Introduction
Acute pulmonary edema is a common presentation in
cardiology. Its clinical manifestations include sudden severe
dyspnea and orthopnea, which are often accompanied by a cough, pink
frothy sputum, restlessness, cyanosis, sweating, tachycardia, and
moist and wheezing rales (1). If
severe, acute pulmonary edema can cause syncope and cardiac arrest
(2).
Acute pulmonary edema is pernicious and the
mortality rate is ~12% (3). The
pathogenesis and physiology of acute pulmonary edema have been
extensively researched in recent years (2,4);
however, the pathogenesis of acute pulmonary edema is yet to be
fully elucidated. Previous studies have demonstrated that the
following are important mechanisms of acute pulmonary edema:
Excessive shrinkage of pulmonary vasculature; increased pulmonary
capillary permeability; and a decreased capacity for the clearance
of hypoxia-induced alveolar fluid (5,6). The
study of pathological and physiological changes of lung tissue in
the human body is challenging. Therefore, the human pathologic
state and pathophysiological processes may be reproduced in an
animal model of lung tissue damage to enable investigation of the
occurrence, development and characteristics of the disease, and to
explore novel therapeutic strategies for the treatment of acute
pulmonary edema.
Animal models of acute pulmonary edema have
previously been established and studies have demonstrated that
changes in the levels of malondialdehyde (MDA), superoxide
dismutase (SOD) and interleukin (IL)-6 may play an important role
in hypoxia-induced acute pulmonary edema (7,8). In the
present study, a rat model of acute pulmonary edema was established
in order to clarify the functions of MDA, SOD and IL-6 in this
condition. In particular, alterations in the levels of SOD and MDA
in the lung tissue of rats with acute pulmonary edema were analyzed
to elucidate the role of hypoxia in acute pulmonary edema.
Materials and methods
Experimental animals and grouping
A total of 48 adult Wistar rats (male, 26; female,
22; weight, 200±20 g) were purchased from the Experimental Animal
Center of Zhengzhou University (Zhengzhou, China). The rats were
divided into four equally sized groups: Group A, normal group;
group B, acute pulmonary edema model induced by hypoxia for 24 h;
group C, acute pulmonary edema model induced by hypoxia for 48 h;
and group D, acute pulmonary edema model induced by hypoxia for 72
h. The rat model of pulmonary edema was prepared in groups B-D by
the injection of 6% ammonium chloride (as described below). The
general condition of the rats was observed in addition to lung
coefficient calculations and pathological sections, and the
successful establishment of the model was verified. The present
study was performed in strict accordance with the recommendations
outlined by the Guide for the Care and Use of Laboratory Animals
(8th edition, 2010) of the National Institutes of Health (Bethesda,
MD, USA). The animal protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of the Second Hospital
of Hebei Medical University (Shijiazhuang, China).
Establishment of the animal model and
experimental procedures
In order to establish a rat model of acute pulmonary
edema, the 36 rats in groups B-D were injected with 6% ammonium
chloride (Bohu Biotechnology Co., Shanghai, China) into the
abdominal cavity. Female rats were administered 6–8 ml/kg, whereas
male rats received 9–12 ml/kg. The animal model was established
according to a previously described procedure (4). After the model was deemed successful,
all rats were given ad libitum access to feed and water.
Rats in the experimental groups were fasted 24 h prior to sampling,
whereas rats in the normal group were fed during this time.
Following successful modeling for 24, 48 and 72 h, rats in groups
B-D were anesthetized by the intraperitoneal injection of 10%
urethane (Nengren Biotechnology Co., Wuhan, China). Heparinized
blood was harvested from the abdominal aorta and centrifuged at
4,500 × g for 15 min at 4°C to separate the plasma, which was
subsequently preserved at −70°C prior to determination of IL-6
levels. The rat lungs were separated and harvested in order to
prepare the 10% lung tissue homogenate using the tissue lysate
(Boster Biological Technology, Ltd., Wuhan, China). Hematoxylin and
eosin staining was performed in strict accordance with the
manufacturer's protocol (Dingguo Biotechnology Co. Ltd., Beijing,
China). The main procedure included the following steps: Sampling
and fixation, dehydration, paraffin embedding, slicing and wax
dyeing. The staining results were observed under a light microscope
(BX51; Olympus Corp., Tokyo, Japan).
