Introduction
Presbycusis is a major neurodegenerative disease in
elderly individuals. Hearing loss and communication difficulty are
common social problems associated with the increasing incidence of
presbycusis (1). Presbycusis with
hearing loss at high frequencies may result in difficulty in
understanding speech in a noisy environment (2). If the hearing loss spreads to the
frequency range of speech, presbycusis may lead to difficulty in
understanding speech in any environment. The loss of hearing and
hair cells are the two primary features of presbycusis (3).
Age-related cochlear hair cell loss has been
reported in humans (4,5) and animals (6). The loss of inner hair cells (IHCs) and
outer hair cells (OHCs) increases with increased age, and the loss
of OHCs is particularly pronounced. The molecular mechanisms
underlying the age-related loss of hair cells remain unclear.
Damage to mitochondrial DNA is speculated to be a cause of
presbycusis (7). Mitochondrial DNA
damage is associated with intracellular calcium (Ca2+)
overload, which induces a cascade of adverse consequences.
Excessive amounts of Ca2+ ions activate
Ca2+-dependent enzymes, which promote the generation of
free radicals that cause damage to cells (8,9). The
high concentration of Ca2+ is hypothesized to cause hair
cell degeneration (10).
Preventing the degeneration of hair cells is crucial
for the prevention of presbycusis. Research into the regeneration
of hair cells in animals has made numerous breakthroughs. For
instance, it is known that, in mammals, inner ear stem cells are
pluripotent and are capable of differentiating into hair cell-like
cells; this implies a possible use of such cells in the replacement
of lost inner-ear sensory cells (11). However, regenerative technologies are
not yet ready for use in clinical applications. Calcium channel
blockers may offer an effective intervention for the prevention of
presbycusis, as these blockers inhibit excessive calcium entry and
thus protect hair cells against degeneration. T-type and L-type
Ca2+ channels serve a crucial function in the synaptic
release of hair cells during the cochlear development of mammals
(12). T-type calcium channels have
been found to be present in the organ of Corti and neurons
(13,14). Furthermore, T-type calcium channel
blockers reportedly protect OHCs from noise damage (14). Similarities between the age-related
pathologies of mice and humans indicate that mice may provide a
good model for presbycusis (15).
The most widely used model is the C57BL/6J mouse, which exhibits
marked progressive age-related hearing loss (16). The results of our previous study
indicate that the hearing threshold of C57BL/6J mice is
significantly higher at 24–26 weeks of age than in 6–8-week-old
mice (17). The aim of the present
study was to investigate the protective effect of T-type calcium
channel blockers against presbycusis, using the C57BL/6J mice
model. Differences in the hearing of the C57BL/6J mice and the
function and morphology of their hair cells were analyzed following
the administration of T-type calcium channel blockers.
Materials and methods
Animals and tissue preparation
A total of 30 male C57BL/6J mice (age, 6–8 weeks)
were randomized into three groups for the detection of three
calcium channel receptor subunits α1G, α1H and α1I, using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). In
addition, a further 30 C57BL/6J male mice (age, 24–26 weeks) were
allocated at random into three treatment groups: Saline, mibefradil
and benidipine. Each group was subjected to auditory brainstem
recording (ABR) and distortion product otoacoustic emission (DPOAE)
tests following treatment. Mibefradil and benidipine were obtained
from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in
physiological saline solution. A preliminary experiment led to the
selection of dosages of 30 mg/kg/day mibefradil and 10 mg/kg/day
benidipine. The drugs were administered to the mice by gavage for
four consecutive weeks. All experiments were performed in
compliance with the Chinese legislation on the use and care of
laboratory animals, and all studies were approved by the animal
care committee of the First Affiliated Hospital of Soochow
University (Suzhou, China).
The mice were anesthetized with an intraperitoneal
injection of 2.5%, 0.1 ml/10 g chloral hydrate and sacrificed by
cervical dislocation following the ABR and DPOAE tests. The
cochleae were immediately removed, and the stapes were discarded.
The cochleae for RT-qPCR were immersed in ice-cold RNA solution to
avoid RNA degradation. The cochleae were rapidly dissected under a
microscope in ice-cold 0.01 M phosphate-buffered saline (PBS) and
stored at −80°C. The cochleae for scanning electron microscopy
(SEM) were perfused with 2.5% glutaraldehyde via a 10-µm drill
hole, created with a needle, in the vestibular and cochlear windows
and then immersed in fixative fluid.
