Introduction
Joint contracture is a major complication following
elbow trauma that restricts joint motion, and additional surgical
procedures are often required for the treatment of severe joint
contracture. In the anatomical context of a contracted joint, the
joint capsule is regarded as the critical motion-limiting structure
(1). In patients with chronic elbow
contractures following trauma, the fibrotic joint capsule becomes
markedly thickened and disorganized compared with normal elbows
(2,3). Cellular, matrix, and growth factor
components of the joint capsule are changed during the formation of
fibrotic joint capsules in a post-traumatic contracture model
(4). Multiple scientific groups are,
therefore, investigating novel strategies to reduce joint capsule
fibrosis in order to prevent joint contracture.
Prior studies have reported that ketotifen, a mast
cell stabilizer, inhibits joint capsule fibrosis in a joint
contracture model following trauma (5,6). The
formation of joint contractures may also be inhibited by the
intra-articular injection of lentivirus (LV)-mediated extracellular
signal-regulated kinase 2 (ERK2) small interfering RNA (siRNA);
myofibroblast hyperplasia and its subsequent reduction also appear
to be affected by the downregulation of pERK2 (7).
In the present study, the effects of LV-mediated
ERK2 siRNA treatment of the fibrotic joint capsule of a contracted
joint were, therefore, investigated; specifically, the effects upon
extracellular matrix components, including total collagen, collagen
I, collagen III, matrix metalloproteinase (MMP)-1, MMP-13 and
tissue inhibitor of metalloproteinase (TIMP)-13 were evaluated.
Materials and methods
Group allocation
All experimental procedures were approved by the
Institutional Animal Review Committee of Shanghai Jiaotong
University Affiliated Sixth People's Hospital (Shanghai, China). A
total of 57 female rats, weighing 0.22–0.28 kg and aged 3 months,
were purchased from the Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China). As previously described (7), the rats were randomly separated into
three groups (n=19 in each), as follows: The operated contracture
(ORC) group, the contracture-treatment (CNT) group and the
non-operated control (CON) group. In the ORC and CNT groups,
representative post-traumatic joint contracture was developed
through 8 weeks of immobilization following surgical
intra-articular injury. Rats of the CNT group then received an
intra-articular injection of 0.1 ml LV particles containing ERK2
siRNA at days 3 and 7 after surgery. The rats of the CON group did
not receive any surgical or pharmacological intervention.
LV-mediated ERK2 siRNA construction
and in vivo bioluminescence detection
The siRNA sequence targeting rat ERK2
(5′-GCACCTCAGCAATGATCAT-3′) has previously been reported to
efficiently downregulate ERK2 expression in rats (7). Pairs of complementary oligonucleotides
containing these sequences were synthesized (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and cloned into the
pshRNA-H1-Luc lentivector (System Biosciences, Mountain View, CA,
USA). Next, 293T producer cells were co-transfected with pPACK
Packaging Plasmid Mix and the pshRNA-H1-Luc lentivectors containing
the shRNA sequences (System Biosciences, Mountain View, CA, USA).
Viral supernatants were collected after 48 h, centrifuged at 5,000
× g at 4°C for 5 min to eliminate cell debris and filtered through
0.45 to l-µm polyvinylidene fluoride filters (EMD Millipore,
Billerica, MA, USA). The viral titers were determined with serial
dilutions of concentrated LV. As previously described (7), the luciferase expression and
distribution of the rats in the CNT group were measured using a
Xenogen IVIS 50 Bioluminescence System (PerkinElmer, Inc., Waltham,
MA, USA) 2 and 8 weeks after surgery. For a basis of comparison,
the rats in the ORC group were also imaged using the with Xenogen
IVIS 50 Bioluminescence System.
Joint interventions
As previously described (7), the rats were placed in a supine
position for surgery under aseptic conditions; general anesthesia
was administered via inhalation, using 2–3% isoflurane and oxygen.
Following a midline skin incision in the right knee joint, a
lateral parapatellar arthrotomy was performed. The patella was
moved medially and the knee joint was flexed to expose the femoral
condyles. Two 1.5×1.5-mm cortical windows were created from the
non-articulating cartilaginous regions of the medial and lateral
femoral condyles using a 1.5-mm drill bit. The anterior and
posterior cruciate ligaments were then incised, and the knee was
overextended to 45° to disrupt the posterior joint capsule. The
right knee was immobilized at flexion of 140° with surgical
sutures. The patellofemoral joint was appropriately reduced prior
to incision closure. Following joint interventions, the rats were
permitted unrestricted movement in their cages (10 rats to a cage
of 0.1 m3).
