Introduction
Hypohidrotic ectodermal dysplasia (HED), also known
as anhidrotic ectodermal dysplasia (AED) or Christ-Siemens-Touraine
Syndrome, is an X-linked recessive genetic disease (1). HED is a rare congenital genetic
disorder with a birth incidence of 1/100,000–1/10,000 (2). It is characterized by the diminution or
absence of eccrine sweat glands, oligodontia and peg-shaped teeth,
and hair that is sparse and fine (1,3).
Previous study indicates that X-linked HED (XLHED) is caused by
mutations of the ectodysplasin A (EDA) gene at Xq12–13.1
(4).
The EDA gene encodes the protein
ectodysplasin A, a member of the tumor necrosis factor (TNF) ligand
family, which is associated with NF-κB signaling mechanisms
(5,6). Bayés et al (7) indicated that the EDA gene
(GenBank Gene ID: 1896) has a variant 1 transcript (EDA-A1) with a
full length of 5,296 bp (NM_001399.4, GI: 54112099), which has an
open reading frame of 1,176 bp, and encodes a protein with 391
amino acids. Our previous clinical and molecular study of a family
with XLHED, it was showed that a missense mutation of EDA-A1
(907A→C; A907C) would cause the change of a glutamine residue to a
proline residue (Gln306Pro), and eukaryotic expression vectors
carrying mutant EDA-A1 (pcDNA3.1 (−)-EDA-A1-M) and wild-type
EDA-A1 (pcDNA3.1 (−)-EDA-A1-W) were constructed (8).
Human umbilical vein endothelial cells (HUVECs) are
cells derived from the endothelium of veins from the umbilical
cord. They are used as a laboratory model system for the study of
the function and pathology of endothelial cells (9). In the present study, the effects of
transfection with the EDA-A1 gene and its mutant on the
proliferation, cell cycle and protein expression of HUVECs were
investigated.
Materials and methods
Cell culture
The ECV304 HUVECs were provided by Professor
Chunming Wang (Lanzhou University, Lanzhou, China). The cells were
cultured in RPMI-1640 (Huamei Biotechnique Co., Ltd., Shanghai,
China). The medium included 10% fetal bovine serum (FBS; Evergreen
Biological Engineering Materials, Co. Ltd., Hangzhou, China) and
100 U/ml penicillin/streptomycin (Gibco; Thermo Fisher Scientific
Inc., Waltham, MA, USA). The cells were maintained in a humidified
incubator in an atmosphere containing 5% CO2 at 37°C,
and subjected to digestion with 0.25% trypsin (Gibco; Thermo Fisher
Scientific, Inc.) overnight. Cells were maintained at
2×105-1×106 cells/ml. An Olympus IX70
inverted microscope (Olympus Corporation, Tokyo, Japan) was used
for the observation of cell morphology.
Plasmid extraction
The eukaryotic plasmids, pcDNA3.1 (−)-EDA-A1-M and
pcDNA3.1 (−)-EDA-A1-W, were constructed as previously described
(8). Plasmid DNA was extracted using
Plasmid Extraction kit (Tiangen Biotech Co., Ltd., Beijing, China),
according to the manufacturer's protocol, and 3 µl DNA was
subsequently diluted to 1 ml with sterile deionized water.
Absorbance (A) values at 260 and 280 nm were measured using a UV
spectrophotometer (UV-2401, Shimadzu Corpoartion, Kyoto, Japan).
The plasmid DNA concentration was calculated as follows: Plasmid
DNA concentration (µg/µl) = A260 × dilution factor × 50/1,000. The
plasmid DNA (positive recombinants and empty control) was
precipitated with ethanol. Each DNA pellet was then resuspended in
sterile deionized water.