Determination of water content in lung
tissue
The surface blood in the left lung of the rats was
dried using an absorbent strip, immediately weighed, and placed
into a 60°C oven for 72 h, until no further weight reduction was
observed. Subsequently, the dry weight of the lung tissue was
calculated and the water content (%) in the lung tissue was
determined as (wet weight - dry weight)/wet weight ×100%.
Determination of MDA and SOD levels in
lung tissue
MDA and SOD levels were detected in the lung tissue
of rats in the four groups. MDA and SOD assay kits were purchased
from Boster Biological Technology, Ltd., and were performed in
strict accordance with the manufacturer's protocol.
Enzyme-linked immunosorbent assay
(ELISA)
IL-6 levels in the plasma and lung tissue were
detected using ELISA (Sigma-Aldrich, St. Louis, MO, USA), according
to the manufacturer's protocol.
Statistical analysis
Data were analyzed using SPSS statistical software,
version 14.0 (SPSS, Inc., Chicago, IL, USA) and were presented as
the mean ± standard deviation. Between-group differences were
compared using single factor analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Observation results in the lung tissue
of rats
Rats in the four groups were sacrificed, and lung
tissue was obtained. In group A, the lung tissue was found to be
uniform, with no significant pulmonary edema, and the wet weight
was not significantly increased. However, significant edema was
detected in the lung tissue of groups B, C and D, while the wet
weight and water content were significantly increased (P=0.046,
0.021 and 0.009, respectively; Fig.
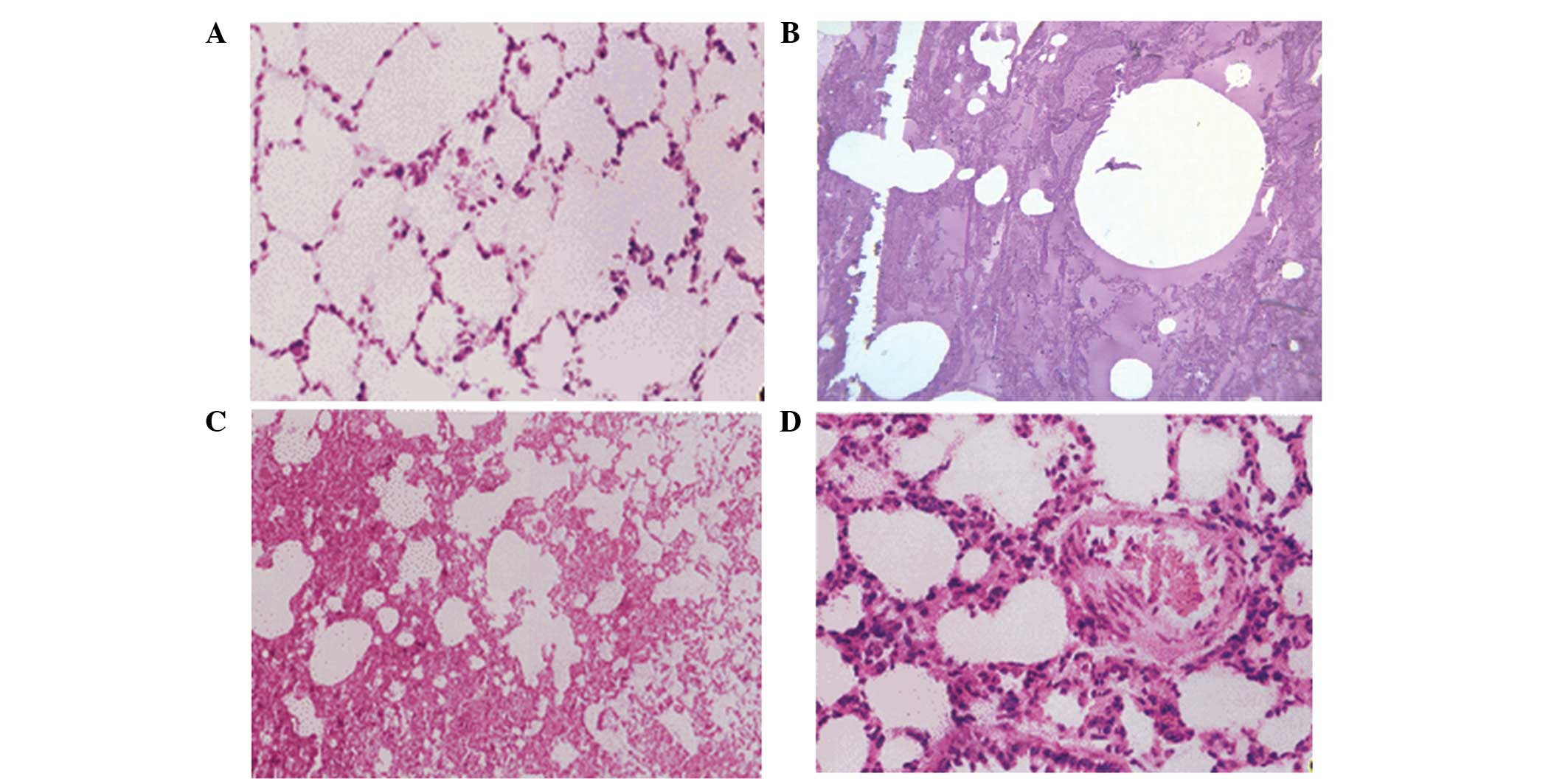
1) compared with group A. The results of hematoxylin and eosin
staining showed that the rat lung tissue in group A did not present
significant edema, and the pulmonary tissue morphology was normal.
By contrast, pulmonary congestion and edema were detected in groups
B, C and D, with a protein rich liquid saturating the pulmonary
interstitium, alveoli and bronchioles, in addition to hyaline
membranes lining the alveolar walls (Fig. 2).
MDA and SOD changes in the lung tissue
of rats
The levels of MDA and SOD in the lung tissue of rats
with acute hypoxia in groups B-D were measured as representative
values in edema at different time points, and compared with those
of group A. The results demonstrated that the MDA levels increased