Western blotting
A total of 15 rats (n=5 per group) were sacrificed
using an overdose of pentobarbital sodium (Sumimoto Dainippon
Pharma Co., Ltd., Osaka, Japan) at 2 and 8 weeks, respectively.
Posterior joint capsules were then gathered and lysed with lysis
buffer. The cell lysates were centrifuged at 13,000 × g for 15 min
at room temperature, and the supernatants collected were used for
western blotting. Equal amounts of protein were separated on a 12%
sodium dodecyl sulfate gel using polyacrylamide gel electrophoresis
and electrotransferred to nitrocellulose membranes (EMD Millipore).
The membranes were blocked with 5% skimmed milk, and incubated with
antibodies against glyceraldehyde 3-phosphate dehydrogenase, ERK2
and pERK (Cell Signaling Technology Inc., Danvers, MA, USA) for 1 h
at room temperature. Following a washing step, the membranes were
incubated with horseradish peroxidase-conjugated immunoglobin G
(Acris Antibodies, GmbH, Herford, Germany) for 1 h and
immunoreactive bands were detected by chemiluminescence (Amersham
Biosciences, Freiburg, Germany).
Histological assessment
As previously described (7), 23 rats across the three groups were
euthanized using an overdose of pentobarbital sodium (Dainippon
Pharmaceutical) 8 weeks after surgery. Posterior capsule tissues of
the right knees were immediately removed and the tissues were cut
into halves. One half was quickly frozen for subsequent
immunohistochemical staining of α-smooth muscle actin for use in
another study (7). The other half
was fixed with formalin, embedded with paraffin and cut into 4-mm
thick sections. A number of sections were stained using the Masson
trichrome staining method for collagen, as follows: First, the
tissue was deparaffinized and hydrated using graded ethanol and
xylene solutions, followed by distilled water. The tissue slides
were incubated in Weigert iron hematoxylin stain, followed by
counterstaining with Biebrich scarlet-acid fuchsin and aniline blue
(Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturers
instructions. Masson trichrome staining was used to demonstrate the
association of muscle cells with collagen.
Immunohistochemistry
Immunohistochemistry was performed on the remaining
sections. Primary rabbit monoclonal antibodies against rat collagen
I (1:1,000; ab34710), collagen III (1:1,000; ab7778), MMP-1 (1:40;
ab118529), MMP-13 (1:100; ab39012) and TIMP-1 (1:40; ab61224;
Abcam, Cambridge, UK) were applied to slides overnight (~18 h at
4°C). Following a washing step, all slides were exposed to the
secondary goat anti-rabbit antibody, Super Sensitive Rabbit Link
(1:1,000; BioGenex, Shanghai, China), for 1 h at room temperature
and washed again. Finally, the sections were stained with
3,3′-diaminobenzidine for 1 min, and counterstained with
hematoxylin. The slides were assessed under an optical microscope
(DP75; Olympus Corporation, Tokyo, Japan), and the observer was
blinded to which specimen was being examined. The
immunohistochemical staining intensities of collagen I and III were
graded on a scale of 0–3 as follows: No staining, 0; weak staining,
1; moderate staining, 2; and intense staining, 3. A total of 9
randomly-selected sections were then examined for collagen I and
III, which were again assessed by the same observer blinded to the
conditions.
Statistical analysis
The data were statistically analyzed using a one-way
analysis of variance with Student-Newman-Keuls post hoc t-test.
Differences were considered to be statistically significant for
values of P<0.05. All statistical analyses were conducted using
SPSS 11.0 (SPSS Inc., Chicago, IL, USA).
Results
Among the 57 rats used in this study, 4 animals were
excluded from the study due to a failure to thrive, a tibial
fracture and immobilization failure. The other animals appeared
healthy, with no signs of impaired wound healing or deep
infection.