Cell transfection
Transfection of the ECV304 cells was performed using
the calcium phosphate co-precipitation method, according to the
protocol provided with the Effectene Transfection Reagent kit
(Qiagen GmbH, Hilden, Germany). Transfection was carried out when
the cell density had reached 70% confluence after 24 h of
cell-passaging. Cells were transferred into a complete medium (CM)
2 h prior to transfection. Then, 2.5 µg plasmid DNA was slowly
added to 2 M CaCl2 and allowed to stand for 10 min. The
DNA-CaCl2 solution was slowly added dropwise to 2X
HEPES-buffered saline (HeBS), containing 280 mM NaCl, 1.5 mM
Na2HPO4, and 50 mM HEPES (pH 7.05), and
allowed to stand for 30 min until tiny particles precipitated. The
precipitate was uniformly dropwise added to the cells (70%
confluence; ~2×105 cells/ml) in the culture flasks.
After a 12-h growth at 37°C in a humidified incubator containing 5%
CO2, cells were washed twice with HeBS, followed by
culturing in CM. Empty vector-transfected cells were used as the
control group.
Reverse transcription-polymerase chain
reaction (RT-PCR)
To semi-quantitatively analyze the expression levels
of EDA-A1 in cells, RT-PCR analysis was performed. Total RNA
was extracted from the cells from each group after culturing for 48
h, using a reverse transcription (RT) kit (Fermentas; Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA). Primers for EDA-A1
were designed using Primer Premier 5.0 software (Premier Biosoft,
Palo Alto, CA, USA) and synthesized by Shanghai Sangon Biological
Engineering Technology and Services Co., Ltd. (Shanghai, China).
The primers used were as follows: EDA-A1 (408 bp), forward:
5′-CGCAGGATCCATGGGCTACCCGGAGGT-3′, and reverse:
5′-ATTAAGCTTGCCAAGCGGGCACCAGGGAGAC-3′; β-actin (230 bp), forward:
5′-TTCACAGGCAGGACAGAAGA-3′, and reverse:
5′-TTGAAGGTCGCAGAGTTCCT-3′. The 50-µl PCR reaction system comprised
cDNA template (2 µl), 1X PCR Buffer (5 µl), deoxynucleotide (dNTP;
1 µl), primer (forward and reverse, 1 µl), Taq DNA polymerase (1
µl) and ddH2O (39 µl). RT-PCR was performed in a thermal
reactor (Thermocycler, Takara Bio Inc., Otsu, Japan). Products were
subjected to electrophoresis (1.5% agarose gel, 120 V, 90 mA).
Western blot analysis
In order to prepare cell lysates, cells were
collected and cell extracts were prepared using
radioimmunoprecipitation assay buffer, according to the
manufacturer's protocol (Biotek Corporation, Beijing, China). Cell
lysates were collected following centrifugation at 9,500 × g for 15
min at 4°C, and were subsequently transferred to clean
microcentrifuge tubes. For western blot analysis, proteins were
extracted from the cells in each group. Proteins were collected
following cell lysis. The Bradford protein assay (10) was used to confirm the protein
content. The proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to a
0.45-µm pore size nitrocellulose (NC) membrane (RPN303E; Amersham;
GE Healthcare Life Sciences, Chalfont, UK). Membranes were blocked
with Tris-buffered saline (TBS; Boster Biological Technology, Ltd.,
Wuhan, China) containing 5% milk and 0.5% Tween for 1 h (37°C), and
then washed three times with 0.1 M TBS (pH 7.6) with 0.1% Tween
(TBST). Anti-EDA-A1 rabbit anti-serum polyclonal antibody was
obtained by custom rabbit immunization using purified FLAG-EDA as
the immunogen. Then, the NC membrane was treated with TBST solution
(containing 2% milk, 1:200 dilution of anti-EDA-A1 rabbit
anti-serum) for 1 h at 37°C, and washed with TBST three times.
Following incubation with horseradish peroxidase-conjugated
anti-rabbit immunoglobulin G secondary antibody (1:2,000; A6154;
Sigma-Aldrich, St. Louis, MO, USA), the expression levels of the
target protein were visualized with SuperSignal West Femto Maximum
Sensitivity Substrate (Pierce Biotechnology, Inc., Rockford, IL,
USA)
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay for the detection of cell proliferation
To determine the proliferation of each group of
ECV304 cells, an MTT assay was performed. The 24-h-infected and
uninfected cells were seeded into a 96-well plate with an
inoculation density of 5,000 cells/well and incubated at 37°C.