whereas SOD activity decreased in the rats in group B, as compared
with those in group A, although not significantly (P>0.05).
However, the MDA levels and SOD activity in the lung tissue of rats
with pulmonary edema were significantly altered as the duration of
edema increased. The MDA levels significantly increased in groups C
and D following hypoxia for 48 h and 72 h whereas SOD activity
significantly decreased, when compared with those in group A
(P<0.05; Table I).
 | Table I.Superoxide dismutase (SOD) activity
and malondialdehyde (MDA) levels in the lung tissue of rats in
groups A-D. |
Table I.
Superoxide dismutase (SOD) activity
and malondialdehyde (MDA) levels in the lung tissue of rats in
groups A-D.
| Indices | Group A | Group B | Group C | Group D |
|---|
| SOD (U/g) | 3.57±0.67 | 3.33±0.56 |
2.74±0.54a |
2.56±0.48a |
| MDA (mmol/g) | 0.97±0.47 | 1.12±0.56 |
1.37±0.62a |
1.52±0.74a |
IL-6 changes in the plasma and lung
tissue of rats
The changes of IL-6 levels in the lung tissue and
plasma of the rats with pulmonary edema induced by acute hypoxia
were measured at three different time points, and compared with
those in group A. The results showed that the IL-6 levels were
significantly increased in the plasma of the rats in groups B-D as
compared with those in group A (P<0.05). The IL-6 levels in the
lung tissue were notably increased in the rats in group B compared
with those in group A (P>0.05), and were significantly increased
in the rats in groups C and D as compared with those in group A
(P<0.05), indicating that the IL-6 levels increased when the
duration of lung edema was prolonged (Table II).
 | Table II.Levels of IL-6 in the plasma and lung
tissue of rats in groups A-D. |
Table II.
Levels of IL-6 in the plasma and lung
tissue of rats in groups A-D.
| Indices | Group A | Group B | Group C | Group D |
|---|
| IL-6 in plasma
(µg/l) | 103.83±32.14 |
157.48±39.59a |
178.53±45.78a,b |
194.91±54.59a,b,c |
| IL-6 in lung tissue
(µg/l) |
97.58±31.67 | 117.79±35.54 |
146.60±43.81d,e |
162.80±46.01d,e,f |
Discussion
Acute pulmonary edema predominantly occurs following
acute extensive myocardial infarction, acute myocarditis, severe
hypertension, aortic or mitral stenosis, infective endocarditis and
cardiac trauma (9). Its onset is
rapid, often inducing cardiac and respiratory failure; therefore,
the mortality rates are high (10).
