Introduction
Radiofrequency technology has been utilized in
medicine for >100 years and is frequently used for reducing
nasal turbinate, palate and tongue base tissues in the management
of nasal obstruction, snoring and obstructive sleep apnea, with
promising results (1–7). The radiofrequency energy delivered to
the tissue is generally considered to determine lesion size
(8). However, according to previous
findings, the lesion size of radiofrequency ablation (RFA) is not
proportional to the total energy delivered (9). Furthermore, temperature-time
integration (TTI) has emerged as a novel predictor of RFA lesion
size, improving upon the current metric of total delivered energy
when applied in clinical practice. In order to implement TTI as a
predictive strategy, it is necessary to know the critical
temperature to use as a starting point in the calculation of TTI.
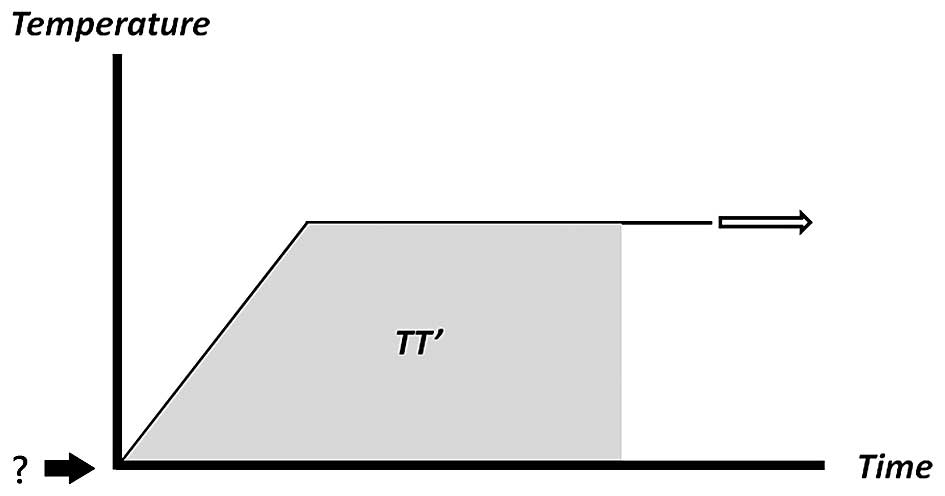
The present study proposes that the RFA lesion size may have a more
marked correlation with TTI (Fig. 1,
shaded area TT') than with total delivered energy. In order to
quantify this shaded area, the starting temperature in the TT' area
calculations must be determined. This critical temperature is
regarded as the temperature threshold for the tissue to be
permanently affected or injured by the radiofrequency energy.
In order to deduce accurate TTI parameters, heat
dispersal must occur in a smooth pattern, with the highest
temperature at the center of the RFA lesion, gradually declining
farther from the lesion center. However, previous studies have
suggested that tissue heating is not correlated with the RFA probe
temperature (10–14) and, in numerous circumstances, the
maximal heating temperature appears farther from the RFA probe.
The present study aimed to provide support for the
use of TTI as an improved indicator of RFA lesion size, initially
through determining the critical temperature threshold for this
integration calculation. The temperature distribution over the
tissue during the RFA process was also evaluated in order to
determine whether the RFA transducer temperature is representative
of this thermal injury, and whether heat dispersal follows
predictable patterns.
Materials and methods
Ethics statement
The protocol for the present study was reviewed and
approved by the Taipei Medical University Institutional Animal Care
and Use Committee (approval no. LAC-2014-0220).
Determination of critical temperature
threshold as a starting point for integration calculation
The ex vivo lesioning model used in the
present study to create RFA lesions was modified from previous
studies (9,15–17). For
the in vivo study, three male New Zealand white rabbits
(Wei-Hsin Co., New Taipei City, Taiwan), aged 10–15 weeks and
weighing 2,500–3,000 g, were used.