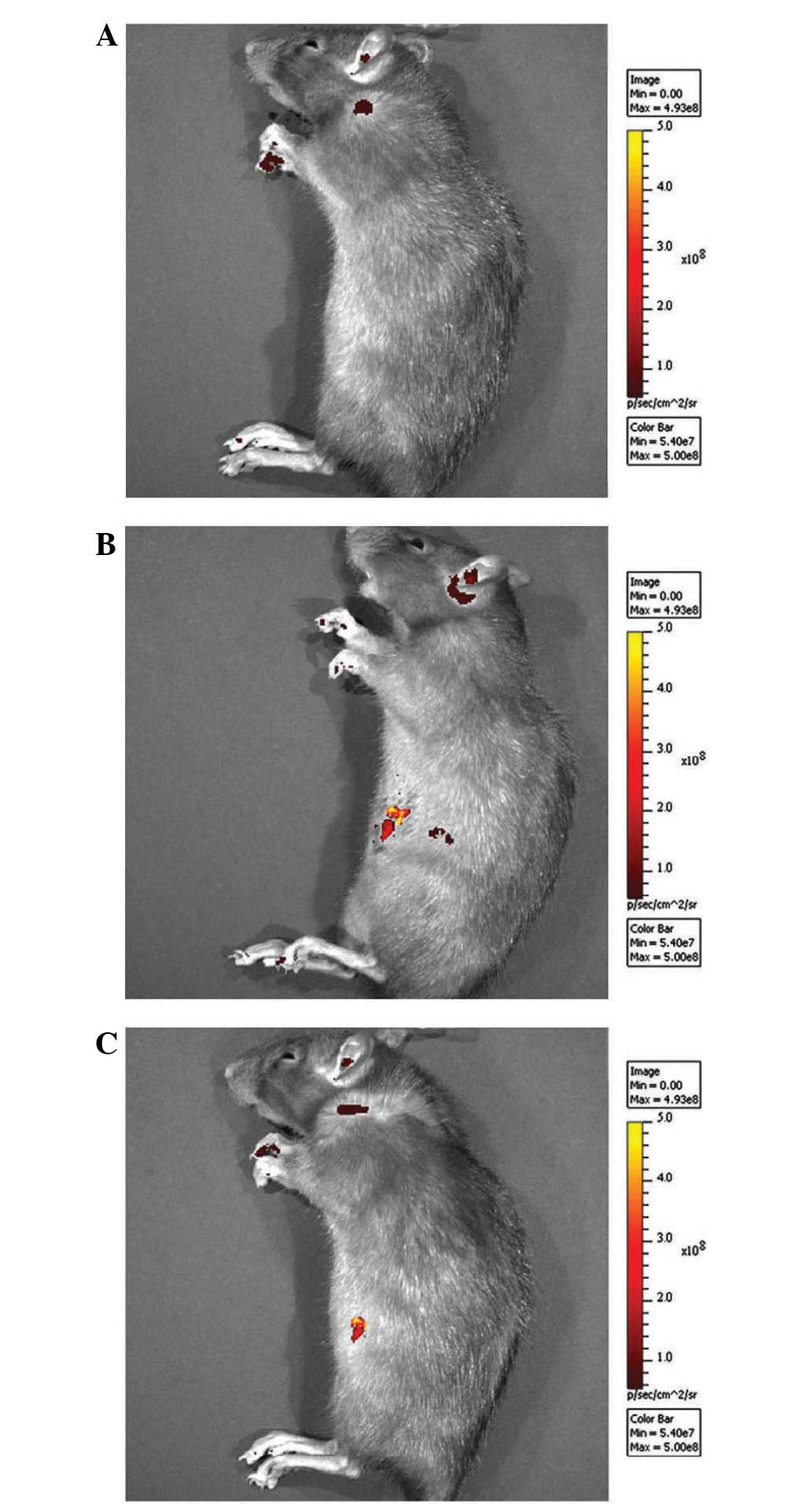
As previously described (7), fluorescence was detected in the right
knee joints of rats of the CNT group 2 weeks after surgery
(Fig. 1). At 8 weeks after surgery,
luciferase fluorescence did not significantly decrease (Fig. 1), and no fluorescence was detected in
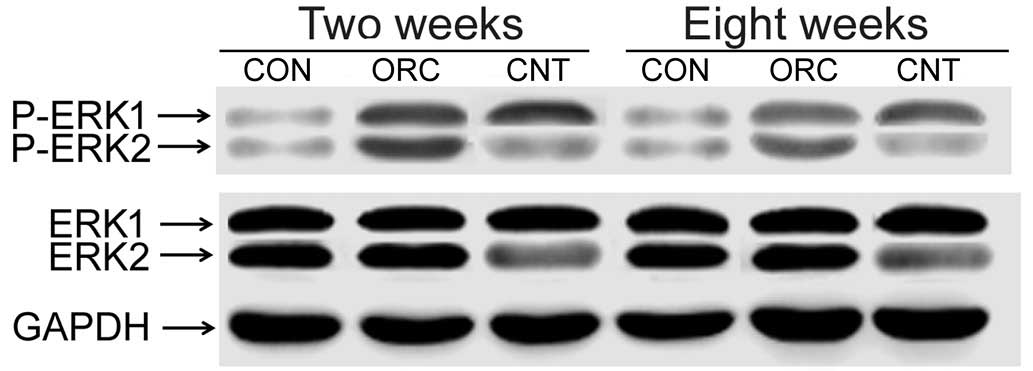
the right knee joints of rats of the ORC group (Fig. 1). Western blot analysis demonstrated
that pERK2 levels of the posterior joint capsule from rats in the
ORC group were significantly increased compared with those in the
CON group at 2 and 8 weeks (Fig. 2).