In recent years, the incidence of acute pulmonary edema has
gradually increased (3). Prevention
and treatment can significantly increase the survival rate of
patients with pulmonary edema and reduce the sequelae and
complications (9). Since this
disease has important social significance, it has been increasingly
investigated by the medical community (11,12).
Study of the pathological and physiological changes
of lung tissue in the human body is difficult. Therefore, it is
useful to reproduce the pathological state and pathophysiological
processes of acute pulmonary edema in humans by stimulating lung
tissue damage in rats. The creation of an animal model of acute
pulmonary edema aids the elucidation of the occurrence, development
and characteristics of the disease, and the exploration of novel
therapeutic strategies for the treatment of acute pulmonary edema.
Previous studies have reported the establishment of animal models
of acute pulmonary edema (7,8). It has previously been demonstrated that
the following are key mechanisms of acute pulmonary edema:
Excessive shrinkage of the pulmonary vasculature; increased
pulmonary capillary permeability and a decreased capacity for the
clearance of alveolar liquid induced by hypoxia (5). Previous studies have demonstrated that
acute hypoxia induces oxidative stress; the latter is capable of
regulating inflammation, reducing the synthesis and release of NO
in lung tissue and reducing pulmonary
Na+-K+ATP enzyme activity (13,14).
Therefore, oxidative stress induced by hypoxia may have a key role
in the occurrence and development of acute pulmonary edema. In
order to verify this hypothesis, the present study investigated the
effects of oxidative stress in acute pulmonary edema by creating a
rat model of hypoxia-induced acute pulmonary edema (8) and analyzing MDA, SOD and IL-6
levels.
Hypoxia can alter the balance of oxygen free
radicals and nitric oxide. Oxygen free radicals are capable of
inducing cell damage via the peroxidation of polyunsaturated fatty
acids in the biofilm and peroxide decomposition products (15). The content or activity of important
indicators of oxidative stress, including SOD, lipid peroxidation
products such as MDA, and IL-6 can reflect the degree of lipid
peroxidation and the extent of cell damage (16).
SOD is an important antioxidant enzyme that has a
specific physiological activity and is the most important substance
for scavenging free radicals in the body; furthermore, a decline in
SOD levels is associated with aging. SOD is capable of resisting
and blocking the damaging effects of oxygen free radicals on cells
and initiating the timely repair of damage to cells caused by free
radicals (17). Oxidative stress
results from the excessive production of free radicals in the body,
which can affect protein expression and other regulatory and
response mechanisms (18). When
induced by free radical and compensated stress, the antioxidant
capacity of a cell or organism can increase, during which the
phenomenon of transiently increased levels of SOD may be observed
(19). SOD levels in the lung
tissues of patients with acute pulmonary edema will increase due to
oxidative stress. In particular, IL-6 is produced by various cells,
including macrophages, T cells and B cells, and can regulate the
growth and differentiation of numerous cells. Disorders in the
expression levels of IL-6 have been associated with various
diseases; for example, polyclonal B cell activation may occur in
patients with autoimmune diseases, including systemic lupus
erythematosus, Reiter syndrome, Castleman disease, scleroderma,
membranoproliferative glomerulonephritis and rheumatoid arthritis
(20). IL-6 expression levels are
significantly increased in patients with these diseases, which may
have an association with the patient's state of illness and
curative effects. Furthermore, IL-6 expression levels are
significantly increased in the lung tissue and plasma of patients
with acute pulmonary edema, due to inflammation and oxidative
stress. In particular, the severity of the patient's state of
illness and prognosis increases as the IL-6 expression level
increases (21).