Ex vivo lesioning model
Ex vivo fresh chicken breast samples (Sung
Ching Commercial Co., Ltd., Taipei, Taiwan) were used as substrates
to create the RFA lesions. Previous surface contact methods
(15) were modified and the needle
was inserted into the chicken muscle tissue with all portions of
the active tip embedded. The advantages of using chicken samples
are well-described in the previous literature (18). Aside from using chicken tissue, fresh
porcine muscle (Sung Ching Commercial Co., Ltd., Taipei, Taiwan)
was also tested. The target temperatures were set at 50, 55, 60 and
65°C. For each temperature, RFA was performed in triplicate.
Following RFA, the tissues were fixed in formalin for subsequent
histological analysis.
In vivo lesioning model
Three male New Zealand white rabbits were used as
the in vivo model. Following anesthetization with 50 mg/kg
ketamine and 20mg/kg xylazine via intramuscular injection, a wound
on the dorsal side of the animal was created by exposing the back
muscles. Similar to the aforementioned ex vivo lesioning
method, the needle was inserted into the rabbit tissue with all
portions of the active tip embedded. The target temperatures were
also set at 50, 55, 60 and 65°C. RFA was performed in triplicate at
each temperature. Following RFA, the rabbits were euthanized via
intramuscular injection of a double dose of anesthesia, and tissues
were excised and fixed in formalin for subsequent histological
analysis.
