Introduction
MicroRNA (miRNA or miR) is a small endogenic
non-coding RNA molecule that regulates gene expression by binding
to 3′-untranslated regions of target mRNA (1). The expression profile of miRNA is
significantly altered in tumor tissues, which suggests that miRNA
molecules may play an important role in the development of tumors.
Therefore, miRNA molecules have great application prospect in the
clinical treatment and diagnosis of tumors (2,3).
miR-503 is a recently-discovered tumor miRNA
molecule (4,5) The expression levels of miR-503 are
associated with various tumor tissue types (6) and have been found to be reduced in oral
cancer (7), hepatocellular carcinoma
(8) and endometrial carcinoma
(6), when compared with the levels
in normal tissues. Xiao et al (9) found that low expression levels of
miR-503 were associated with tumor node metastasis staging of
hepatocellular carcinoma (10).
However, in adrenal carcinoma, parathyroid carcinoma and
retinoblastoma, the expression levels of miR-503 are higher
compared with those in normal tissues (11). Kangai-1 (KAI1) is a common tumor
suppressor gene that is associated with the invasion, migration and
prognosis of numerous tumors, including laryngeal squamous cell
carcinoma and prostate, lung, liver and breast cancer (12,13).
B-cell non-Hodgkin's lymphoma (B-NHL) is a common
cancer of the immune system, which mainly occurs in the lymph node
tissues (14,15). In China, B-NHL accounts for
61.6–74.2% of all NHL cases (15).
Due to the fast progression and high invasiveness of this disease,
the prognosis of B-NHL patients is relatively poor (16). To the best of our knowledge, the
expression levels of miR-503 and KAI1 have not been previously
reported in B-NHL tissues. Through the analysis of the Kyoto
Encyclopedia of Genes and Genomes (KEGG) database that was
performed in the present study, it is hypothesized that KAI1 may be
regulated by miR-503 in B-NHL tissues. Therefore, in the present
study, the expression levels of miR-503 and KAI1 were detected in
B-NHL tissues and the peripheral blood of patients with B-NHL.
Subsequently, the association of the miR-503 and KAI1 expression
levels with the occurrence and development of B-NHL was
analyzed.
Materials and methods
Reagents
The TRIzol reagent used for RNA extraction was
purchased from Invitrogen (Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA). The PrimeScript RT Reagent kit and One Step
SYBR PrimeScript RT-PCR kit II (Perfect Real Time) were purchased
from Takara Bio Inc. (Tokyo, Japan). The rabbit anti-human KAI1
polyclonal antibody (cat no. ab66400; dilution 1:1,000),
biotinylated goat anti-rabbit IgG (cat no., ab128978; dilution
1:5,000) rabbit anti-human anti-β-actin polyclonal antibody (cat
no., 189073; dilution 1:5,000) and horseradish peroxidase
(HRP)-conjugated goat anti-rabbit antibody (cat no. ab6721;
dilution 1:5,000) were purchased from Abcam (Cambridge, MA, USA).
Streptavidin-peroxidase immunohistochemical staining kits were
purchased from Zhongshan Golden Bridge Biotechnology Co., Ltd.
(Beijing, China). Radioimmunoprecipitation assay (RIPA) buffer and
BeyoECL Plus enhanced chemiluminescence reagent were purchased from
Beyotime Institute of Biotechnology (Haimen, China).
Clinical data
A total of 45 patients with B-NHL admitted to the
First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China) between January 2013 and April 2014 were enrolled in the
present study. There were no specific inclusion criteria in this
study. These 45 patients with B-NHL included 23 males and 22
females, with an age range of 17–66 years and a mean age of 45.7
years. Among them, there were 29 cases with stage III/IV and 16
cases with stage I/II disease (Ann Arbor staging classification)
(17). In addition, 26 patients with
reactive lymphoid hyperplasia (RLH), including 12 males and 14
females with a mean age of 38 years, were included in this study as
the control group. Lymphoma or healthy tissues were obtained from
the B-NHL patients by surgery prior to treatment, and peripheral
blood samples were collected from the cubital vein of the RHL
patients prior to surgery. The diagnostic criteria for B-NHL or RHL
were based on the 2008 revision of the World Health Organization
classification of myeloid neoplasms and acute leukemia (18). B-NHL patients were treated with
surgery and routine chemotherapy, while RHL patients were treated
with surgery. Prior written informed consent was obtained from each
patient. The study was approved by the Ethics Review Board of
Zhengzhou University.
