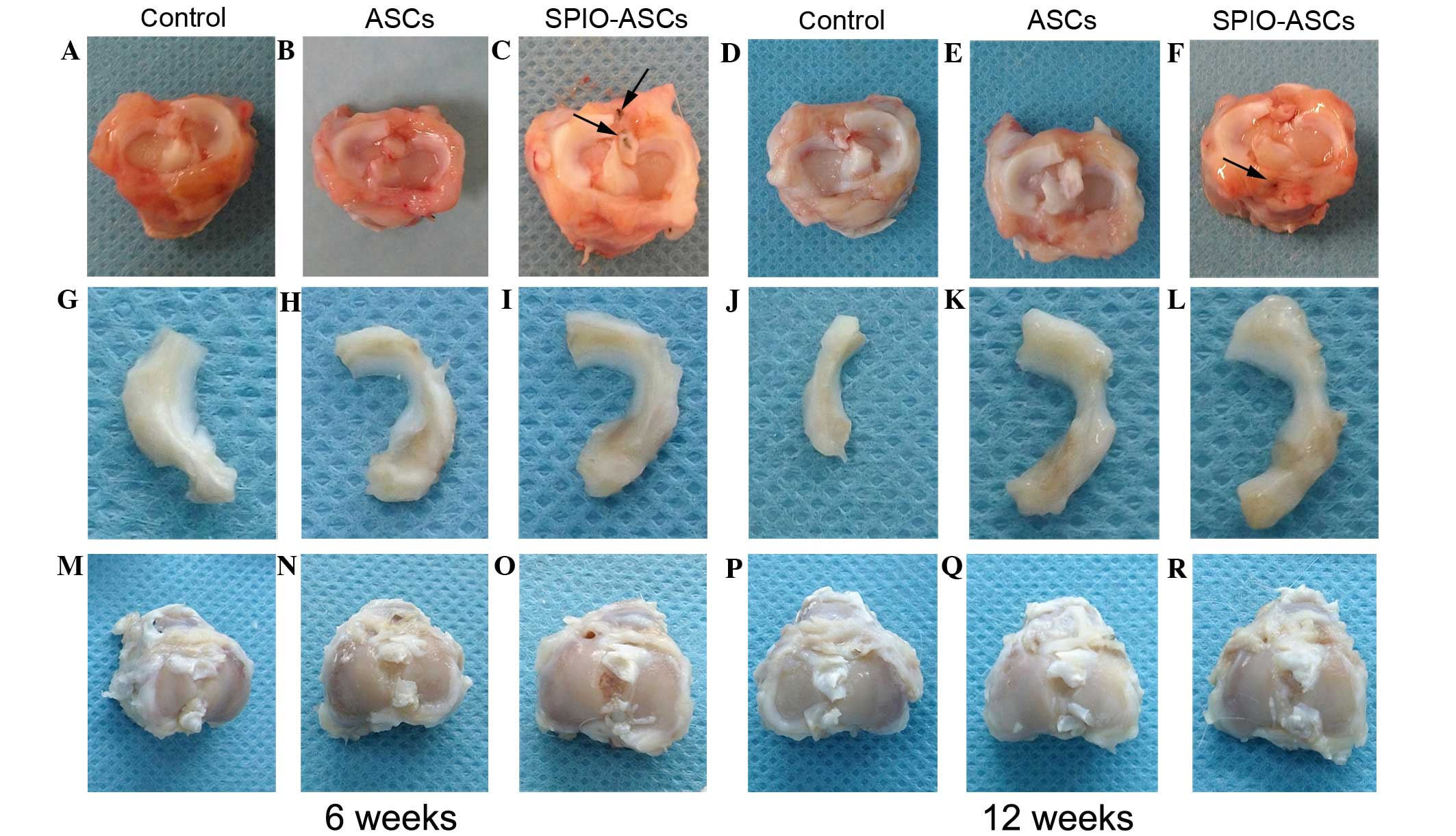
Fig. 3 demonstrates
representative images of the post-surgery tibial joint, regenerated
meniscus and joint surface of the tibia. At 6 weeks, the anterior
section of the meniscal defect of the ASC and SPIO-ASC injection
groups exhibited improved meniscal regeneration in contrast to the
control group (Fig. 3A–C and G–I).
The SPIO-ASCs group reported the highest level of meniscal
regeneration and a good meniscal shape. In addition, SPIO-positive
staining was observed in the surrounding tissues (Fig. 3C).