Radiofrequency equipment and RFA
RFA was performed using the temperature-controlled
S-1500 RF generator (MedSphere International Holdings, Inc.,
Shanghai, China). For the RFA procedure, 100-mm, 22-gauge, 10-mm
curved, active-tip cannulas (model 10–141221; MedSphere
International Holdings, Inc., Shanghai, China) were used. For the
ex vivo and in vivo models, the lesions were created
using a power of 25 W. The RFA application time was 2 min, based on
the saturation point in a previous study (9).
Histological analysis
Samples were fixed for 24 h in a 10%
neutral-buffered formalin solution in phosphate-buffered saline (pH
7.4) at room temperature. The samples were then washed in distilled
water, dehydrated in graded alcohol, embedded in paraffin (Merck,
Darmstadt, Germany) and cut into 5-mm sections. Adjacent sections
were stained with Masson's trichrome stain (Sigma-Aldrich, St.
Louis, MO, USA) to evaluate thermal injury.
Infra-red thermal imaging analysis to
measure temperature distribution
Using the ex vivo model, the infra-red
thermal image and temperature at the lesion center were captured
and recorded at 10-sec intervals using a VT02 Visual IR Thermometer
(Fluke, Norwich, UK). For this experiment, the target temperatures
were set at 75 and 85°C, with the RFA application time set to 2
min.
Statistical analysis
In the ex vivo chicken tissue RFA lesioning
model, the lesion center temperatures were recorded between 10 and
120 sec and SPSS 17.0 software for Windows was used to compare the