RT-qPCR
Total RNA was extracted using TRIzol reagent (Gibco;
Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the
manufacturer's protocol. Reverse transcribed cDNA was synthesized
using MMLV reverse transcriptase (Promega Corporation, Madison, WI,
USA). PCR was performed using an ABI-7500 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific) according to the
manufacturer's instructions. RT was conducted in a 20-µl reaction
mixture containing 4 µl 5X RT buffer, 0.5 µl oligo(dT), 0.5 µl
dNTPs, 1 µl MMLV reverse transcriptase, 10 µl
diethylpyrocarbonate-treated water and 4 µl RNA template. The
reaction conditions to inactivate MMLV were 37°C for 1 h and 95°C
for 5 min. PCR was performed using a SYBR Green PCR kit (Thermo
Fisher Scientific), according to the manufacturer's instructions,
in a total volume of 50 µl. The mixture contained 32.5 µl SYBR
Green Master mix, 0.5 µl forward primer, 0.5 µl reverse primer,
14.5 µl ddH2O and 2 µl cDNA template. The PCR cycling
conditions were as follows: 95°C for 30 min, followed by 40 cycles
of 95°C for 30 sec, 58°C for 30 sec and 73°C for 90 sec.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an
endogenous control for the quantification of the PCR. The relative
quantification was based on the Cq (the number of PCR cycles)
values.
The DNA sequences of primers (forward and reverse)
were as follows: GAPDH, 5-CCTGGCCAAGGTCATCCATGACAAC-3′ and
5′-TGTCATACCAGGAAATGAGCTTGAC-3′; α1G subunit,
5′-AATGGCAAGTCGGCTTCAGG-3′ and 5′-TGTCAGAGACCATGGACACCAG-3′; α1H
subunit, 5′-ATGTTCCGGCCCTGTGAGGA-3′ and
5′-CCATGACGTAGTACATGATGTCC-3′; and α1I subunit,
5′-ATCTGCTCCCTGTCGG-3′ and 5′-GAGAACTGGGTCGCTATG-3′. The primers
were designed using Primer Premier 5.0 software (Premier Biosoft
International, Palo Alto, CA, USA) and synthesized at the Shanghai
Institute of Cell Biology (Shanghai, China).
ABR test
Each mouse was anesthetized by an intramuscular
injection of 25 mg/kg xylazine and 100 mg/kg ketamine
(Sigma-Aldrich). The mice were placed in a soundproof anechoic room
with a thermostat prone experimental platform that maintained a
body temperature of 37°C. The ABRs were recorded subcutaneously
using specialized needle electrodes (Tucker-Davis Technologies,
Alachua, FL, USA) placed at the vertex, mastoid prominence and
contralateral mastoid prominence. The speaker was placed in the
external auditory meatus. The stimulus signal was generated and the
evoked potential was recorded by a System II evoked potential
workstation (Tucker-Davis Technologies, Alachua, FL, USA). Tone
burst stimuli (duration, 5 msec; rise-fall time, 0.5 msec) were
generated, and the average response was determined on the basis of
1,000 repetitive stimuli. A repetition rate of 11 times/sec was
applied at frequencies of 8, 16, 24 and 32 kHz. The neuronal
activity was amplified (×100,000) and filtered (0.3–3.0 kHz).
Recording was initiated at a sound pressure level (SPL) of 100 dB,
and 10-dB decrements were applied, followed by 5-dB decrements
until a clear wave response was elicited.
DPOAE test
Following the ABR test, all mice were prepared for
the DPOAE test under anesthesia in the same manner as they were for
the ABR test. An acoustic probe was inserted into the external
auditory meatus near the tympanic membrane. The DPOAE was measured
using an amplifier system that provided two stimulation tones, F1
and F2, which were generated using a dual channel synthesizer (AD3;
Tucker-Davis Technologies). The frequency ratio of the F1 and F2
primary stimulation tones was 1.25, and their intensities were
L1=65 dB SPL and L2=55 dB SPL. The amplitude of DPOAEs was measured
at 2F1-F2. The threshold from low to high frequency was determined
when the DPOAE was >5 dB SPL higher compared with the noise
floor. In order to measure the frequency-specific responses, the F2
stimulation tone was set between 6 and 40 kHz, and the amplitudes
of DPOAEs were recorded at 12 test point frequencies: 6.7, 8.3,
9.5, 11.3, 13.4, 17.9, 21.5, 23.8, 26.7, 33.6, 36.3 and 39.8
kHz.