However the rate of increase was greater at 2 weeks than at 8 weeks
(Fig. 2). The successful injection
of LV-mediated ERK2 siRNA into the joints of rats of the CNT group
(Fig. 2) specifically downregulated
pERK2 levels of the posterior joint capsule by inhibiting ERK2
protein expression levels compared with those in the ORC group at 2
and at 8 weeks (Fig. 2).
The posterior joint capsules of the right knees of 8
rats in the CON group, 8 rats in the ORC group and 7 rats in the
CNT group were examined by histology and immunohistochemistry.
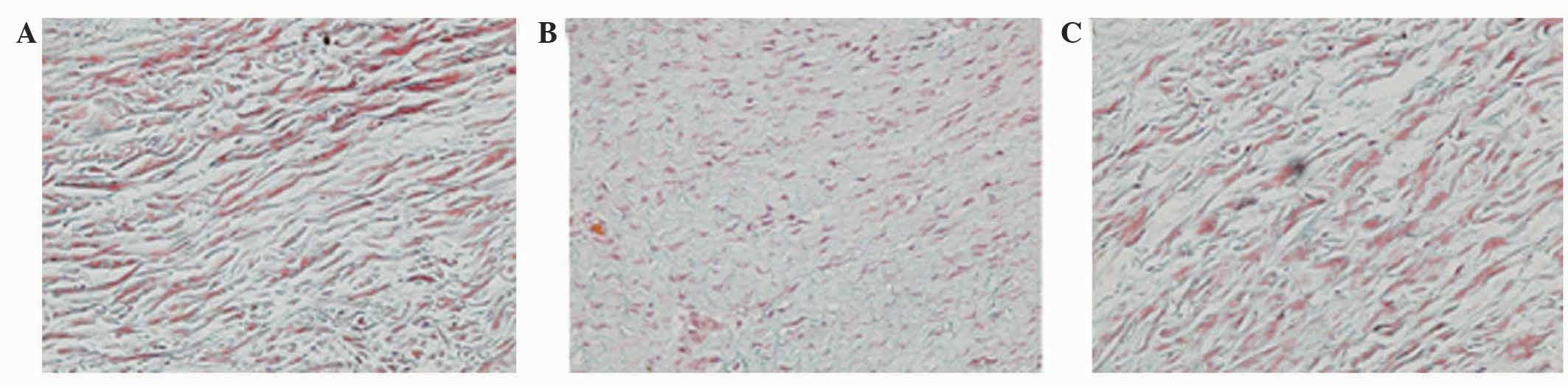
Under light microscopy, Masson trichrome staining of all control
specimens revealed well-organized, parallel, sparse collagen fibers
between intervening cells (Fig. 3A).
All capsules from the ORC group demonstrated extensive
disorganization of the collagen fiber bundle arrangement with a
dense structure (Fig. 3B). Similar
to the capsule wall structure of the CON group, tissue of the CNT
group became sparse, with well-organized collagen fibers (Fig. 3C). Evaluation of the staining
intensity of collagen demonstrated significant differences between
the ORC and CON groups, and between the ORC and CNT groups
(P<0.05; Table I).
 | Table I.Mean staining intensities of total
collagen, collagen type I, collagen type III, MMP-1, MMP-13 and
TIMP-13. |
Table I.