data using one-way analysis of variance (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
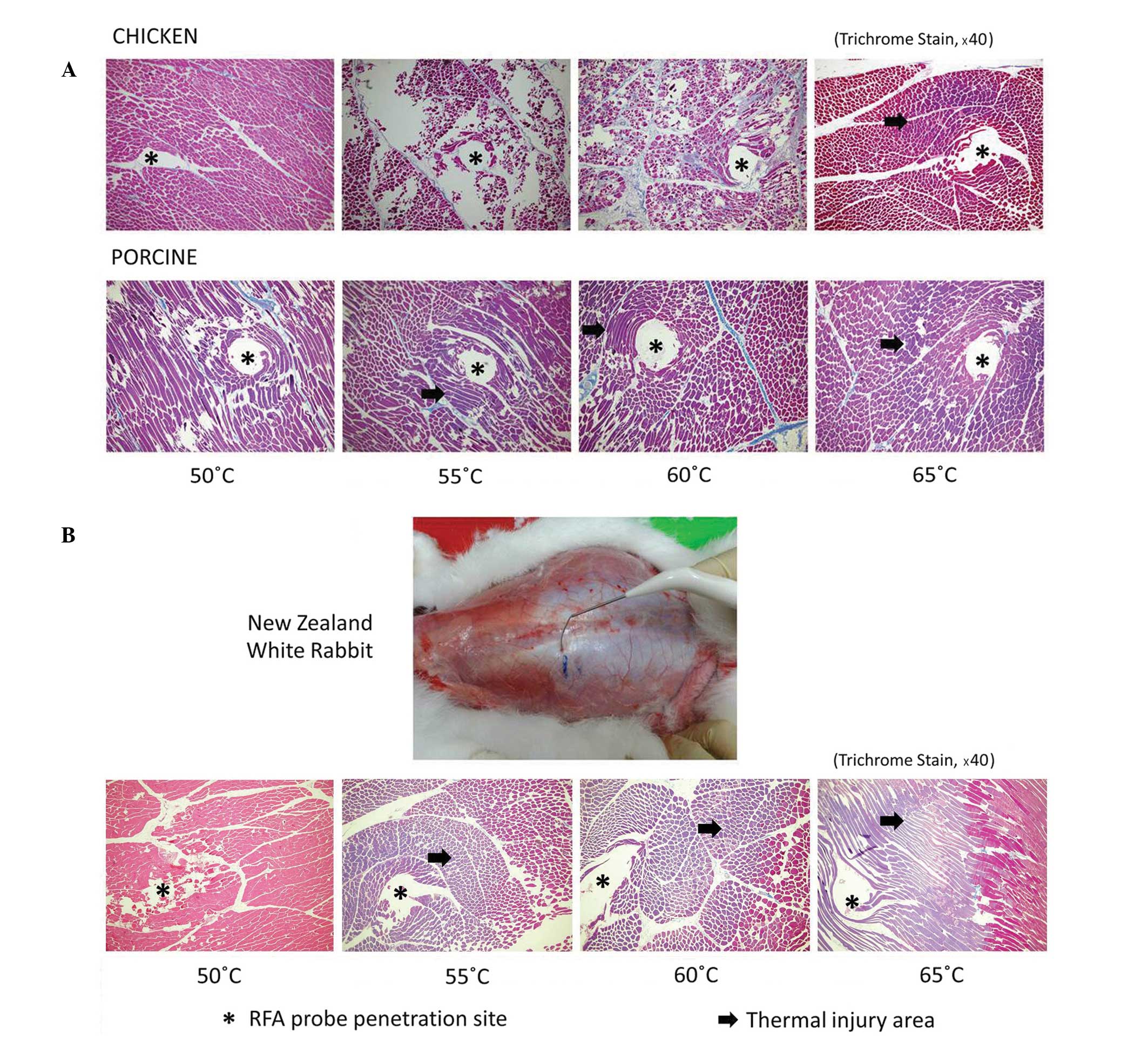
The results for the ex vivo model RFA
temperature threshold measurement are indicated in Fig. 2A. In the chicken tissues, thermal
injury (indicated by purple Trichrome stain; Fig. 2) was not significant until the
temperature setting reached 65°C. In porcine tissues, thermal
injury became apparent at temperatures ≥55°C.
The results for the in vivo model RFA
temperature threshold measurement are demonstrated in Fig. 2B. When lesions were created in the
dorsal muscles of living New Zealand white rabbits, thermal injury
was not significant until the target temperature reached 55°C.
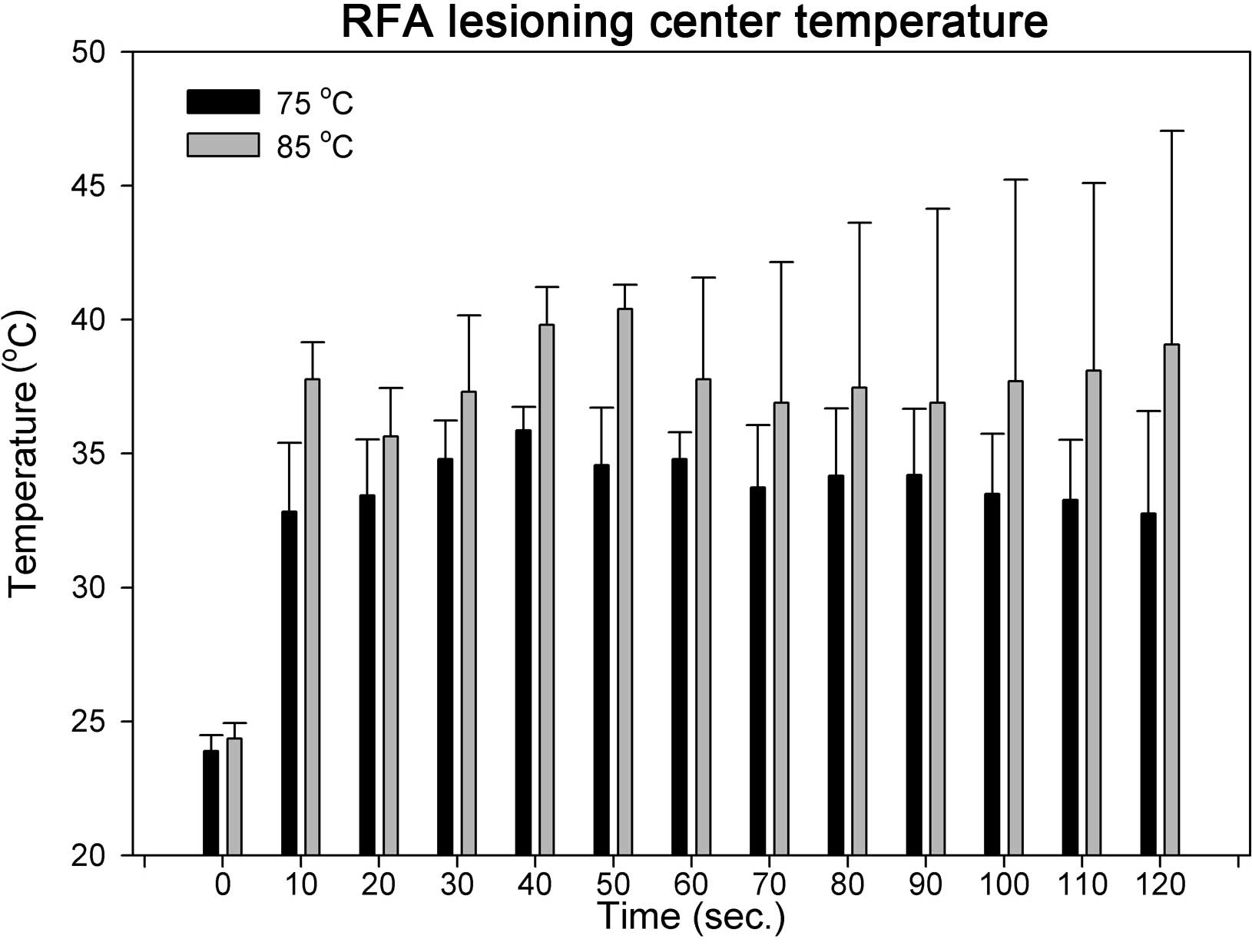
In the ex vivo chicken tissue RFA lesioning
model, two target temperatures, 75 and 85°C, were used. The lesion
center temperatures were recorded and the IR thermal images were
captured from the beginning of the RFA procedure for 120 sec, at
10-sec intervals. The lesion center rapidly reached 32.8±0.6 and
37.8±1.4°C, within 10 sec after starting RFA, at 75 and 85°C,
respectively (Fig. 3). Lesion center
temperatures reached a plateau subsequent to the rapid temperature
increase in the first 10 sec. No significant differences were
identified between the temperatures recorded at the center of the
lesion between 10 and 120 sec at either temperature setting (75 and
85°C, respectively).