After 12 h, 100 µl serum-free Dulbecco's modified Eagle's medium
was added to each well. After 72 h, 20 µl MTT was added to each
well and incubation was continued at 37°C for 4 h. Then, the medium
was removed and the precipitate was dissolved in dimethylsulfoxide.
The absorbance (optical density, OD) at 560 nm was measured using a
SpectraMax 190 microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA). The inhibition rate of cell growth was
calculated (n=10) on the basis of the experimentally measured OD
value.
Cell cycle analysis
Flow cytometry was used to investigate the cell
cycle. Following incubation for 48 h, the cells were collected and
washed with cold phosphate-buffered saline. The washed cells were
fixed in 70% cold ethanol with incubation overnight at 4°C. To
stain the cells, propidium iodide (PI) solution was added. A flow
cytometer (Coulter Epics XL; Beckman Coulter, Inc., Brea, CA, USA)
was used to analyze the samples. CellQuest 6.0 software (BD
Biosciences, San Jose, CA, USA) was used to analyze the percentage
of cells in the G0/G1, S and G2/M
phases (n=5).
Statistical analysis
SPSS statistical analysis software, version 13.0
(SPSS, Inc., Chicago, IL, USA) was used to conduct analysis of
variance testing for all data, which are expressed as the mean ±
standard deviation. P-values less than 0.05 were considered to
indicate a statistically significant difference.
Results
EDA-A1 expression pattern in ECV304
cells is influenced by plasmid-mediated transfection
To determine the expression level of ED1-A1 in the
transfected ECV304 cells, the RNA samples with an OD260/OD280 ratio
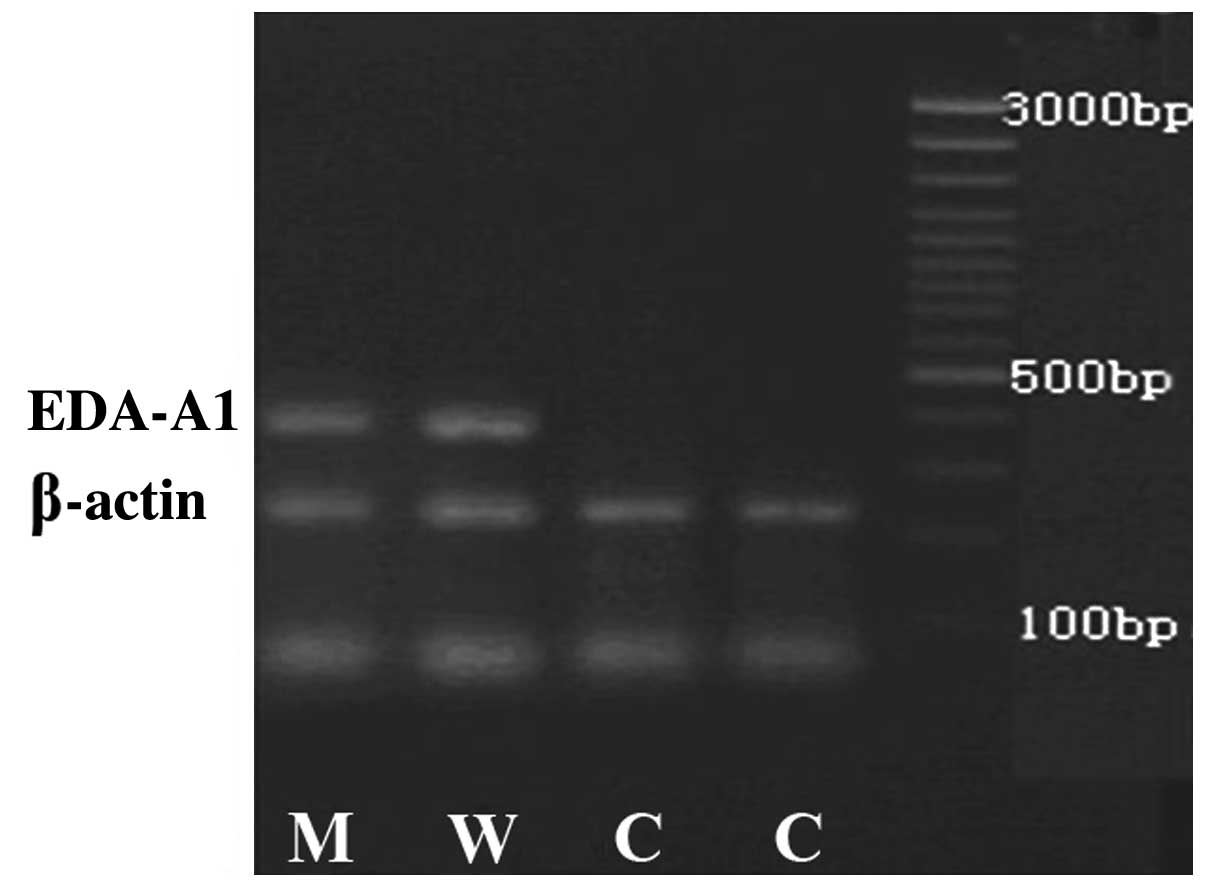
of 1.8–2.0 were selected for RT-PCR. The ECV304 cells transfected
with pcDNA3.1 (−)-EDA-A1-M or pcDNA3.1 (−)-EDA-A1-W showed a band
at ~400 bp that was not observed for the empty vector-transfected
control cells when examined using semi-quantitative PCR and primers
specific to EDA-A1 (Fig. 1).
Additionally, a β-actin band between 200 bp and 300 bp was observed
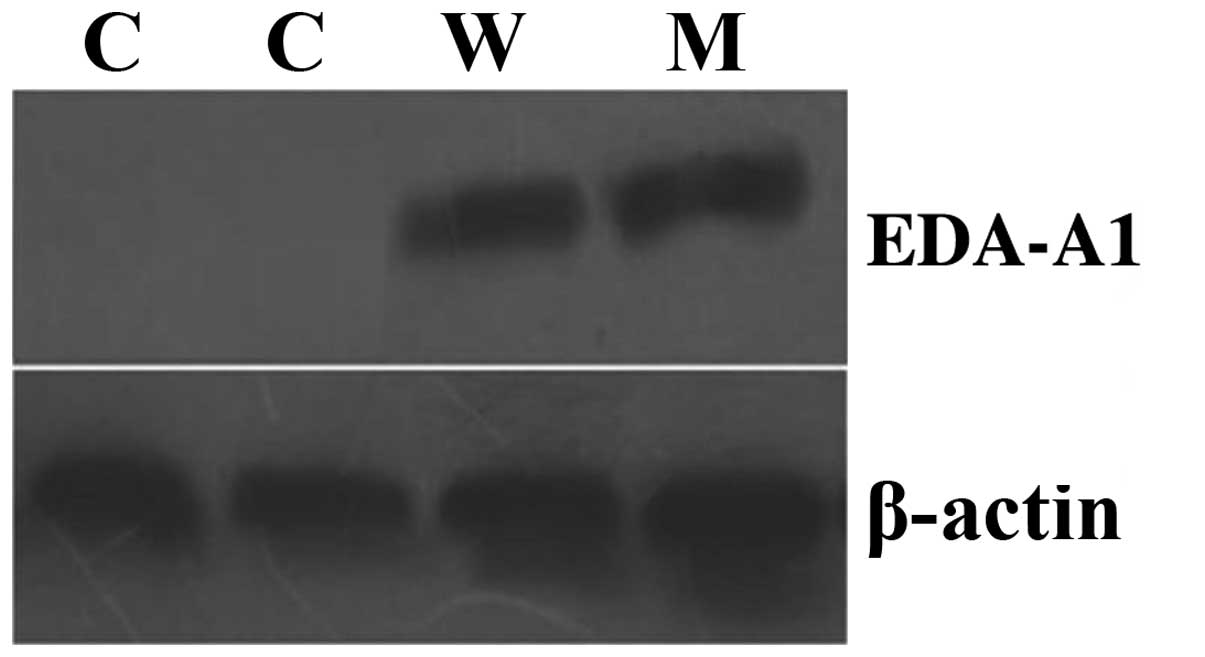
for all groups. Then, EDA-A1 protein expression levels in the
ECV304 cells were detected by western blotting. Fig. 2 shows that the EDA-A1 protein was
expressed in the cells infected with pcDNA3.1 (−)-EDA-A1-M or
pcDNA3.1 (−)-EDA-A1-W vector, but was not expressed in the control
group. In conclusion, EDA-A1 mRNA and protein was expressed in
ECV304 cells following the exogenous delivery of EDA-A1, but not in
control cells.