Mean staining intensities of total
collagen, collagen type I, collagen type III, MMP-1, MMP-13 and
TIMP-13.
| Target | CON (n=8) | ORC (n=8) | CNT (n=7) |
|---|
| Total collagen | 1.9 (0.5) | 2.8 (0.5) | 2.0 (0.4) |
| Collagen type I | 1.9 (0.6) | 2.7 (0.5) | 1.9 (0.7) |
| Collagen type
III | 2.7 (0.4) | 2.1 (0.6) | 2.8 (0.5) |
| MMP-1 | 0.8 (0.5) | 1.3 (0.5) | 0.9 (0.5) |
| MMP-13 | 1.2 (0.9) | 1.7 (0.3) | 1.3 (0.4) |
| TIMP-13 | 1.6 (0.5) | 1.2 (0.9) | 1.5 (0.6) |
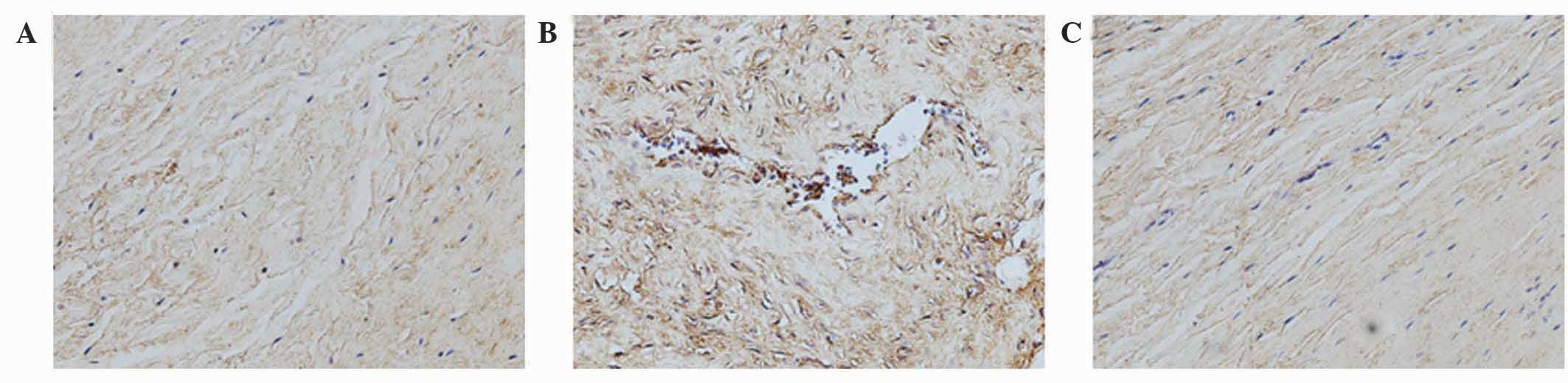
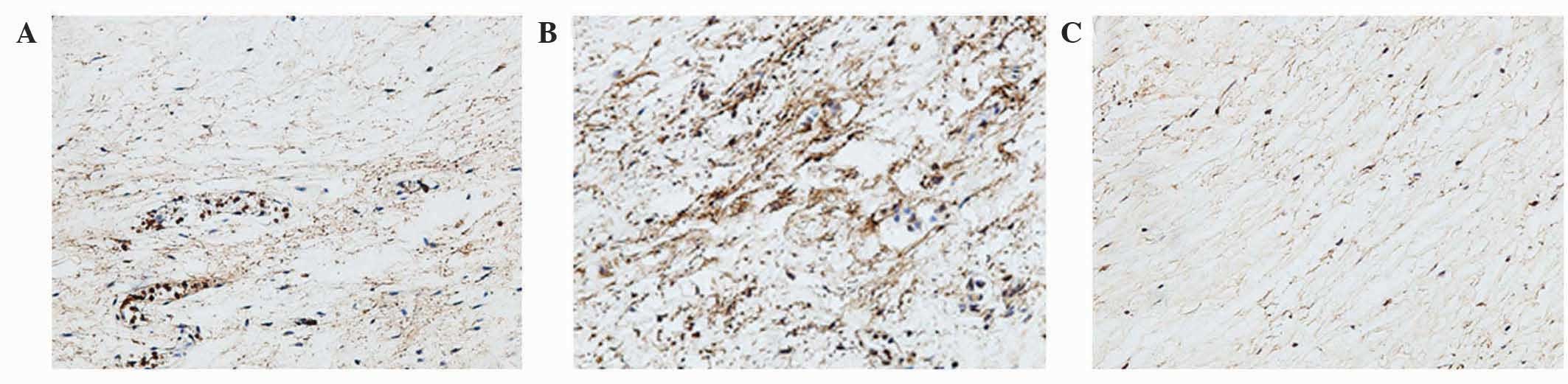
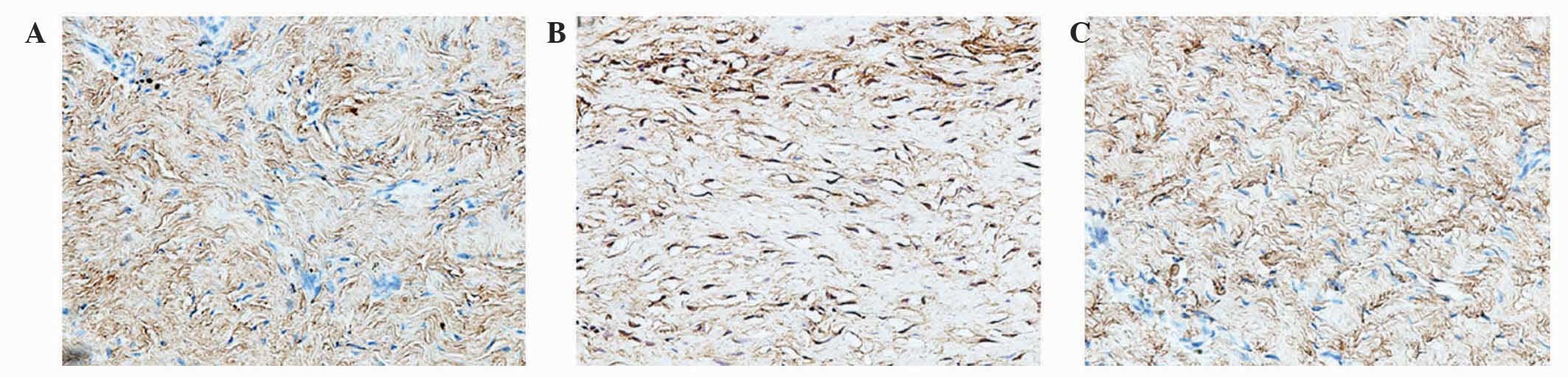
Analysis of the staining intensities of collagen I,
MMP-1 and MMP-13 demonstrated a marked difference between the