At 12 weeks, in the control group, the meniscal
tissue had deteriorated (Fig. 3D and
J). The majority of the meniscuses had regenerated in the ASC
group (Fig. 3E and K) and the
SPIO-ASCs group reported almost normally-shaped meniscuses
(Fig. 3F and L). SPIO-positive
staining was also reported in the surrounding tissues of the
SPIO-ASCs group (Fig. 3F).
Protection from osteoarthritic
damage
Besides meniscal regeneration, degenerative changes,
including cartilage erosion, and osteophyte formation on the
surface of the medial condyle of femur and medial tibial plateau,
were evaluated (Fig. 3). The
degenerative changes on the surface of the tibial plateau were more
severe than those of the condyle of the femur, which was less
affected by meniscal degeneration, in all groups. From 6–12 weeks,
the degenerative changes were more severe in the control group; in
this group, a large region of osteophytes developed on the surface
of the tibial plateau (Fig. 3A and
G). In the ASC- and SPIO-ASC-treated joints, the degree of
cartilage destruction and osteophyte formation were all markedly
reduced compared with that of the control joints (Fig. 3B, C, H and I). At 12 weeks, based on
the ICRS grading system, in the control group, 1 knee had grade I
lesions, 3 knees had grade II lesions and 2 knees had grade III
lesions. By contrast, in the ASC-treated group, 2 knees had grade 0
lesions and 4 knees had grade I lesions. In the SPIO-ASC-treated
group, 3 knees had grade 0 lesions and 3 knees had grade I lesions.
All results indicated that ASC and SPIO-ASC transplantation had the
overall effect of protection from OA.
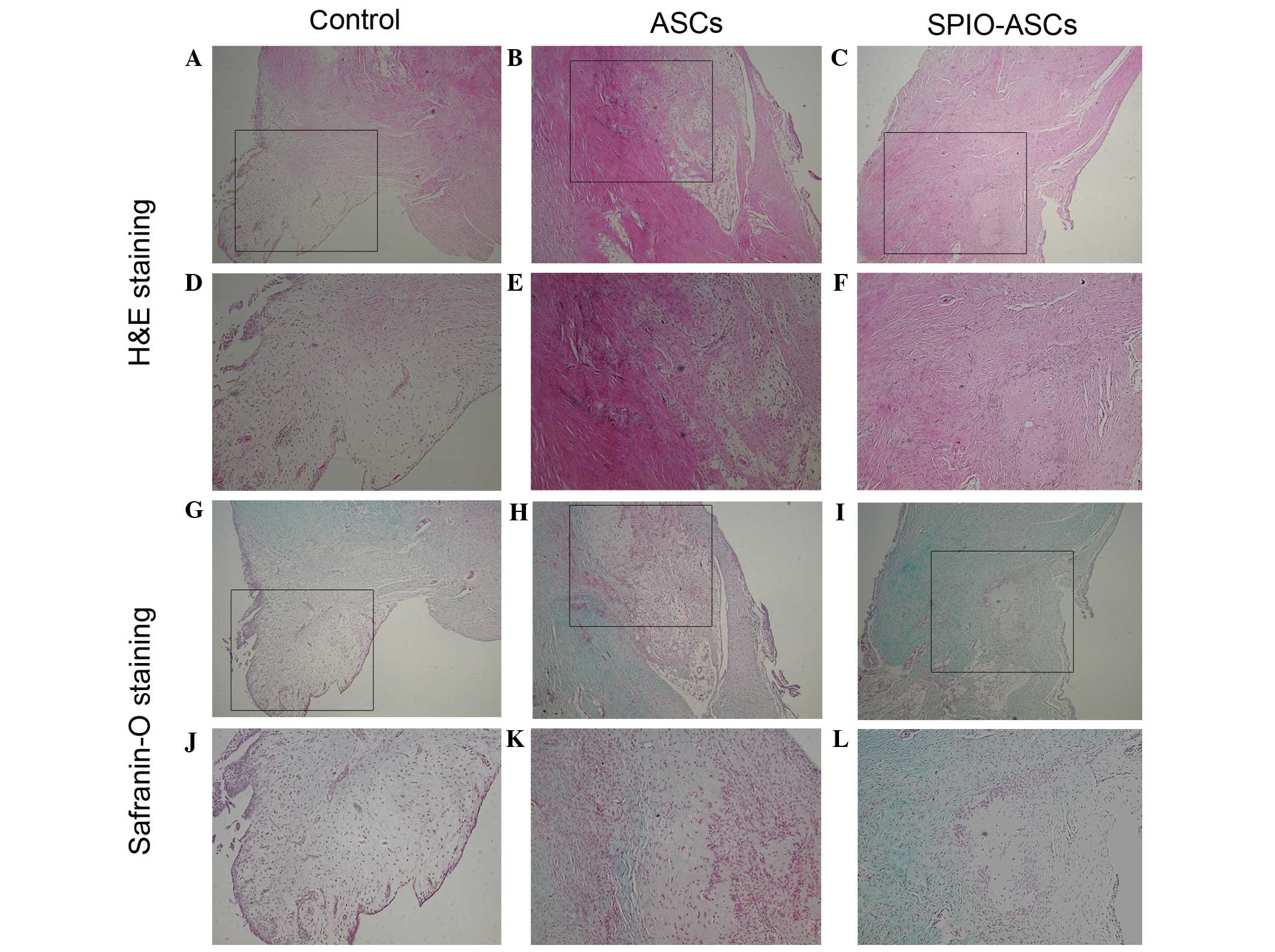
Histological observation
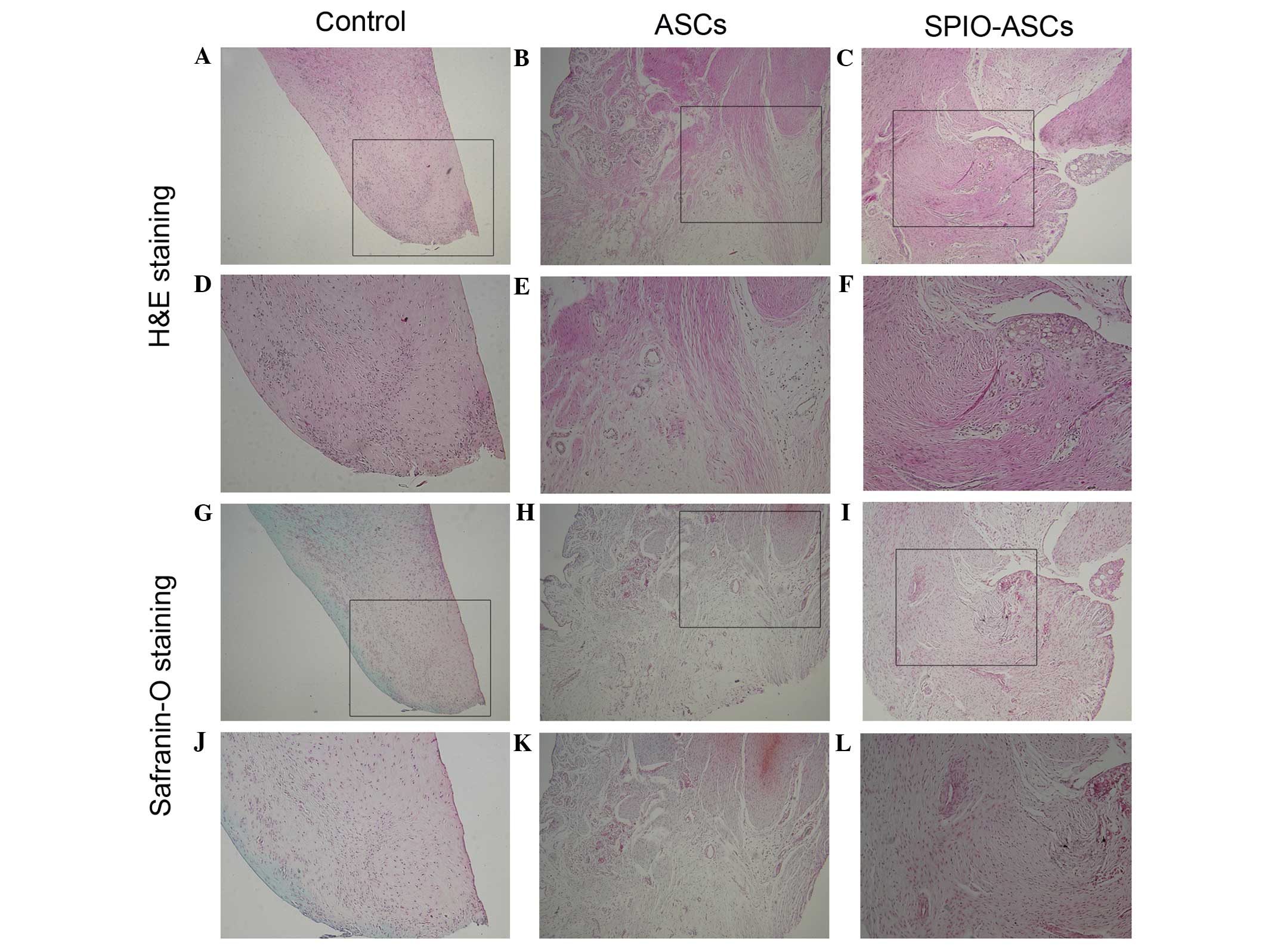
At 6 weeks post-implantation, the control group
demonstrated regeneration of more fibroblast-like tissue and less
hypercellular fibrocartilaginous tissue at the anterior portion of
the meniscus (Fig. 4A, D, G and J).