SEM
The cochlea was removed and immersed in 2.5%
glutaraldehyde for 6 h at 4°C, followed by two washes in PBS for 10
min each. The volute, spiral ligament, vestibular membranes and
covering film were removed from the cochlea under a SGO-45T1
dissecting microscope (Shenzhen Shenshi Guanggu Optical Instrument
Co., Ltd, Shenzhen, China) after rinsing in PBS. The basilar
membrane and cochlear spiral shaft were then exposed. The samples
were post-fixed in 1% osmium tetroxide for 2 h at 4°C. The samples
were dehydrated using a graded series of ethanol (50, 70, 80, 90
and 100% for 10 min each) and incubated in aqueous 90% potassium
tert-butoxide for 10 min. The samples were critical-point
dried in a Leica EM CPD300 dryer (Leica Microsystems, Inc., Buffalo
Grove, IL, USA) and coated with gold (Au) using a Hitachi E-1045
ion sputter coater (Hitachi, Ltd., Tokyo, Japan). The hair cells of
the base turn of the basilar membrane were visualized using a
SU8010 scanning electron microscope (Hitachi, Ltd., Tokyo,
Japan).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean and were analyzed using SPSS software, version 17.0
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was
used to analyze the RT-qPCR, ABR test, and DPOAE test data sets.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of three T-type channel
subunits by RT-qPCR
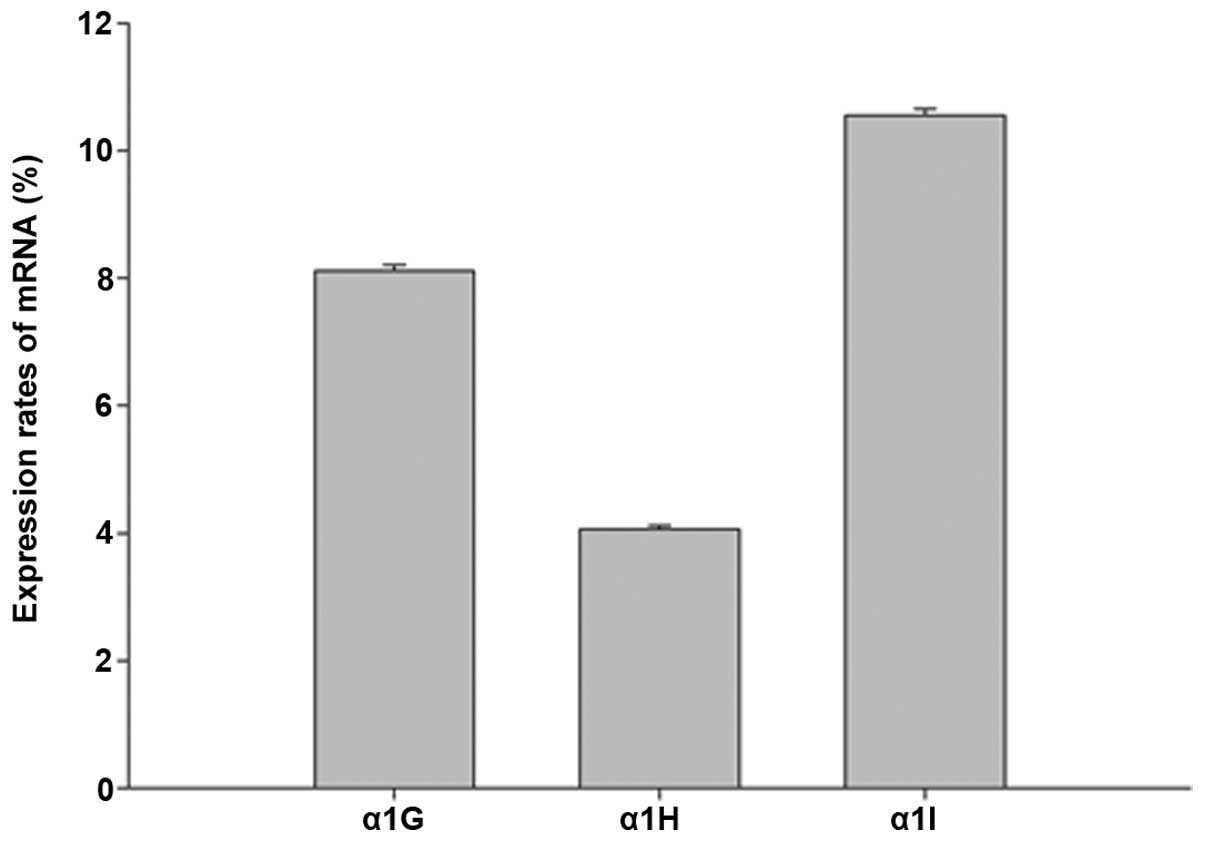
The expression rate of each subunit was calculated
using the formula 2−ΔΔCq (Fig. 1). It was found that all three
subunits were expressed in the cochlea of the 6–8-week-old C57BL/6J
mice. Among the three subunits, the expression levels of the α1H
subunit were lower compared with those of α1G and α1I (P<0.05).