contracture and the control capsules (Figs. 4–6).
However, the intra-articular delivery of LV-mediated ERK2 siRNA
significantly reduced the staining intensities of collagen I, MMP-1
and MMP-13 in capsule tissue of the CNT group compared with the ORC
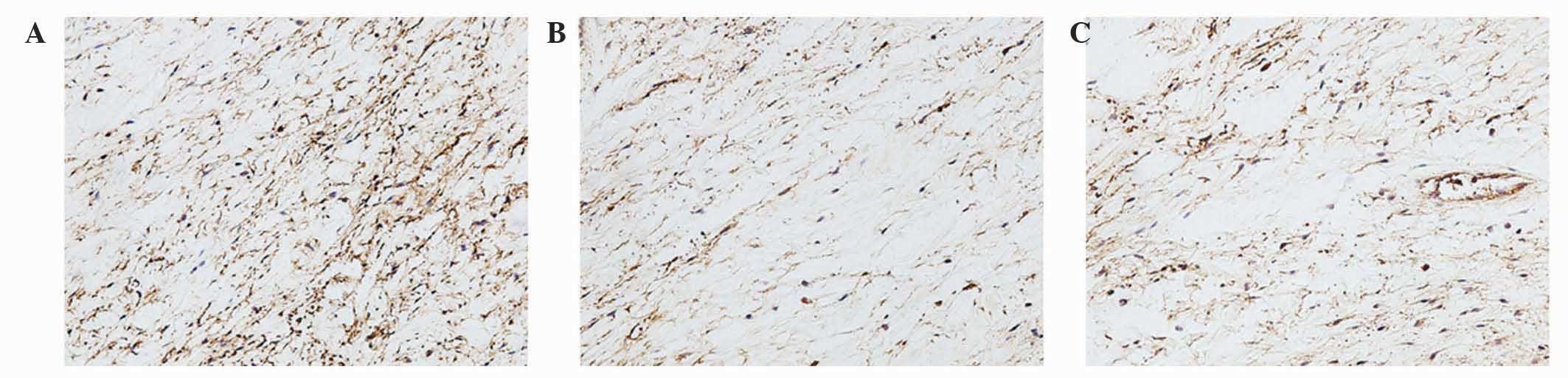
group (Figs. 4–6). However, the staining intensities of
collagen III and TIMP-13 were weaker in the ORC group compared with
the CON group, but were elevated following LV-mediated ERK2 siRNA
treatment of the fibrotic joint capsule in the CNT group (Figs. 7 and 8). Assessment by blinded observers revealed
significant differences in staining intensities between the ORC and
CON groups, and between the ORC and CNT groups (P<0.05; Table I).