The anterior portion of the meniscus was partially repaired by
hypercellular fibrocartilaginous tissue and less fibrous-like
tissue in the ASC group (Fig. 4B, E, H
and K). In the SPIO-ASC group, the anterior portion of the
meniscus was regenerated, with a greater mass of hypercellular
fibrocartilaginous tissue and extracellular matrix (ECM) (Fig. 4C, F, I and L).
At 12 weeks post-implantation, the regenerated
meniscus deteriorated in the control group, and was filled with
fibroblastic cells and reduced extracellular matrix; the edge of
this region was also atrophied (Fig. 5A,
D, G and J).
In the ASC group, the anterior portion of the
meniscus was occupied by hypercellular fibrocartilaginous tissue
with a low quantity of ECM. However, the neomeniscus was not
normally shaped (Fig. 5B, E, H and
K). In the SPIO-ASC group, the anterior portion of the meniscus
was similar to the native tissue, demonstrating typical
fibrochondrocytes surrounded by collagen-rich ECM that bridged the
interface; the neomeniscus also integrated well with the host
meniscus (Fig. 5C, F, I and L). Scar
tissue was not observed in any groups at any time point.
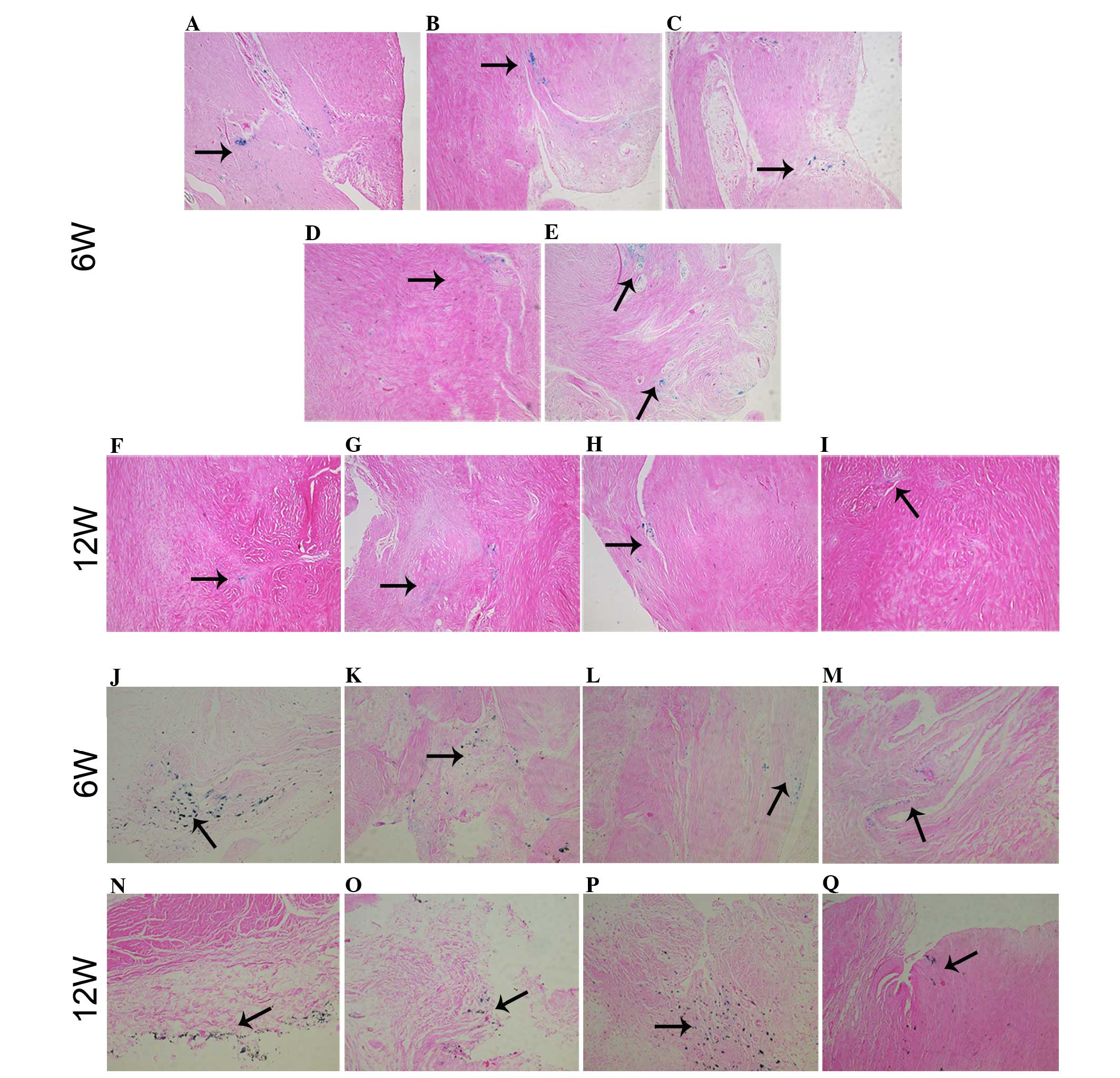
ASC grafting
Prussian blue staining of sections of neomeniscal
tissue in each sample indicated that the implanted ASCs were
directly associated with the regenerated tissue (Fig. 6A–I). SPIO-positive cells were
detected in all five samples at 6 weeks (Fig. 6A–E) and in four of five samples at 12
weeks (Fig. 6F–I). The SPIO-positive
cells were typically associated with the edge of the tissue and, in
numerous cases, were also detected in the interior of the tissue. A
number of typical fibrochondrocytes were stained with Prussian
blue, indicating that implanted ASCs differentiated directly into
fibrochondrocytes and contributed to meniscus regeneration
(Fig. 6E). The injected ASCs also
colonized and integrated into the surface layers of soft tissues
within the joint, including the synovial capsule, fat tissue and
posterior cruciate ligament at 6 and 12 weeks post-injection
(Fig. 6J–Q). The synovial capsule,
fat tissue and posterior cruciate ligament demonstrated a high
incidence of SPIO-positive cells. However, evidence of cell
grafting onto articular cartilage from the femoral condyles and the
tibial plateaus was not detected.
Discussion
The present study demonstrated that targeted