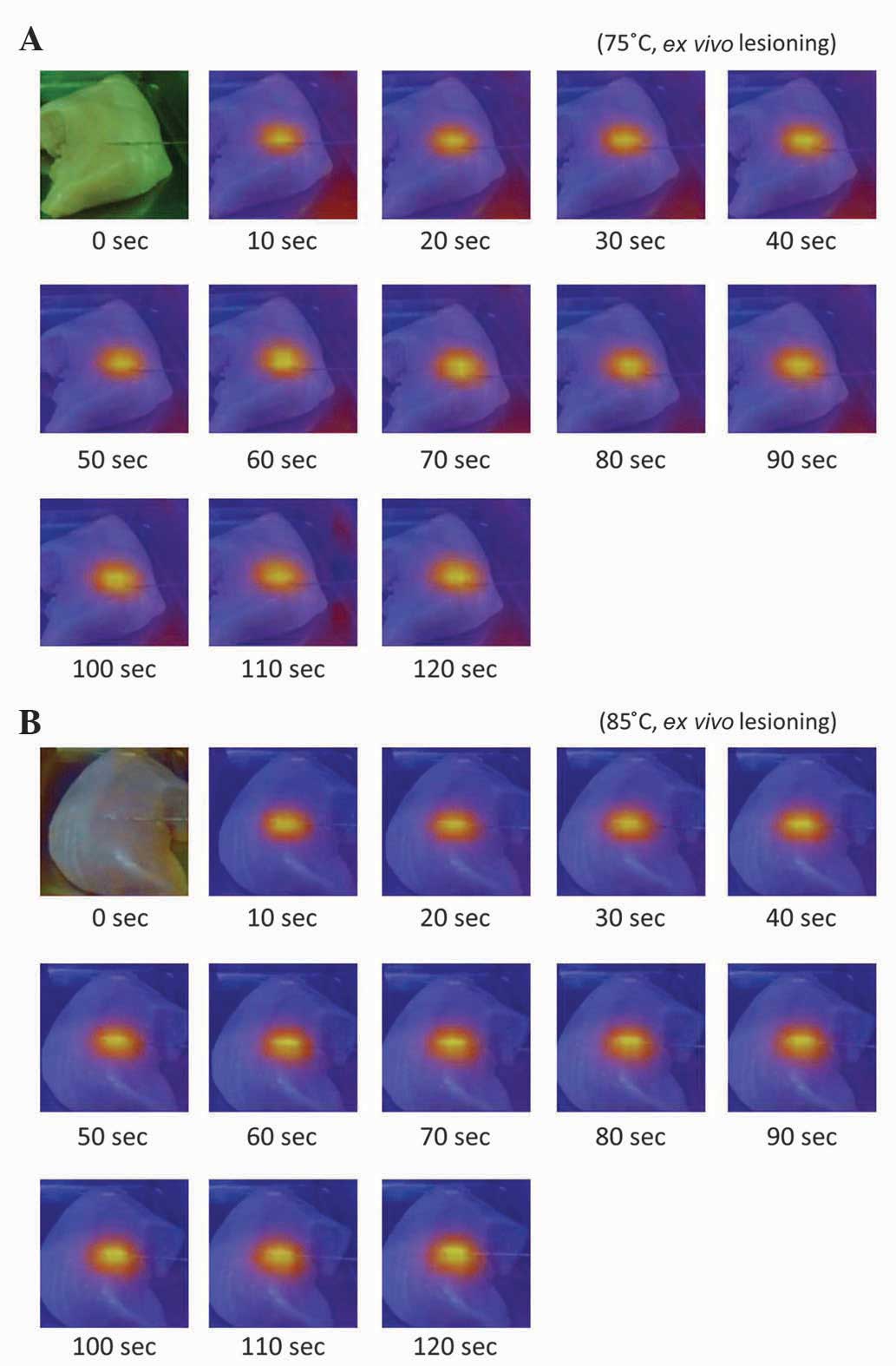
The IR thermal color gradient images of the ex
vivo chicken tissue RFA lesioning model at the two temperature
settings are indicated in Fig. 4A and
B. As expected, no thermal differences were detected at 0 sec,
therefore the whole image was automatically set to the same green
color tone. The color gradient images demonstrate the uniform heat
diffusion pattern. The temperature gradient reached its equilibrium
after 10 sec, with no clear gradient pattern change from 10–120
sec. There were no unexpected temperature rises or hot spots
throughout the RFA lesioning field.
Discussion
The present study revealed that the temperature
threshold during RFA ranges above the established protein
denaturing temperature. The data presented in the current study
suggests that the thermal injury threshold for RFA in chicken
tissues is ~65°C. For mammals, the temperature threshold is likely
to range from 55–65°C, presumably closer to 55°C for humans due to
greater similarity in physiological and histological muscular
tissue structures to pigs and rabbits. Furthermore, the present
study revealed that, during RFA lesioning, the temperature gradient
(from central to peripheral) has a uniform heat diffusion pattern.