Overexpression of EDA-A1 affects
ECV304 cell proliferation
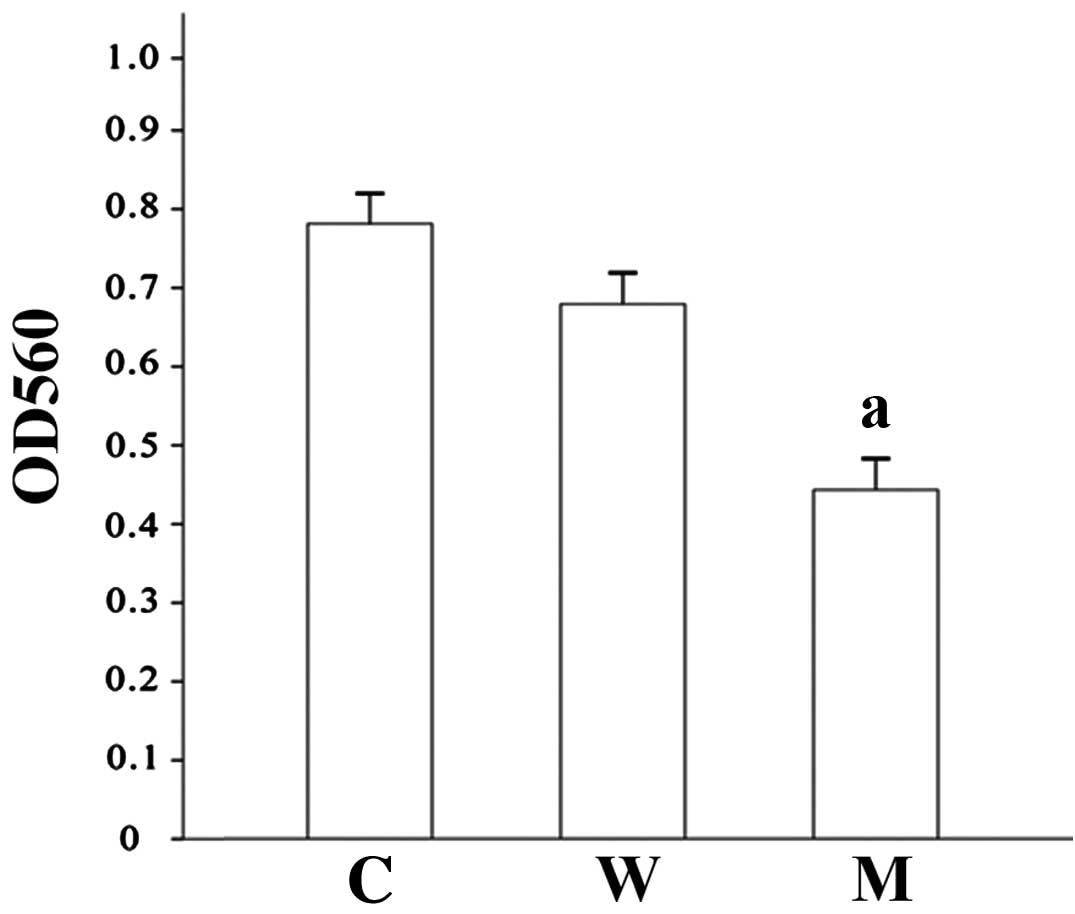
To elucidate whether EDA-A1 has an effect on ECV304
cell proliferation, MTT assays were performed. As shown in Fig. 3, the ECV304 cell viability at 96 h
infection was decreased significantly in the mutant group by
comparison with that in the wild type and control groups. The
proliferation of mutant group cells was suppressed by 45.7%
relative to control, while the proliferation of the wild type group
was suppressed by 16.0% (Table I,
Fig. 3).
 | Table I.OD560 value of ECV304 cells
transfected with the EDA-A1 gene following 96-h culture. |
Table I.