The combined observation and detection of SOD, MDA,
and IL-6 in the lung tissue of patients with acute pulmonary edema
can comprehensively reflect alterations in the physiological state
of patients, and is conducive to the effective diagnosis, treatment
and prognosis of patients with acute pulmonary edema (22). In the present study, changes in the
levels of MDA and SOD in the lung tissue of a rat model of
hypoxia-induced acute pulmonary edema were compared at various
durations of acute hypoxia and against the group A control. The
results demonstrated that the levels of MDA increased in the lung
tissue of the rats following the induction of acute pulmonary edema
for 24 h whereas the SOD levels decreased at this time point
(P>0.05). As the duration of pulmonary edema increased from 24 h
in group B to 48 and 72 h in groups C and D, the MDA and SOD levels
in the lung tissue of the rats following hypoxia were significantly
increased, as compared with those of the rats in group A
(P<0.05).
IL-6 levels in the lung tissue and plasma of rats in
the three groups with various durations of induced acute hypoxia
were compared with those in group A. The results demonstrated that
plasma IL-6 levels were significantly increased in groups B-D, as
compared with those in group A (P<0.05). IL-6 levels in the lung
tissue of the rats in group B notably increased following 24 h
acute pulmonary edema, as compared with those in group A
(P>0.05). With the increased duration of pulmonary edema, the
IL-6 levels in the lung tissue of the rats in groups C and D were
significantly increased as compared with those in group A
(P<0.05). The results of the present study were consistent with
the initial hypothesis.
Hypoxia-induced alterations in the oxidative stress
indices of rats with acute pulmonary edema were observed in the
present study, and the effects on the activity level of the
antioxidant enzyme SOD, and the levels of the lipid peroxidation
product MDA and IL-6 in were investigated the lung tissue, so as to
explore their effects and related mechanisms in a rat model of
acute pulmonary edema (23,24). The results of the present study
demonstrated that the antioxidant capacity and associated SOD
enzyme activity of the lung tissue significantly decreased when the
duration of pulmonary edema was extended; whereas the levels of the
MDA lipid peroxidation product significantly increased, and the
levels of IL-6 in the plasma and lung tissue significantly
increased, which suggested that the induction of oxidative stress
may have an important role in the pulmonary tissue damage
associated with acute pulmonary edema. Furthermore, the alterations
in the levels of the MDA, SOD and IL-6 oxidative stress-related
indices in the lung tissue of rats with hypoxia suggested that the
pathogenesis of acute pulmonary edema is complicated, and may be a
result numerous factors (25,26).
MDA, SOD and IL-6 were detected in the present
study, and the results demonstrated that oxidative stress may have
an important role in the occurrence of acute pulmonary edema. The
present study had certain limitations. The number of rats used to
model acute pulmonary edema was small and, at only 72 days, the
observation time was relatively short. Furthermore, the present
study was not a randomized prospective double-blind study. These
limitations will be improved in further research. Therefore,
definitive conclusions cannot be drawn from the results of the
present study. Future studies of acute pulmonary edema with longer
follow-up times and a larger sample size are required. However, the
findings of the present study suggest that the simultaneous
measurement of MDA, SOD and IL-6 levels in the lung tissue and
plasma of rats with acute pulmonary edema may provide key guidance
for the assessment of disease pathology, therapeutic strategies and
prognosis for patients with acute pulmonary edema.
References
|
1
|
Obiagwu C, Paul V, Chadha S, Hollander G
and Shani J: Acute pulmonary edema secondary to hyperbaric oxygen
therapy. Oxf Med Case Reports. 2015:183–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith WS and Matthay MA: Evidence for a
hydrostatic mechanism in human neurogenic pulmonary edema. Chest.
111:1326–1333. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang XT, Liu DW, Zhang HM and Chai WZ:
Integrated cardiopulmonary sonography: a useful tool for assessment
of acute pulmonary edema in the intensive care unit. J Ultrasound
Med. 33:1231–1239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
MacIver DH and Clark AL: The vital role of
the right ventricle in the pathogenesis of acute pulmonary edema.
Am J Cardiol. 115:992–1000. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Theodore J and Robin ED: Pathogenesis of
neurogenic pulmonary oedema. Lancet. 2:749–751. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mammoto T, Jiang E, Jiang A, Lu Y, Juan
AM, Chen J and Mammoto A: Twist1 controls lung vascular
permeability and endotoxin-induced pulmonary edema by altering Tie2
expression. PLoS One. 8:e734072013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minnear FL, Kite C, Hill LA and van der
Zee H: Endothelial injury and pulmonary congestion characterize
neurogenic pulmonary edema in rabbits. J Appl Physiol (1985).