The expression levels of α1G and α1I did not significantly differ
from each other (P>0.05).
ABR test
The hearing thresholds of the 24–26-week-old
C57BL/6J mice differed following the 4-week treatment period. The
hearing threshold at 24 kHz was significantly decreased in the
mibefradil-treated and benidipine-treated groups compared with the
saline-treated group (P<0.05). The hearing threshold was also
decreased at 32 kHz in the mibefradil-treated and
benidipine-treated groups compared with the saline-treated group;
however, this difference was not found to be statistically
significant (P>0.05; Table
I).
 | Table I.Hearing threshold at various
frequencies in 24–26-week-old C57BL/6J mice after treatment [sound
pressure level (dB)]. |
Table I.
Hearing threshold at various
frequencies in 24–26-week-old C57BL/6J mice after treatment [sound
pressure level (dB)].
| Group | 8 Hz | 16 Hz | 24 Hz | 32 Hz |
|---|
| Saline-treated | 56.5±5.7975 | 54.5±3.6890 | 74. 0±3.1620 | 93.0±2.5820 |
|
Mibefradil-treated | 58.0±3.4960 | 54.5±4.9721 |
69.0±3.9441a | 90.5±2.8382 |
|
Benidipine-treated | 57.0±4.2164 | 53.5±3.3747 |
68.5±5.2968a | 91.0±3.9441 |
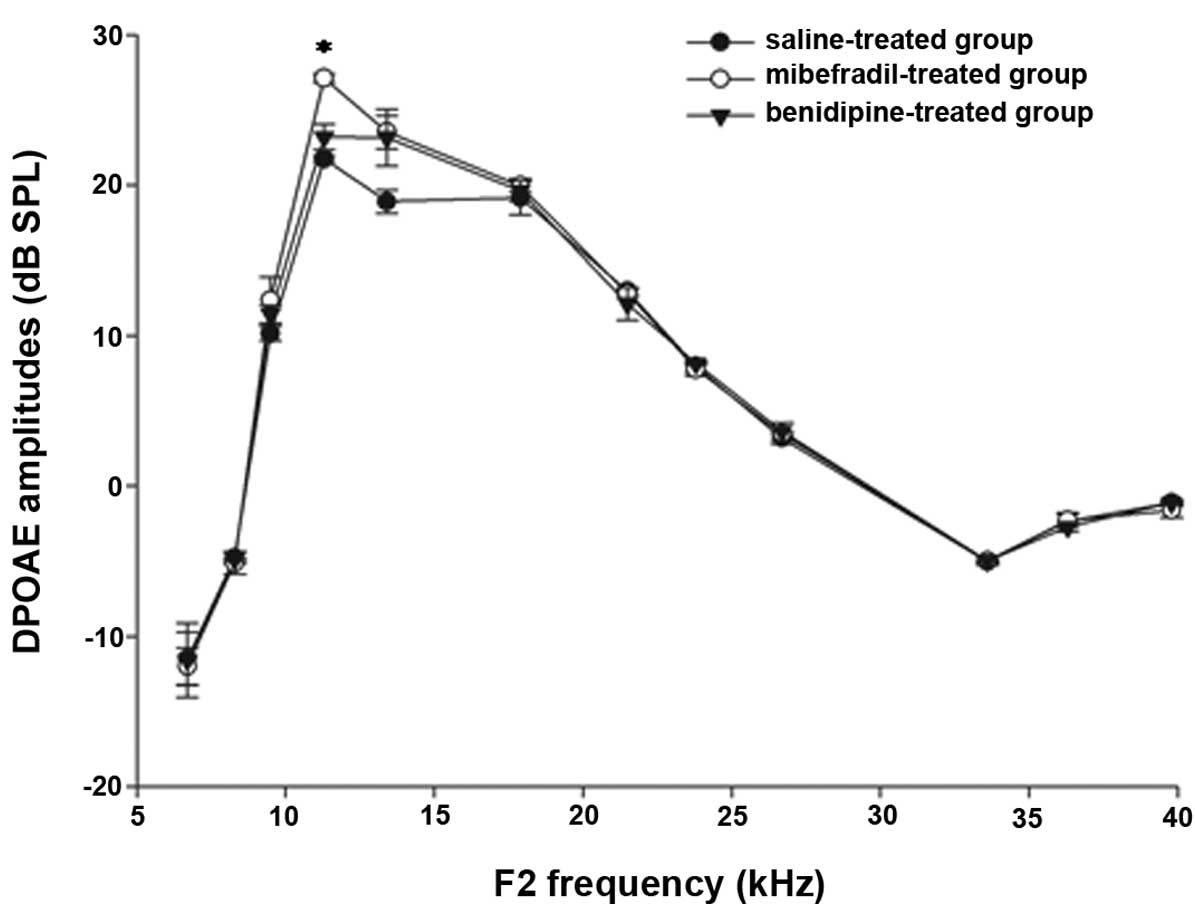
DPOAE amplitudes
The DPOAE amplitudes were measured in the
24–26-week-old C57BL/6J mice at F2 frequencies between 6 and 40 kHz
(Fig. 2). The DPOAE amplitudes in
the mibefradil-treated group were increased compared with those in
the saline-treated group at the F2 frequencies of 11.3 and 13.4 kHz
(P<0.05). The DPOAE amplitudes in the benidipine-treated group
were increased compared with those in the saline-treated group at a
F2 frequency of 13.4 kHz (P<0.05). The DPOAE amplitudes did not
significantly differ at other F2 frequencies between the
mibefradil-treated or benidipine-treated groups and the
saline-treated group.
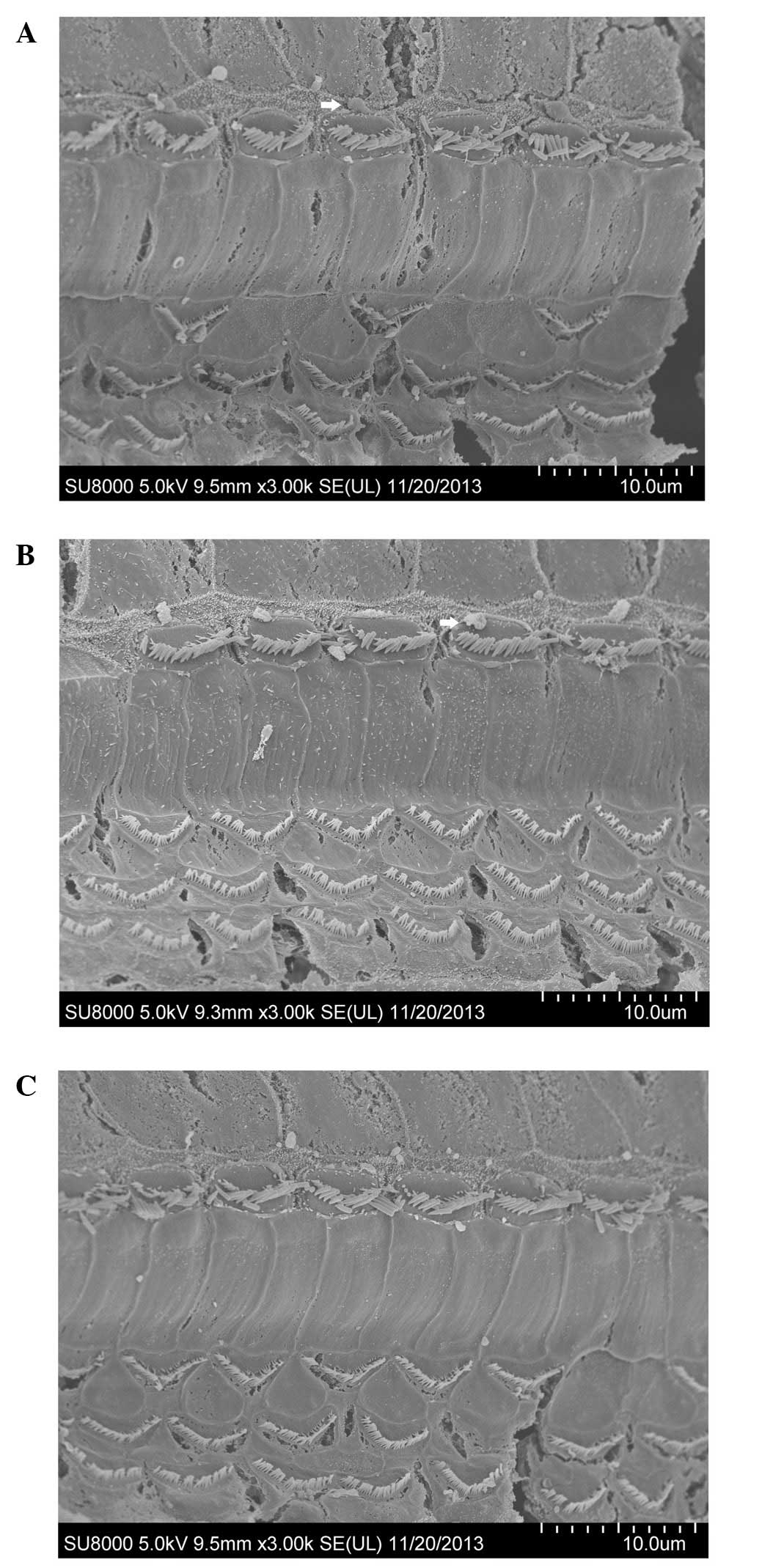
Morphology of hair cells observed
using SEM
The hair cells of the cochleae in the 24–26-week-old
C57BL/6J mice observed by SEM showed differences following the
administration of treatment for four consecutive weeks.