These data support TTI as a critical parameter in determining RFA
lesion size, and suggest that it is clinically applicable to
replace joules as a unit of measurement, using the equation:
[Target temperature − 55 (°C)] × time (sec) is proportional to RFA
lesion size.
Clinically, if RFA is used to destroy a certain
tissue volume (for instance, in otorhinolaryngology to reduce
normal tissue), lack of control of thermal energy leads to
undesirable therapeutic outcomes. If RFA is used to treat cancer
(for example, hepatocellular carcinoma), insufficient RFA may
induce additional malignant transformation (19). Control of the RFA procedure is
therefore vital to precisely determine how much tissue is
destroyed.
The elevation of cell and tissue temperature is the
principal property of radiofrequency currents used to achieve the
desired clinical effects (20).
There are three basic mechanisms through which radiofrequency
currents increase tissue temperature. The first of these is the
conversion of electromagnetic energy to mechanical energy.
High-frequency alternating currents cause rapid oscillations of
electrically-charged particles (ions) within the cellular
cytoplasm. Rapid ion movement leads to frictional forces that
induce thermal energy production and cause an elevation of
intracellular and tissue temperature. The second of these is
resistive heating, based on the physical concept of an increased
temperature in a resistor due to the current flow. The third
mechanism is indirect conductive heat transfer; the increase in
tissue temperature leads to the conduction of thermal energy to the
adjacent tissue, resulting in the elevation of tissue temperature
(21). Thermal injury is known to
directly lead to tissue injury and cell death (22).
At a certain temperature, protein denaturation
occurs, which may cause cell death. Thermal injury is
well-documented in numerous previous cellular studies; for example,
Nikfarjam et al (23)
documented the importance of focal hyperthermia in the destruction
of liver tumors. In a study regarding RFA, Goldberg et al
(22) reported that the diameter of
tissue coagulation in a pig liver model may be predicted by the
local temperature along the exposed electrode, also demonstrating
that the diameter of local coagulation necrosis is a function of
the mean local temperature.
No coagulation is observed when the local
temperature is <50°C in in vitro experiments.
Temperatures above this threshold have previously been demonstrated
to lead to progressively greater lesion diameter, with a minimum of
1 cm of necrosis occurring at 71°C. Additional increases in lesion
diameter (1.4–1.6 cm) have previously been observed at ~90°C;
however, the time setting of this experiment was uniformly set at 6
min, indicating that temperature is important in the prediction of
RFA lesion size (22).
Previous evidence in cellular models also suggested
that temperature and time are critical parameters to be considered.
Moriyama-Gonda et al (24)
revealed that a qualitative change in cell death was associated
with the degree and duration of thermotherapy of PC-3 cells, from
apoptosis to necrosis. Leber et al (25) demonstrated, in a hepatocellular
carcinoma cell culture model, that heating resulted in early
apoptosis in 20–30% of HepG2 cells and in 10–15% of LX-1 cells.
Late apoptosis is observed in a large percentage of cells 24 h
after heating at 65°C for 15 min or at 75°C for 5 min; by contrast,
heating to 65°C for 10 min resulted in only a moderate increase of
late apoptotic cells, whilst heating to 55°C for 15 min resulted in
a smaller proportion than this of late apoptotic cells. In the
present study, the concept of heating time is documented as under a
higher temperature, therefore, a shorter duration of exposure is
required to achieve comparable thermal injury.
Evidence examined in previous studies supports the
current hypothesis of the present study that the TTI may be a
better indicator of RFA lesion size. However, there are numerous
studies revealing evidence to the contrary. In a previous
cardiology study, Petersen et al (10) revealed that, for an established
target temperature, power consumption is positively correlated with
lesion volume, whereas measured tip temperature is not, suggesting
that power is a critical factor in determining the lesion size;
however, in this previous study, increased convective cooling was
achieved through induction of a flow surrounding the electrode tip,
increasing lesion dimensions and power consumption.
Nakagawa et al (11), using a canine thigh model, also
reported that saline irrigation maintains a low electrode-tissue
interface temperature during RFA use at high power, which prevents
a rise in electrical impedance, and produces deeper and larger
lesions. A similar study by Skrumeda and Mehra (13) demonstrated that irrigated ablation
creates larger lesions than standard. Importantly, these
contradictory studies have been performed in lesion-irrigating
models, in contrast with the present study. In the cardiology study
(10), for example, a model was
likely used in which RFA would be used to treat arrhythmias.