intra-articular delivery of SPIO-ASCs promoted meniscus
regeneration whilst providing protection from osteoarthritic
damage, unaffected by SPIO labeling; this is likely to be
attributable to paracrine effects or in situ modulation of
the healing response. Furthermore, the implanted SPIO-labeled ASCs
adhered to the injured meniscus and were directly associated with
meniscal regeneration, observed using Prussian blue staining and
MRI.
SPIO has been widely used for stem cell labeling
(10). The present results revealed
that ASCs may be efficiently labeled with 100 µg/ml SPIO without
using a transfection agent; the labeling efficiency was almost 100%
after a 24-h incubation, which was consistent with previous studies
(15,18). Using transfection agents may increase
intracellular SPIO uptake; however, transfection agents may have
adverse effects on MSCs. Arbab et al (19) investigated the effects of ferumoxides
with various transfection agents on MSCs; following an increase in
iron concentration from 50 to 125 µg/ml, the MSC intracellular iron
concentration demonstrated a 3-fold rise after 24 h, leading to
~40% cell mortality when compared with unlabeled MSCs. These
results suggest that a higher intracellular free iron concentration
may be toxic to the cells and that an iron concentration safety
threshold exists. This toxic effect may be due, in part, to the
transfection agents. Direct SPIO labeling of cells without
transfection agents would therefore advance the clinical use of
these particles (14,20). The direct iron uptake of the cells
could reach an amount comparable to that of SPIO labeling with a
transfection agent by increasing SPIO concentration (14), which can be observed in the present
study. In the current study, ASCs were incubated in SPIO at
concentrations up to 100 µg Fe/ml of ferucarbotran for 24 h to
achieve appropriate and optimized cell labeling. A previous study
has reported that cell viability was not significantly affected
following incubation with high ferucarbotran concentrations of
>500 µg Fe/ml (21). In the
present study, ASCs were labeled with numerous concentrations of
SPIO and no significant difference was identified in the
proliferation of ASCs between these groups. Furthermore, another
previous study revealed that direct labeling with ferucarbotran did
not impair the function or toxicity, or inhibit the differentiation
capacity of MSCs (15).