Degeneration of hair cells was evident in the saline-treated group
(Fig. 3A). The rupture of the
cuticular plate, loss of OHCs, and dispersed stereociliary bundles
on IHCs were observed. Spherical extrusions appeared on the outside
of the stereocilia of the IHCs. The loss of OHCs was not obvious in
the mibefradil-treated group (Fig.
3B); however, the stereocilia of IHCs were disorganised and
sparse. A limited number of the OHCs were lost in the
benidipine-treated group (Fig.
3C).
Discussion
The mechanisms underlying the pathogenesis of
presbycusis, including mechanisms associated with the auditory
system, remain unclear. This ambiguity has prevented scientists
from discovering improved treatments for age-related hearing loss.
Mutations of mitochondrial DNA are known to accumulate with aging
(18). These mutations have been
associated with Ca2+ overload (19). The homeostasis of Ca2+ is
crucial for cell survival and for numerous physiological processes,
including hearing (20,21). An elevated concentration of
intracellular Ca2+ may induce the release of
neurotrophins (22), attenuate
action potentials in hair cells and improve neuronal connections
(23).
Calcium channel blockers may provide a novel
intervention for the protection of hair cells against degeneration
during presbycusis. Calcium channels are divided into L, N, P/Q, R
and T-types, according to their electrophysiological and
pharmacological properties (24). In
the present study, the effects of T-type calcium channels blockers
were investigated, as the protective effects of L-type calcium
channels are controversial (25,
26).
C57BL/6J mice, which have an age-related hearing
loss gene (Ahl), were selected as our experimental model, as the
degeneration of cochlear hair cells begins early in adulthood and
progresses with advancing age. To clarify the impact of T-type
calcium channel blockers on the cochlear hair cells, the
distribution of calcium channels in the cochlea was determined.
CaV3.1 is a T-type calcium channel subunit that has been shown to
be involved in intracellular Ca2+ regulation in mature
rat OHCs (27). In the present
study, the three receptor subunits α1G, α1H and α1I corresponding
to calcium channels CaV3.1, CaV3.2 and CaV3.3, respectively, were
confirmed to be expressed in the cochlea, although the expression
levels were low. These results provided a theoretical basis for the
use of T-type calcium channel blockers. The calcium channel
blockers mibefradil and benidipine were selected, and saline was
administered to the animals in the control group. Mibefradil
selectively blocks T-type calcium channels and has been
demonstrated to exert a cardioprotective effect in rats with acute
myocardial infarction (28).
Benidipine has been widely used for hypertension therapy as it is
able to block the L-type and T-type calcium channels in various
cell types (29).
To determine whether hair cells are protected by
T-type calcium channel blockers, the hearing level, function, and
morphology of hair cells were observed following treatment of
C57BL/6J mice with mibefradil and benidipine. The results of the
ABR analysis showed that the hearing threshold decreased at 24 and
32 kHz, particularly at 32 kHz, both in the mibefradil-treated and
benidipine-treated groups compared with the saline-treated group.