Discussion
A feature of the post-traumatic joint contracture
model used in the present study is irreversible loss of joint
motion, irrespective of the period of remobilization (8). By contrast, other animal models of
post-traumatic immobilization have revealed reversible joint motion
loss following periods of remobilization equal to the duration of
initial immobilization (9–11), bringing into question the unique
pathological changes occurring following trauma that render the
joint susceptible to irreversible joint contractures. Based on
previous studies in animals and humans, joint capsule fibrosis
observed in post-traumatic joint contractures may account for this
irreversible loss of joint motion; myofibroblasts are considered to
be the cell type predominantly involved in the development of joint
capsule fibrosis (12–14), and elbow extension-flexion motion is
inversely proportional to the number of myofibroblasts present
within the capsule of the elbow joint (15). A previous study using the current
model also supports this hypothesis, as the joint capsules from
rats of the ORC group were densely filled with myofibroblasts
(7). In addition, treatment of the
CNT group with LV-mediated ERK2 siRNA significantly reduced (or
prevented) the number of myofibroblasts within the joint capsule
following trauma-induced joint contracture (7). However, this previous study did not
investigate extracellular matrix turnover of the fibrotic joint
capsule, leading to its evaluation in the current study.
The joint capsule is composed mainly of collagen
fibers and the normal joint capsule is compliant, well-organized
and thin. The present study evaluated the morphological
characteristics and the expression of specific types of collagen in
the fibrotic capsule in order to reveal the structural and
biochemical changes of the capsule that may lead to motion
limitation of the joint following traumatic injury. Collagen
content is a key factor that determines the mechanical strength of
healing tissue (16–18). The relative proportions of collagen I
and III as a proportion of the total collagen are another
determinant of the mechanical properties of a tissue, with a higher
proportion of collagen III hypothesized to reduce the strength of
the fibrotic joint capsule by decreasing fiber diameter (16–18). One
previous study demonstrated that the expression levels of collagen
I and III proteins, which are major constituents of contracted
tissue, were also increased in fibrotic joint capsules (19). Concordantly, the present study
revealed that staining of total collagen and collagen I was more
intense in the contracted capsules than in the control capsules. In
contrast to total collagen and collagen I, staining for collagen
III was more intense in the control capsules, which is in agreement
with the results of the study by Matsumoto et al (20). These results demonstrate a mechanism
of contracture tissue formation that differs from that of wound
healing, with increased levels of collagen type III (21,22).
Similar to total collagen and collagen I, the levels of MMP-13 and
MMP-1 were increased in the capsule of the ORC group compared with
the CON group in the present study. By contrast, TIMP-13 was
decreased in the control capsules compared with the contracted
capsules. This high matrix turnover of joint capsule in the
fibrotic joint capsule has also been reported in previous studies
(21,22). An increase of pERK2 in rats with
trauma-induced joint capsule fibrosis was observed in the present
study, which may be responsible for these pathological alterations.
Furthermore, LV-mediated ERK2 siRNA was responsible for
pathological alterations in the joint capsular tissue of the CNT
group by downregulating pERK2 levels, which may reflect the
important role of pERK2 in these pathological alterations.
The results of the present study demonstrated that
high extracellular matrix turnover has an important role in the
pathogenesis of the fibrotic joint capsule, and that the
phosphorylation of ERK2 is a key factor in reducing joint capsule
fibrosis by changing extracellular matrix turnover.
Acknowledgements
The present study was supported by the Chinese
National Natural Science Foundation (grant no. GSCX0818005).
References
|
1
|
Lindenhovius AL and Jupiter JB: The
posttraumatic stiff elbow: A review of the literature. J Hand Surg
Am. 32:1605–1623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gallay SH, Richards RR and O'Driscoll SW:
Intraarticular capacity and compliance of stiff and normal elbows.