63:335–341. 1987.PubMed/NCBI
|
|
8
|
Zhang XM, Sun DY, Tang L and Yuan YJ:
Preliminary experimental research on glucocorticoid for treatment
of nitrogen dioxide induced acute pulmonary edema in rats. Zhonghua
Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 28:822–826. 2010.(In
Chinese). PubMed/NCBI
|
|
9
|
Viswanathan S, Muthu V and Remalayam B:
Pulmonary edema in near hanging. J Trauma Acute Care Surg.
72:297–301. 2012.PubMed/NCBI
|
|
10
|
Hamdy O, Maekawa H, Shimada Y, Feng GG and
Ishikawa N: Role of central nervous system nitric oxide in the
development of neurogenic pulmonary edema in rats. Crit Care Med.
29:1222–1228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lionte C, Sorodoc L and Laba V:
Respiratory syndromes in acute poisoning. Rev Med Chir Soc Med Nat
Iasi. 108:544–548. 2004.(In Romanian). PubMed/NCBI
|
|
12
|
Ekman I, Ekstrand L and Schaufelberger M:
Pulmonary oedema - a life threatening disease. Eur J Cardiovasc
Nurs. 6:259–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greenbaum R, Bay J, Hargreaves MD, Kain
ML, Kelman GR, Nunn JF, Prys-Roberts C and Siebold K: Effects of
higher oxides of nitrogen on the anaesthetized dog. Br J Anaesth.
39:393–404. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lavie L: Oxidative stress in obstructive
sleep apnea and intermittent hypoxia - revisited - the bad ugly and
good: Implications to the heart and brain. Sleep Med Rev. 20:27–45.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wojtczak L and Slyshenkov VS: Protection
by pantothenic acid against apoptosis and cell damage by oxygen
free radicals - the role of glutathione. Biofactors. 17:61–73.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prys-Roberts C: Principles of treatment of
poisoning by higher oxides of nitrogen. Br J Anaesth. 39:432–439.
1967. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mansuroğlu B, Derman S, Yaba A and
Kizilbey K: Protective effect of chemically modified SOD on lipid
peroxidation and antioxidant status in diabetic rats. Int J Biol
Macromol. 72:79–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li K, Gao B, Li J, Chen H, Li Y, Wei Y,
Gong D, Gao J, Zhang J, Tan W, et al: ZNF32 protects against
oxidative stress-induced apoptosis by modulating C1QBP
transcription. Oncotarget. 6:38107–38126. 2015.PubMed/NCBI
|
|
19
|
Parsons PE: Respiratory failure as a
result of drugs, overdoses, and poisonings. Clin Chest Med.
15:93–102. 1994.PubMed/NCBI
|
|
20
|
Fang LL and Ye JJ: Investigated progress
of expression of interleukin-6,-10 in herniated disc. Yi Xue Zong
Shu. 13:569–572. 2007.(In Chinese).
|
|
21
|
Rassler B: Role of α- and β-adrenergic
mechanisms in the pathogenesis of pulmonary injuries characterized
by edema, inflammation and fibrosis. Cardiovasc Hematol Disord Drug
Targets. 13:197–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sarada S, Himadri P, Mishra C, Geetali P,
Ram MS and Ilavazhagan G: Role of oxidative stress and NFkB in
hypoxia-induced pulmonary edema. Exp Biol Med (Maywood).
233:1088–1098. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chatterjee K and Parmley WW: The role of
vasodilator therapy in heart failure. Prog Cardiovasc Dis.
19:301–325. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sonnenblick EH, Mancini DM and LeJemtel
TH: New positive inotropic drugs for the treatment of congestive
heart failure. Am J Cardiol. 55:41A–44A. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Francois G, Faizende J and Reboul J:
Pulmonary edemas due to acute heroin poisoning. Ann Anesthesiol Fr.
16:77–83. 1975.(In French). PubMed/NCBI
|
|
26
|
Hamdy O, Maekawa H, Shimada Y, Feng GG and
Ishikawa N: Role of central nervous system nitric oxide in the
development of neurogenic pulmonary edema in rats. Crit Care Med.
29:1222–1228. 2001. View Article : Google Scholar : PubMed/NCBI
|