MSCs are multipotent cells and have been
demonstrated to effect positive roles in cartilage regeneration
(22). MSCs have demonstrated their
ability to migrate and graft onto the site of injury, and undergo
site-specific differentiation (23).
MSCs may frequently be obtained from adipose tissue (24). The present in vivo study
revealed that direct intra-articular delivery of ASCs markedly
improved the meniscus regeneration compared with an untreated
group. This method creates a possibility of regenerating the
meniscus with less invasiveness when compared with meniscal
transplantation. Previous studies described that the injection of
MSCs from different sources into the joint contributed to meniscus
regeneration (25–27), concordant with the results of the
current study.
A massive meniscal defect would inevitably lead to
OA in the long term. In the present study, all groups succumbed to
OA. However, intra-articular ASCs can provide a protective effect
from osteoarthritic damage compared with the control group. Stem
cells represent a possibility for relief of the tumor burden in
degenerative joint diseases via direct intra-articular injection
(28). Stem cells are known to
produce numerous, diverse bioactive molecules with immunoregulatory
(29,30) and/or regenerative functions (31). Through direct cell-cell interaction
or the secretion of a variety of factors, stem cells may exert a
marked effect on local tissue repair through modulation of the
local microenvironment and activation of endogenous progenitor
cells (32). Stem cells, therefore,
not only have the ability to contribute structurally to tissue
repair, but also possess potent immunomodulatory and
anti-inflammatory effects; stem cells thereby provide protection
from osteoarthritic damage, as observed in the ASCs and SPIO-ASCs
groups of the present study.
Cell migration and/or differentiation are necessary
for endogenous meniscal healing and repair. The implanted stem
cells adheres to the meniscal defect first, and subsequently induce
the chondrogenic differentiation of adhered stem cells, which may
be more useful for meniscal repair. Stem cells have revealed an
ability to migrate and graft onto multiple musculoskeletal tissues,
particularly sites of injury, and to undergo site-specific
differentiation (23). However,
in-site migration is much less prevalent. In the present study,
under an external magnetic force, SPIO-labeled ASCs were
successfully targeted and adhered to the meniscal defect and then
differentiated into fibrochondrocytes. The regenerated meniscus was
similar to the native tissue, demonstrating typical
fibrochondrocytes surrounded by collagen-rich ECM. Due to the
paracrine and trophic effects of ASCs, the SPIO-ASC group reported
the highest level of meniscus regeneration and less osteoarthritic
damage.
Monitoring implanted ASCs is vital to successful
cell-based therapies. MRI provides a non-invasive method for
studying the fate of transplanted cells labeled with SPIO, and
allows imaging at the cellular and molecular levels (33). The MRI signal intensity is directly
associated with the amount of intracytoplasmatic SPIO in the
surviving cells. In the present study, in vitro,
centrifuged, labeled ASC precipitations in test tubes were
visualized using high-resolution MRI. In vivo, the implanted
ASCs were successfully tracked by MRI, and Prussian blue staining
indicated that the ASCs not only adhered to the meniscal defect,
but also to other structures, including the synovial capsule, fat
tissue and the anterior cruciate ligament. Use of MRI, combined
with histological observation, may accurately confirm the location
of implanted SPIO-labeled ASCs. However, whether the ASCs
additionally grafted to distant organs was not detected. A previous
study demonstrated that the intra-articularly injected MSCs
remained in the knee joint and did not migrate to other organs
(25). However, subsets of the
implanted ASCs migrated to untargeted sites in the current study. A
method by which to effectively guide the stem cells to the targeted
sites therefore requires additional study. SPIO-labeled MSC-based
targeted therapy has the potential for treating human diseases.
Either a magnetic field or MRI may be used to guide and monitor
SPIO-labeled MSCs to target organs. Furthermore, the dilution of
iron oxide particles following cell proliferation, migration of
labeled cells, and biodegradation of iron oxide particles may
decrease the intensity of hypointense signals (19), which may limit the duration that MSCs
could be accurately detected by MRI.
Despite a high degree of cell loss, labeled and
unlabeled MSCs provide a possible therapeutic benefit in meniscal
regeneration and OA prevention. The therapeutic effects of
transplanted cells may be independent of implanted cell survival or
transdifferentiation. Furthermore, factors secreted by the MSCs
(paracrine effect) or an alternative association with the healing
response may have a beneficial effect in meniscal regeneration.