Arthroscopy. 9:9–13. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen MS, Schimmel DR, Masuda K, Hastings
H 2nd and Muehleman C: Structural and biochemical evaluation of the
elbow capsule after trauma. J Shoulder Elbow Surg. 16:484–490.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hildebrand KA, Zhang M, Germscheid NM,
Wang C and Hart DA: Cellular, matrix, and growth factor components
of the joint capsule are modified early in the process of
posttraumatic contracture formation in a rabbit model. Acta Orthop.
79:116–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Monument MJ, Hart DA, Befus AD, Salo PT,
Zhang M and Hildebrand KA: The mast cell stabilizer ketotifen
reduces joint capsule fibrosis in a rabbit model of post-traumatic
joint contractures. Inflamm Res. 61:285–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monument MJ, Hart DA, Befus AD, Salo PT,
Zhang M and Hildebrand KA: The mast cell stabilizer ketotifen
fumarate lessens contracture severity and myofibroblast
hyperplasia, A study of a rabbit model of posttraumatic joint
contractures. J Bone Joint Surg Am. 92:1468–1477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li F, Liu S and Fan C: Lentivirus-mediated
ERK2 siRNA reduces joint capsule fibrosis in a rat model of
post-traumatic joint contracture. Int J Mol Sci. 14:20833–20844.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hildebrand KA, Zhang M and Hart DA: Joint
capsule matrix turnover in a rabbit model of chronic joint
contractures, Correlation with human contractures. J Orthop Res.
24:1036–1043. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akeson WH, Woo SL, Amiel D and Doty DH:
Rapid recovery from contracture in rabbit hindlimb. A correlative
biomechanical and biochemical study. Clin Orthop Relat Res.
359–365. 1977.PubMed/NCBI
|
|
10
|
Finsterbush A and Friedman B:
Reversibility of joint changes produced by immobilization in
rabbits. Clin Orthop Relat Res. 290–298. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schollmeier G, Sarkar K, Fukuhara K and
Uhthoff HK: Structural and functional changes in the canine
shoulder after cessation of immobilization. Clin Orthop Relat Res.
310–315. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hildebrand KA, Zhang M, van Snellenberg W,
King GJ and Hart DA: Myofibroblast numbers are elevated in human
elbow capsules after trauma. Clin Orthop Relat Res. 189–197. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdel MP, Morrey ME, Barlow JD, Kreofsky
CR, An KN, Steinmann SP, Morrey BF and Sanchez-Sotelo J:
Myofibroblast cells are preferentially expressed early in a rabbit
model of joint contracture. J Orthop Res. 30:713–719. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hildebrand KA, Sutherland C and Zhang M:
Rabbit knee model of post-traumatic joint contractures, The
long-term natural history of motion loss and myofibroblasts. J
Orthop Res. 22:313–320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Germscheid NM and Hildebrand KA: Regional
variation is present in elbow capsules after injury. Clin Orthop
Relat Res. 450:219–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukui N, Tashiro T, Hiraoka H, Oda H and
Nakamura K: Adhesion formation can be reduced by the suppression of
transforming growth factor-beta1 activity. J Orthop Res.
18:212–219. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukui N, Nakajima K, Tashiro T, Oda H and
Nakamura K: Neutralization of fibroblast growth factor-2 reduces
intraarticular adhesions. Clin Orthop Relat Res. 250–258. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukui N, Fukuda A, Kojima K, Nakajima K,
Oda H and Nakamura K: Suppression of fibrous adhesion by
proteoglycan decorin. J Orthop Res. 19:456–462. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hildebrand KA, Zhang M and Hart DA: High
rate of joint capsule matrix turnover in chronic human elbow
contractures. Clin Orthop Relat Res. 439:228–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumoto F, Trudel G and Uhthoff HK: High
collagen type I and low collagen type III levels in knee joint
contracture, An immunohistochemical study with histological
correlate. Acta Orthop Scand. 73:335–343. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robins SP, Milne G, Duncan A, Davies C,
Butt R, Greiling D and James IT: Increased skin collagen
extractability and proportions of collagen type III are not
normalized after 6 months healing of human excisional wounds. J
Invest Dermatol. 121:267–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sculean A, Junker R, Donos N, Berakdar M,
Brecx M and Dünker N: Immunohistochemical evaluation of matrix
molecules associated with wound healing following regenerative
periodontal treatment in monkeys. Clin Oral Investig. 6:175–182.
2002.PubMed/NCBI
|