OD560 value of ECV304 cells
transfected with the EDA-A1 gene following 96-h culture.
| Group | OD560 | Inhibition rate
(%) |
|---|
| Control | 0.79±0.037 |
2.5 |
| Wild type | 0.68±0.016 | 16.0 |
| Mutant |
0.44±0.033a | 45.7a |
EDA-A1 overexpression regulates the
cell cycle of ECV304 cells
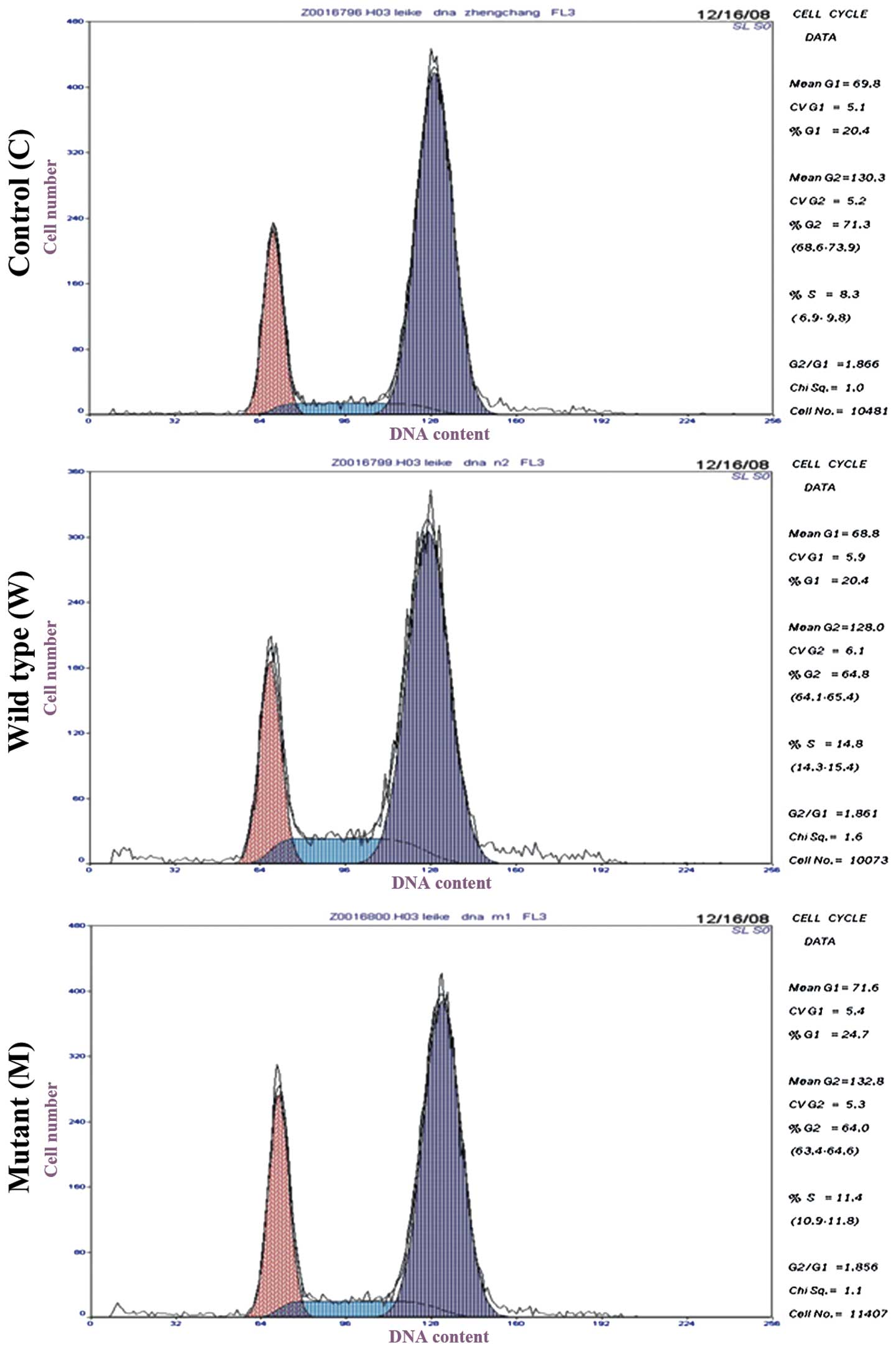
To determine the effect of plasmid-mediated EDA-A1
infection on the cell cycle of ECV304 cells, flow cytometric
analysis was conducted (Fig. 4). It
was observed that 25.45±1.89% of cells were arrested at the
G0/G1 phase of the cell cycle in the mutant
group, compared with 20.37±0.68 and 20.30±0.68% of cells in the
wild type and control groups, respectively (Table II). During the S phase, the mutant
and wild type groups showed significantly higher cell percentages
(12.40±1.75 and 14.80±1.45%, respectively) than the control group
(8.55±0.57%). However, the two EDA-A1-transfected groups had
lower cell percentages than the control in the G2/M
phase. The lowest cell percentage in the G2/M phase was
62.15±1.94% in the mutant group. It may be concluded that the cell
cycle distribution in the G0/G1, S and
G2/M phases of ECV304 cells were regulated by EDA-A1
overexpression.