The targeting efficiency was not high in the present
study, demonstrating that SPIO-ASCs also migrated to other,
untargeted tissues. Additional studies are required to investigate
novel methods in order to improve the targeting of SPIO-ASCs.
Magnetic resonance targeting (MRT) is a promising method for the
magnetic targeting of deep organs; MRT may steer SPIO-labeled cells
to their target region by means of magnetic field gradients
inherent to all MRI systems. It remains uncertain whether the
regenerated meniscus functions in a normal manner and prevents
secondary osteoarthritic change in the long term. Future studies
should, therefore, include biomechanical and biochemical analysis
of the regenerated meniscus.
In conclusion, in the present study, the targeted
intra-articular delivery of SPIO-ASCs promoted meniscus
regeneration whilst providing protective effects from
osteoarthritic damage. SPIO-labeled ASCs injected into the massive
meniscectomized knee were successfully and non-invasively tracked
by MRI. In addition, implanted ASCs adhered to the injured meniscus
and differentiated specifically into meniscal cells. This
less-invasive and targeted intra-articular delivery of ASCs may
have great therapeutic potential in promoting the regeneration of
meniscus or articular cartilage.
Acknowledgements
The present study was supported by the Natural
Science Youth Foundation of Zhejiang Province (grant no.
LQ14H060001), the Natural Science Grants of Zhejiang Province
(grant no. Y210061), the Natural Science Youth Foundation of China
(grant nos. 81401779, 81201414, 81201408) and the Science
Technology Program of Zhejiang Province (grant no. 2013C23104).
References
|
1
|
Fairbank TJ: Knee joint changes after
meniscectomy. J Bone Joint Surg Br. 30B:664–670. 1948.PubMed/NCBI
|
|
2
|
Fox JM, Blazina ME and Carlson GJ:
Multiphasic view of medial meniscectomy. Am J Sports Med.
7:161–164. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jørgensen U, Sonne-Holm S, Lauridsen F and
Rosenklint AL: Long-term follow-up of meniscectomy in athletes. A
prospective longitudinal study. J Bone Joint Surg Br. 69:80–83.
1987.PubMed/NCBI
|
|
4
|
Sweigart MA and Athanasiou KA: Toward
tissue engineering of the knee meniscus. Tissue Eng. 7:111–129.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baker P, Coggon D, Reading I, Barrett D,
McLaren M and Cooper C: Sports injury, occupational physical
activity, joint laxity, and meniscal damage. J Rheumatol.
29:557–563. 2002.PubMed/NCBI
|
|
6
|
Boyd KT and Myers PT: Meniscus
preservation; rationale, repair techniques and results. Knee.
10:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cook JL: The current status of treatment
for large meniscal defects. Clin Orthop Relat Res. 88–95. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Setton LA, Guilak F, Hsu EW and Vail TP:
Biomechanical factors in tissue engineered meniscal repair. Clin
Orthop Relat Res (Suppl). S254–S272. 1999. View Article : Google Scholar
|
|
9
|
Fraser JK, Wulur I, Alfonso Z and Hedrick
MH: Fat tissue: An underappreciated source of stem cells for
biotechnology. Trends Biotechnol. 24:150–154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi Y, Feng G, Huang Z and Yan W: The
application of super paramagnetic iron oxide-labeled mesenchymal
stem cells in cell-based therapy. Mol Biol Rep. 40:2733–2740. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saldanha KJ, Doan RP, Ainslie KM, Desai TA
and Majumdar S: Micrometer-sized iron oxide particle labeling of
mesenchymal stem cells for magnetic resonance imaging-based
monitoring of cartilage tissue engineering. Magn Reson Imaging.
29:40–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hill JM, Dick AJ, Raman VK, Thompson RB,
Yu ZX, Hinds KA, Pessanha BS, Guttman MA, Varney TR, Martin BJ, et
al: Serial cardiac magnetic resonance imaging of injected
mesenchymal stem cells. Circulation. 108:1009–1014. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
An C, Cheng Y, Yuan Q and Li J: IGF-1 and
BMP-2 induces differentiation of adipose-derived mesenchymal stem
cells into chondrocytes-like cells. Ann Biomed Eng. 38:1647–1654.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsiao JK, Tai MF, Chu HH, Chen ST, Li H,
Lai DM, Hsieh ST, Wang JL and Liu HM: Magnetic nanoparticle
labeling of mesenchymal stem cells without transfection agent:
Cellular behavior and capability of detection with clinical 1.5 T
magnetic resonance at the single cell level. Magn Reson Med.