For the hypothesis in the present study to be
applied to solid compartment tissue ablation contexts, including in
the management of nasal turbinate reductions, tongue base
reductions or liver tumor ablations, it is therefore necessary to
ensure that the tip temperature represents a valid reflection of
the RFA lesioning area temperature, and that the temperature is
dispersed evenly in a smooth gradient pattern. The present study
reveals evidence supporting the posited hypothesis and demonstrates
that the tip temperature increases rapidly, reaching a plateau
within only 10 sec; this latter conclusion is important for the
application of the suggested equation.
Recalling the RFA lesioning equation proposed in a
previous study (9) (target
temperature 75°C, y = 0.92× − 2.2987; target temperature 65°C, y =
0.3582× + 2.4539, where y = mm2 and × = sec), if the
temperature threshold is set at 55°C for an established time, the
area under 75°C is twice as large as the area under 65°C. At 30 and
40 sec, the areas calculated using this equation for 75 and 65°C
will be 34.5:16.8 and 25.2:13.2, respectively, which markedly
correlates with the hypothesis equation.
The major limitation of the present study is that
the temperature threshold is based on animal tissues; although an
in vivo mammal model is used, the equation may not be
appropriate for use in human beings. Additional human trials are
required to reveal the usefulness of the proposed equation. It
should be noted that the equation developed is based on animal
muscle tissue and, although it might be useful in the field of
otolaryngology for the treatment of sleep apnea, the equation may
not be suitable for RFA that is intended for tumor ablations,
including the treatment of liver cancer. However, the present study
does provide support to this previously proposed hypothesis. The
current data suggest that the use of temperature-controlled
radiofrequency devices with a target temperature set to >65°C
should be lowered to the temperature threshold of 55°C for RFA in
otorhinolaryngology. The current study also indicates that the RFA
time should be considered a reliable parameter in determining the
desired tissue ablation volume.
The thermal injury threshold for RFA in chicken
tissues is ~65°C and ranges from 55–65°C for mammals. During RFA
lesioning, the temperature gradient from the center to the
periphery demonstrates a uniform heat diffusion pattern. TTI
appears to be a critical parameter in determining RFA lesion size
and is clinically applicable as the following equation [Target
temperature − 55 (°C)] × time (sec) is proportional to RFA lesion
size.
Acknowledgements
The present study was supported by the Taipei
Medical University Hospital Research Fund (grant no.
103TMU-TMUH-20).
References
|
1
|
Pirsig W: Has tongue base reduction with
radiofrequency energy in sleep apnea syndrome been adequately
evaluated? HNO. 49:501–502. 2001.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stuck BA, Maurer JT and Hörmann K: Tongue
base reduction with radiofrequency energy in sleep apnea. HNO.
49:530–537. 2001.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blumen MB, Dahan S, Fleury B, Hausser-Hauw
C and Chabolle F: Radiofrequency ablation for the treatment of mild
to moderate obstructive sleep apnea. Laryngoscope. 112:2086–2092.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li KK, Powell N and Riley R:
Radiofrequency thermal ablation therapy for obstructive sleep
apnea. Oral Maxillofac Surg Clin North Am. 14:359–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Powell NB, Riley RW, Troell RJ, Li K,
Blumen MB and Guilleminault C: Radiofrequency volumetric tissue
reduction of the palate in subjects with sleep-disordered
breathing. Chest. 113:1163–1174. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Powell NB, Riley RW and Guilleminault C:
Radiofrequency tongue base reduction in sleep-disordered breathing:
A pilot study. Otolaryngol Head Neck Surg. 120:656–664. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin HC, Lin PW, Friedman M, Chang HW, Su
YY, Chen YJ and Pulver TM: Long-term results of radiofrequency
turbinoplasty for allergic rhinitis refractory to medical therapy.
Arch Otolaryngol Head Neck Surg. 136:892–895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eick OJ: Temperature controlled
radiofrequency ablation. Indian Pacing Electrophysiol J. 2:66–73.