Immunohistochemical staining
All the tissue samples were rapidly frozen in liquid
nitrogen. After fixing in formaldehyde and embedding in paraffin,
the samples were cut into 4 µM sections. Next, the sections were
dewaxed and rehydrated in graded alcohols. In order to inactivate
endogenous peroxidase, 3% freshly-prepared hydrogen peroxide was
added and the sections were incubated at room temperature for 10
min. Following antigen retrieval, rabbit anti-human KAI1 polyclonal
antibody (dilution, 1:200) was added and the samples were incubated
in the dark for 1 h. After rinsing with phosphate-buffered saline,
biotinylated goat anti-rabbit IgG antibody was added and incubated
for 30 min at 37°C. The sections were developed with DAB
chromogenic reagent and counterstained with hematoxylin (Beyotime
Institute of Biotechology, Haimen, China). Subsequent to
hydrochloric acid differentiation and dimethylbenzene transparency
(19), the tissue sections were
mounted with neutral gum (Bioworld Technology Co., Ltd., St Louis
Park, MN, USA).
The samples were observed under a microscope (BX53;
Olympus Optical Co., Ltd., Tokyo, Japan) and cells exhibiting brown
staining were defined as KAI1-positive. Five fields at
high-magnification were randomly selected and positive cells were
counted. A positive rate referred to the ratio of the positive cell
number relative to the total cell number. A minimum of 200 cells
were counted in each sample. Based on the percentage of positive
staining, the immunohistochemical staining results were scored
based on a scoring system developed by our group: Score 0, 0%
positive staining; score 1, 1–25% positive staining; score 2,
26–50% positive staining; and score 3, 51–100% positive staining.
The immunohistochemical staining results were also scored based on
the staining intensity, as follows: score 0, no staining; score 1,
light yellow staining; score 2, brownish yellow staining; and score
3, brown staining. Subsequently, the degree of staining was
calculated by combining the scores of the positive staining
percentage and the staining intensity. The overall degree of
staining was defined as follows: score 0–1, negative staining;
score 2–3, weakly positive staining; score 4–6, moderately positive
staining; and score >6, strongly positive staining.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted from the tissues and
peripheral blood samples using TRIzol reagent. All RNAs were
reverse-transcribed into cDNA using the PrimeScript RT Reagent kits
and qPCR assay was conducted using One Step SYBR PrimeScript RT-PCR
kit II (Perfect Real Time), according to the manufacturer's
instructions. According to the miR-503 and KAI1 sequences obtained
from the GenBank database (KAI1, U20770.1; miR-503, MI0003188),
primer sets were designed using the online Pubmed primer blast and
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.). The
primer sequences used were as follows: For miR-503,
5′-TAGCAGCGGGAACAGTTC-3′ (forward) and universal primer [reverse;
One Step SYBR PrimeScript RT-PCR kit II (Perfect Real Time)]; for
KAI1, 5′-GTTCTCTTATCAACTCAG-3′ (forward) and
5′-TACAATATACACACCCTT-3′ (reverse); for β-actin,
5′-CTCCATCCTGGCCTCGCTGT-3′ (forward) and 5′-GCTGTCACCTTCACCGTTCC-3′
(reverse). β-actin was used as an internal control. qPCR was
performed on the ABI One-Step Plus thermal cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc., Foster City, CA, USA).
The PCR system included 10 ul qRT-PCR-Mix, 0.5 ul forward primer,
0.5 ul reverse primer, 1 ul cDNA and 8 ul ddH2O. The PCR
cycle conditions for miR503 were as follows: Pre-denaturation at
95°C for 1 min, and then 40 cycles of 95°C for 1 min and 60°C for
30 sec. The PCR cycle conditions for KAI1 were as follows:
Pre-denaturation at 95°C for 5 min and then 40 cycles of 95°C for 1
min, 58°C for 30 sec and 72°C for 30 sec. For each sample, the PCR
reaction was repeated at least 3 times. The 2−ΔΔCt
method was used to calculate the relative expression levels of
miR-503 and KAI1.