The hearing threshold reduction at high-frequencies indicated the
status of the hair cells of the base turn. The improvement in
hearing may be associated with the cochlea, the spiral ganglion
neurons, the auditory cortex or a combination of these. Future
studies are required to confirm whether the hair cells are
affected. To understand the function of hair cells, the DPOAE test
was conducted immediately after the ABR test. The DPOAE measurement
is recognized as an effective method of investigating the function
of OHCs (30). The results indicated
that the DPOAE amplitudes in the mibefradil-treated group were
higher at the F2 frequencies of 11.3 and 13.4 kHz compared with
those in the saline-treated group, and in the benidipine-treated
group. the DPOAE amplitudes were higher at an F2 frequency of 13.4
kHz. The results of the ABR and DPOAE tests suggested that the
function of OHCs was significantly improved, particularly in the
base turn. However, the present results cannot verify the effect of
calcium channel blockers on IHCs. Morphological changes are
typically associated with a change of function. The SEM images
clearly display the morphology of hair cells, including the IHCs.
The progressive loss of hair cells has been previously observed in
the C57BL/6J mouse strain (31). The
present results showed that degeneration of OHCs and IHCs at the
base turn of the cochlea was evident after the 4-week
administration of saline in 24–26-week-old C57BL/6J mice, which
indicated that the saline did not affect the hair cells of
24–26-week-old C57BL/6J mice. The loss of OHCS was reduced after 4
weeks of T-type calcium channel blocker administration,
particularly in the mibefradil-treated group. However, the
stereocilia of IHCs continued to be disorganised and sparse.
Collectively, the present results indicate that
hearing thresholds and DPOAE amplitudes improve at high frequencies
following the administration of T-type calcium blockers. This
result suggests that T-type calcium blockers, such as mibefradil or
benidipine, can protect the OHCs of the base turn in 24–26-week-old
C57BL/6J mice, and this result was confirmed by SEM. In addition,
the SEM images showed that IHCs were not protected following the
administration of T-type calcium blockers. We hypothesize that the
distribution of T-type calcium channel differed between OHCs and
IHCs, which requires confirmation in future studies.
The improvements observed in the present study may
be due to the manner and dose with which T-type calcium channel
blockers were administered. However, these results are experimental
and insufficient to recommend the clinical application of T-type
calcium channel blockers. The underlying mechanisms involved in the
function of calcium ions of the cochlea remain to be further
elucidated in future studies. The use of calcium channel blockers
is required to be specific and individualized. The treatment of
presbycusis may require interventions beyond calcium channel
blockers, as presbycusis is, like aging, an irreversible natural
process. The two calcium channel blockers used in the present study
may affect L-type or other ion channels in addition to exerting an
effect on T-type calcium channels.
In conclusion, the results of the present study
demonstrated that three T-type calcium channel subunits were
expressed in the cochlea of 6–8-week-old C57BL/6J mice. The
expression levels of the α1H subunit were lower compared with those
of α1 G and α1I. The hearing threshold and DPOAE amplitudes of the
24–26-week-old C57BL/6J mice were significantly improved at high
frequencies following the administration of mibefradil or
benidipine for four consecutive weeks. The degeneration of OHCs was
not evident following the treatment with mibefradil, although the
stereocilia of the IHCs continued to be disorganised and sparse.
Therefore, the administration of a T-type calcium channel blocker
for four consecutive weeks appears to protect OHCs, but not IHCs,
against presbycusis-associated damage.
Acknowledgements
The present study was supported by grants from
Science and Technology Bureau of Suzhou (nos. SYS201228 and
SYS201449).
References
|
1
|
Corso JF: Auditory processes and aging:
Significant problems for research. Exp Aging Res. 10:171–174. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olshansky SJ, Carnes BA and Cassel CK: The
aging of the human species. Sci Am. 268:46–52. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gates GA and Mills JH: Presbycusis.
Lancet. 366:1111–1120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnsson LG and Hawkins JE Jr: Sensory and
neural degeneration with aging, as seen in microdissections of the
human inner ear. Ann Otol Rhinol Laryngol. 81:179–193. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schuknecht HF and Gacek MR: Cochlear
pathology in presbycusis. Ann Otol Rhinol Laryngol. 102:1–16.