 | Table II.Effect of EDA-A1 gene
transfection on the cell cycle of ECV304 cells. |
Table II.
Effect of EDA-A1 gene
transfection on the cell cycle of ECV304 cells.
| Group |
G0/G1 phase | S phase | G2/M
phase |
|---|
| Control | 20.30±0.68 |
8.55±0.57 | 71.15±0.57 |
| Wild type | 20.37±0.68 |
14.80±1.45a |
64.83±0.85a |
| Mutant |
25.45±1.89a,b |
12.40±1.75a |
62.15±1.94a |
Discussion
Until now, the exact pathological mechanism of HED
has remained unclear. In the present study, the effect of a
HED-associated gene (EDA-A1) on the proliferation and cell
cycle of ECV304 cells was investigated. The results indicated that
mutant and wild-type EDA-A1 genes might have distinct
biological functions affecting the proliferation and cell cycle
distribution of cultured HUVECs.
EDA-A1, which is a variant of the major
causative gene of HED (EDA), is located on chromosome
Xq12–13.1 and encodes a protein containing 391 amino acids
(4,11). The EDA-A1 protein, a type II
transmembrane protein, is a member of the TNF ligand family. It
consists of a short extracellular domain, a transmembrane region, a
collagen area, and a TNF ligand subunit (6,11–13). The
combination of EDA-A1 and the ectodysplasin receptor can promote
programmed cell death and activate NF-κB signaling (11,14).
Currently, the research relating to HED mostly
comprises case reports and mutation analysis; however, few studies
have reported on the function of the EDA-A1 gene,
particularly in cell activity. Immunohistochemical analysis of
human MCF-7 and COS-1 cells transfected with pCMV5-EDA-A2
identified strong signals at the cell surface for some transfected
cells, and changes in the cell morphology of MCF-7 were found to be
associated with the expression of EDA-A2 (7). Another previous study demonstrated that
pIRES2-EGFP-EDA eukaryotic plasmids could be successfully
transfected into dental pulp mesenchymal cells and stably expressed
(15). In the present study,
compared with the control group, the proliferation of ECV304 cells
in the mutant group was decreased significantly, and cell growth
was inhibited. This may be due to a change in the spatial
configuration and biological activity of the EDA-A1 protein caused
by the EDA-A1 gene mutation. However, the reduction of cell
proliferation of the ECV304 cells transfected with wild-type
EDA-A1 was not significant.
The cell cycle, which consists of a series of highly
ordered phases (G1, S, G2 and M), is
important to both normal and cancer cells (16). The actions of antitumor agents are
also characterized by an association with cell cycle phase
(17). The results of the present
study indicated that the EDA-A1 gene mutant had an impact on
the cell cycle, and blocked cell cycle progression in the
G0/G1 and S phases. However, the present
study was limited, since it lacked transfection experiments with
oral-related cell lines.