2002.PubMed/NCBI
|
|
9
|
Chang YL, Tseng TM, Chen PY, Lin CJ and
Hung SH: Using temperature-time integration as a critical parameter
in using monopolar radiofrequency ablations. Eur Arch
Otorhinolaryngol. 271:1973–1979. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petersen HH, Chen X, Pietersen A, Svendsen
JH and Haunsø S: Lesion size in relation to ablation site during
radiofrequency ablation. Pacing Clin Electrophysiol. 21:322–326.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakagawa H, Yamanashi WS, Pitha JV, Arruda
M, Wang X, Ohtomo K, Beckman KJ, McClelland JH, Lazzara R and
Jackman WM: Comparison of in vivo tissue temperature profile and
lesion geometry for radiofrequency ablation with a saline-irrigated
electrode versus temperature control in a canine thigh muscle
preparation. Circulation. 91:2264–2273. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dorwarth U, Fiek M, Remp T, Reithmann C,
Dugas M, Steinbeck G and Hoffmann E: Radiofrequency catheter
ablation: Different cooled and noncooled electrode systems induce
specific lesion geometries and adverse effects profiles. Pacing
Clin Electrophysiol. 26:1438–1445. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Skrumeda LL and Mehra R: Comparison of
standard and irrigated radiofrequency ablation in the canine
ventricle. J Cardiovasc Electrophysiol. 9:1196–1205. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petersen HH, Chen X, Pietersen A, Svendsen
JH and Haunsø S: Tissue temperatures and lesion size during
irrigated tip catheter radiofrequency ablation: An in vitro
comparison of temperature-controlled irrigated tip ablation,
power-controlled irrigated tip ablation, and standard
temperature-controlled ablation. Pacing Clin Electrophysiol.
23:8–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Provenzano DA, Lassila HC and Somers D:
The effect of fluid injection on lesion size during radiofrequency
treatment. Reg Anesth Pain Med. 35:338–342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Provenzano DA, Liebert MA and Somers DL:
Increasing the NaCl concentration of the preinjected solution
enhances monopolar radiofrequency lesion size. Reg Anesth Pain Med.
38:112–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Provenzano DA, Watson TW and Somers DL:
The interaction between the composition of preinjected fluids and
duration of radiofrequency on lesion size. Reg Anesth Pain Med.
40:112–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmed M, Liu Z, Afzal KS, Weeks D, Lobo
SM, Kruskal JB, Lenkinski RE and Goldberg SN: Radiofrequency
ablation: Effect of surrounding tissue composition on coagulation
necrosis in a canine tumor model. Radiology. 230:761–767. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Obara K, Matsumoto N, Okamoto M, Kobayashi
M, Ikeda H, Takahashi H, Katakura Y, Matsunaga K, Ishii T, Okuse C,
et al: Insufficient radiofrequency ablation therapy may induce
further malignant transformation of hepatocellular carcinoma.
Hepatol Int. 2:116–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ihnat P, Rudinska Ihnat L and Zonca P:
Radiofrequency energy in surgery: state of the art. Surg Today.
44:985–991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Massarweh NN, Cosgriff N and Slakey DP:
Electrosurgery: History, principles, and current and future uses. J
Am Coll Surg. 202:520–530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goldberg SN, Gazelle GS, Halpern EF,
Rittman WJ, Mueller PR and Rosenthal DI: Radiofrequency tissue
ablation: Importance of local temperature along the electrode tip
exposure in determining lesion shape and size. Acad Radiol.
3:212–218. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nikfarjam M, Muralidharan V and Christophi
C: Mechanisms of focal heat destruction of liver tumors. J Surg
Res. 127:208–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moriyama-Gonda N, Igawa M, Shiina H,
Urakami S, Shigeno K and Terashima M: Modulation of heat-induced
cell death in PC-3 prostate cancer cells by the antioxidant
inhibitor diethyldithiocarbamate. BJU Int. 90:317–325. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leber B, Mayrhauser U, Leopold B,
Koestenbauer S, Tscheliessnigg K, Stadlbauer V and Stiegler P:
Impact of temperature on cell death in a cell-culture model of
hepatocellular carcinoma. Anticancer Res. 32:915–921.
2012.PubMed/NCBI
|