1993.PubMed/NCBI
|
|
6
|
Willott JF, Parham K and Hunter KP:
Comparison of the auditory sensitivity of neurons in the cochlear
nucleus and inferior colliculus of young and aging C57BL/6J and
CBA/J mice. Hear Res. 53:78–94. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pickles JO: Mutation in mitochondrial DNA
as a cause of presbyacusis. Audiol Neurootol. 9:23–33. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trump BF and Berezesky IK: The role of
cytosolic Ca2+ in cell injury, necrosis and apoptosis. Curr Opin
Cell Biol. 4:227–232. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orrenius S, McCabe MJ Jr and Nicotera P:
Ca(2+)-dependent mechanisms of cytotoxicity and programmed cell
death. Toxicol Lett 64–65 Spec No: 357–364. 1992. View Article : Google Scholar
|
|
10
|
Fridberger A, Flock A, Ulfendahl M and
Flock B: Acoustic overstimulation increases outer hair cell Ca2+
concentrations and causes dynamic contractions of the hearing
organ. Proc Natl Acad Sci USA. 95:7127–7132. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Liu H and Heller S: Pluripotent stem
cells from the adult mouse inner ear. Nat Med. 9:1293–1299. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Levic S and Dulon D: The temporal
characteristics of Ca2+ entry through L-type and T-type Ca2+
channels shape exocytosis efficiency in chick auditory hair cells
during development. J Neurophysiol. 108:3116–3123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hafidi A and Dulon D: Developmental
expression of Ca(v)1.3 (alpha1d) calcium channels in the mouse
inner ear. Brain Res Dev Brain Res. 150:167–175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen H, Zhang B, Shin JH, Lei D, Du Y, Gao
X, Wang Q, Ohlemiller KK, Piccirillo J and Bao J: Prophylactic and
therapeutic functions of T-type calcium blockers against
noise-induced hearing loss. Hear Res. 226:52–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Willott JF: Aging and the Auditory System:
Anatomy, Physiology, and Psychophysics. San Diego, CA: Singular
Publishing Group, Inc. 1–295. 1991.
|
|
16
|
Johnson KR, Erway LC, Cook SA, Willott JF
and Zheng QY: A major gene affecting age-related hearing loss in
C57BL/6J mice. Hear Res. 114:83–92. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu YF, Zhai F, Dai CF and Hu JJ: The
relationship between age-related hearing loss and synaptic changes
in the hippocampus of C57BL/6J mice. Exp Gerontol. 46:716–722.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kujoth GC, Hiona A, Pugh TD, Someya S,
Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA,
et al: Mitochondrial DNA mutations, oxidative stress and apoptosis
in mammalian aging. Science. 309:481–484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Z, Zhang J and Zhao B: Superoxide anion
regulates the mitochondrial free Ca2+ through uncoupling proteins.
Antioxid Redox Signal. 11:1805–1818. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giacomello M, De Mario A, Primerano S,
Brini M and Carafoli E: Hair cells, plasma membrane Ca2+
ATPase and deafness. Int J Biochem Cell Biol. 44:679–683. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karlstad J, Sun Y and Singh BB: Ca(2+)
signaling: An outlook on the characterization of ca(2+) channels
and their importance in cellular functions. Adv Exp Med Biol.
740:143–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eatock RA and Hurley KM: Functional
development of hair cells. Curr Top Dev Biol. 57:389–448. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spitzer NC: Activity-dependent neuronal
differentiation prior to synapse formation: The functions of
calcium transients. J Physiol Paris. 96:73–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Triggle DJ: The pharmacology of ion
channels: With particular reference to voltage-gated Ca2+ channels.
Eur J Pharmacol. 375:311–325. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Niu YG, Li WX, Yuan YY, Han WJ, Yu
N, Yang SM and Li XQ: Interaction of a calcium channel blocker with
noise in cochlear function in guinea pig. Acta Otolaryngol.
132:1140–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kansu L, Ozkarakas H, Efendi H and Okar I:
Protective effects of pentoxifylline and nimodipine on acoustic
trauma in Guinea pig cochlea. Otol Neurotol. 32:919–925. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inagaki A, Ugawa S, Yamamura H, Murakami S
and Shimada S: The CaV3.1 T-type Ca2+ channel contributes to
voltage-dependent calcium currents in rat outer hair cells. Brain
Res. 1201:68–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sandmann S, Spitznagel H, Chung O, Xia QG,
Illner S, Jänichen G, Rossius B, Daemen MJ and Unger T: Effects of
the calcium channel antagonist mibefradil on haemodynamic and
morphological parameters in myocardial infarction-induced cardiac
failure in rats. Cardiovasc Res. 39:339–350. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao K, Nagashima K and Miki H:
Pharmacological, pharmacokinetic and clinical properties of
benidipine hydrochloride, a novel, long-acting calcium channel
blocker. J Pharmacol Sci. 100:243–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kemp DT: Otoacoustic emissions, their
origin in cochlear function and use. Br Med Bull. 63:223–241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spongr VP, Flood DG, Frisina RD and Salvi
RJ: Quantitative measures of hair cell loss in CBA and C57BL/6 mice
throughout their life spans. J Acoust Soc Am. 101:3546–3553. 1997.
View Article : Google Scholar : PubMed/NCBI
|