In conclusion, the present study revealed the
inhibitory effect of an EDA-A1 gene mutant on the
proliferation of ECV304 cells. The aim of our future research is to
focus on the transfection of the EDA-A1 gene in other oral
cavity-related cell lines, and to further elucidate the effect of
the EDA-A1 gene on tooth, jaw and craniofacial
development.
References
|
1
|
Clarke A, Phillips D, Brown R and Harper
PS: Clinical aspects of X-linked hypohidrotic ectodermal dysplasia.
Arch Dis Child. 62:989–996. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Callea M, Vinciguerra A, Willoughby CE,
Deroma L and Clarich G: Infantile bilateral glaucoma in a child
with ectodermal dysplasia. Ophthalmic Genet. 34:58–60. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yildirim M, Yorgancilar E, Gun R and Topcu
I: Ectodermal dysplasia, Otolaryngologic evaluation of 23 cases.
Ear Nose Throat J. 91:E28–E33. 2012.PubMed/NCBI
|
|
4
|
Mues G, Tardivel A, Willen L, Kapadia H,
Seaman R, Frazier-Bowers S, Schneider P and D'Souza RN: Functional
analysis of ectodysplasin-A mutations causing selective tooth
agenesis. Eur J Hum Genet. 18:19–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mikkola ML: Molecular aspects of
hypohidrotic ectodermal dysplasia. Am J Med Genet A.
149A:2031–2036. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ulvmar MH, Sur I, Mémet S and Toftgård R:
Timed NF-kappaB inhibition in skin reveals dual independent effects
on development of HED/EDA and chronic inflammation. J Invest
Dermatol. 129:2584–2593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bayés M, Hartung AJ, Ezer S, Pispa J,
Thesleff I, Srivastava AK and Kere J: The anhidrotic ectodermal
dysplasia gene (EDA) undergoes alternative splicing and encodes
ectodysplasin-A with deletion mutations in collagenous repeats. Hum
Mol Genet. 7:1661–1669. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lei K, Che TJ, Wang JM, Deng N, Zhang L
and He XY: Mutation analysis of the eda-A1 gene for hypohidrotic
ectodermal dysplasia and construction of recombined eukarytoic
expression vector. Hua Xi Kou Qiang Yi Xue Za Zhi. 27:610–613.
2009.(In Chinese). PubMed/NCBI
|
|
9
|
Park HJ, Zhang Y, Georgescu SP, Johnson
KL, Kong D and Galper JB: Human umbilical vein endothelial cells
and human dermal microvascular endothelial cells offer new insights
into the relationship between lipid metabolism and angiogenesis.
Stem Cell Rev. 2:93–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kruger NJ: The Bradford method for protein
quantitation. Methods in Molecular Biology: Basic Protein and
Peptide Protocols. Walker JM: 32:(Totawa, NJ). Humana Press. 9–15.
1994.
|
|
11
|
Cluzeau C, Hadj-Rabia S, Jambou M, Mansour
S, Guigue P, Masmoudi S, Bal E, Chassaing N, Vincent MC, Viot G, et
al: Only four genes (EDA1, EDAR, EDARADD, and WNT10A) account for
90% of hypohidrotic/anhidrotic ectodermal dysplasia cases. Hum
Mutat. 32:70–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hashimoto T, Cui CY and Schlessinger D:
Repertoire of mouse ectodysplasin-A (EDA-A) isoforms. Gene.
371:42–51. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ezer S, Schlessinger D, Srivastava A and
Kere J: Anhidrotic ectodermal dysplasia (EDA) protein expressed in
MCF-7 cells associates with cell membrane and induces rounding. Hum
Mol Genet. 6:1581–1587. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Courtney JM, Blackburn J and Sharpe PT:
The ectodysplasin and NFkappaB signalling pathways in
odontogenesis. Arch Oral Biol. 50:159–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gan YN, Wang ZY, Chen SM, Zhao GW, Ye XL
and Shen LJ: Establishment of human dental papilla mesenchymal
cells stably-transfected with EDA gene. Kouqiang Yixue. 26:270–272.
2006.(In Chinese).
|
|
16
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhuyan BK, Scheidt LG and Fraser TJ: Cell
cycle phase specificity of antitumor agents. Cancer Res.
32:398–407. 1972.PubMed/NCBI
|