58:717–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang CY, Hsiao JK, Tai MF, Chen ST, Cheng
HY, Wang JL and Liu HM: Direct labeling of hMSC with SPIO: The
long-term influence on toxicity, chondrogenic differentiation
capacity, and intracellular distribution. Mol Imaging Biol.
13:443–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mankin HJ, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteo-arthritic human hips. II. Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971.PubMed/NCBI
|
|
17
|
Pritzker KP, Gay S, Jimenez SA, Ostergaard
K, Pelletier JP, Revell PA, Salter D and van den Berg WB:
Osteoarthritis cartilage histopathology: Grading and staging.
Osteoarthritis Cartilage. 14:13–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Henning TD, Sutton EJ, Kim A, Golovko D,
Horvai A, Ackerman L, Sennino B, McDonald D, Lotz J and
Daldrup-Link HE: The influence of ferucarbotran on the
chondrogenesis of human mesenchymal stem cells. Contrast Media Mol
Imaging. 4:165–173. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arbab AS, Bashaw LA, Miller BR, Jordan EK,
Lewis BK, Kalish H and Frank JA: Characterization of biophysical
and metabolic properties of cells labeled with superparamagnetic
iron oxide nanoparticles and transfection agent for cellular MR
imaging. Radiology. 229:838–846. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mailänder V, Lorenz MR, Holzapfel V,
Musyanovych A, Fuchs K, Wiesneth M, Walther P, Landfester K and
Schrezenmeier H: Carboxylated superparamagnetic iron oxide
particles label cells intracellularly without transfection agents.
Mol Imaging Biol. 10:138–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Metz S, Bonaterra G, Rudelius M, Settles
M, Rummeny EJ and Daldrup-Link HE: Capacity of human monocytes to
phagocytose approved iron oxide MR contrast agents in vitro. Eur
Radiol. 14:1851–1858. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi Y, Zhao T, Xu K, Dai T and Yan W: The
restoration of full-thickness cartilage defects with mesenchymal
stem cells (MSCs) loaded and cross-linked bilayer collagen
scaffolds on rabbit model. Mol Biol Rep. 39:1231–1237. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen FH and Tuan RS: Mesenchymal stem
cells in arthritic diseases. Arthritis Res Ther. 10:2232008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kern S, Eichler H, Stoeve J, Klüter H and
Bieback K: Comparative analysis of mesenchymal stem cells from bone
marrow, umbilical cord blood, or adipose tissue. Stem Cells.
24:1294–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horie M, Sekiya I, Muneta T, Ichinose S,
Matsumoto K, Saito H, Murakami T and Kobayashi E: Intra-articular
injected synovial stem cells differentiate into meniscal cells
directly and promote meniscal regeneration without mobilization to
distant organs in rat massive meniscal defect. Stem Cells.
27:878–887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murphy JM, Fink DJ, Hunziker EB and Barry
FP: Stem cell therapy in a caprine model of osteoarthritis.
Arthritis Rheum. 48:3464–3474. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruiz-Ibán MA, Díaz-Heredia J, García-Gómez
I, Gonzalez-Lizán F, Elías-Martín E and Abraira V: The effect of
the addition of adipose-derived mesenchymal stem cells to a
meniscal repair in the avascular zone: An experimental study in
rabbits. Arthroscopy. 27:1688–1696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi Y, Feng G and Yan W: Mesenchymal stem
cell-based treatment for cartilage defects in osteoarthritis. Mol
Biol Rep. 39:5683–5689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uccelli A, Pistoia V and Moretta L:
Mesenchymal stem cells: A new strategy for immunosuppression?
Trends Immunol. 28:219–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen X, Armstrong MA and Li G: Mesenchymal
stem cells in immunoregulation. Immunol Cell Biol. 84:413–421.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caplan AI and Dennis JE: Mesenchymal stem
cells as trophic mediators. J Cell Biochem. 98:1076–1084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koelling S and Miosge N: Stem cell therapy
for cartilage regeneration in osteoarthritis. Expert Opin Biol
Ther. 9:1399–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bulte JW and Kraitchman DL: Iron oxide MR
contrast agents for molecular and cellular imaging. NMR Biomed.
17:484–499. 2004. View
Article : Google Scholar : PubMed/NCBI
|