Western blotting
Western blotting was performed as previously
described (20). Briefly, tissues
were homogenized in RIPA buffer. Total proteins were separated by
12% SDS-PAGE and then analyzed by immunoblotting. β-actin was used
as an internal control. The primary antibodies used in western
blotting were the rabbit anti-human KAI1 polyclonal antibody
(dilution, 1:500) and rabbit anti-human anti-β-actin polyclonal
antibody (dilution, 1:5,000). The secondary antibody used in the
present study was the HRP-conjugated goat anti-rabbit antibody
(dilution, 1:2,000). Detection of the blots was performed using
BeyoECL Plus enhanced chemiluminescence reagent and the blots were
quantified using Quantity One software version 4.62 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The experiments were
repeated at least 3 times.
Predicting the possible target genes
of miR-503
The target genes of miR-503 were predicted using
TargetScan version 7.0 (http://www.targetscan.org/). The signaling pathways
that were found to be associated with the target genes of miR-503
were then analyzed using the KEGG database (http://www.genome.jp/kegg/). KAI1 was identified as
one of the target genes of miR-503. The prediction using TargetScan
and the analysis using KEGG were performed according to standard
procedures (21).
Statistical analysis
All the results are expressed as the mean ± standard
deviation and statistical analyss were performed using SPSS version
16.0 for Windows (SPSS Inc., Chicago, IL, USA). Paired t-test was
used to analyze comparisons between groups and for the analysis of
paired data. χ2 test was used to analyze the
relationship of KAI1 positive expression with the clinical staging
of B-NHL. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of miR-503 are
increased in B-NHL tissues
In order to detect the expression levels of miR-503
in B-NHL and RLH tissues, RT-qPCR assay was performed and the
results are demonstrated in Fig. 1.
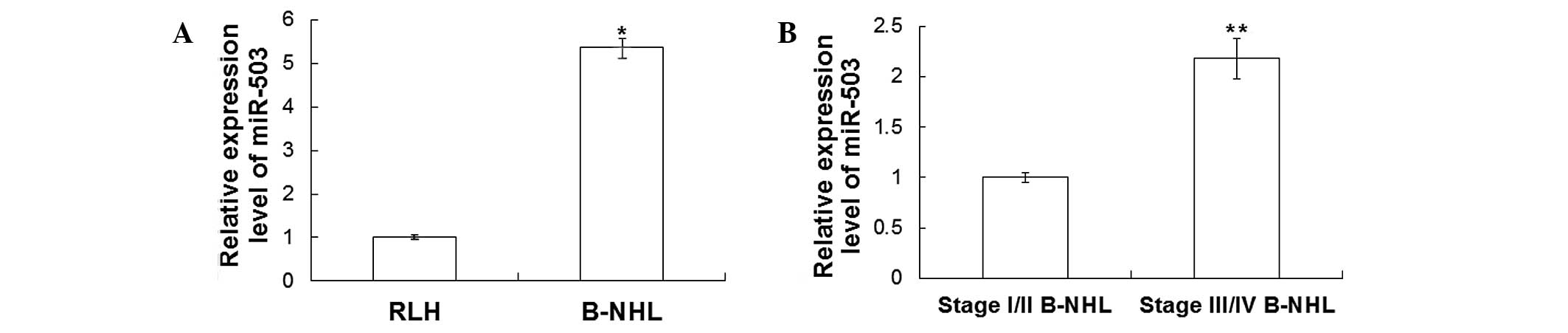
As shown in Fig. 1A, the expression
levels of miR-503 in B-NHL tissues were ~5.36±0.21-fold higher
compared with those in RLH tissues (P<0.05). As shown in
Fig. 1B, the miR-503 expression
levels in B-NHL tissues of patients with stage III/IV disease were
~2.18±0.20-fold higher compared with those in patients with stage
I/II disease (P<0.05; Fig. 1B).
These results indicated that miR-503 was highly expressed in B-NHL
tissues and that its expression was higher in stage III/IV B-NHL
tissues.
Expression levels of KAI1 are
decreased in B-NHL tissues
To determine the expression of KAI1 protein in B-NHL
and RLH tissues, immunohistochemical staining was conducted.
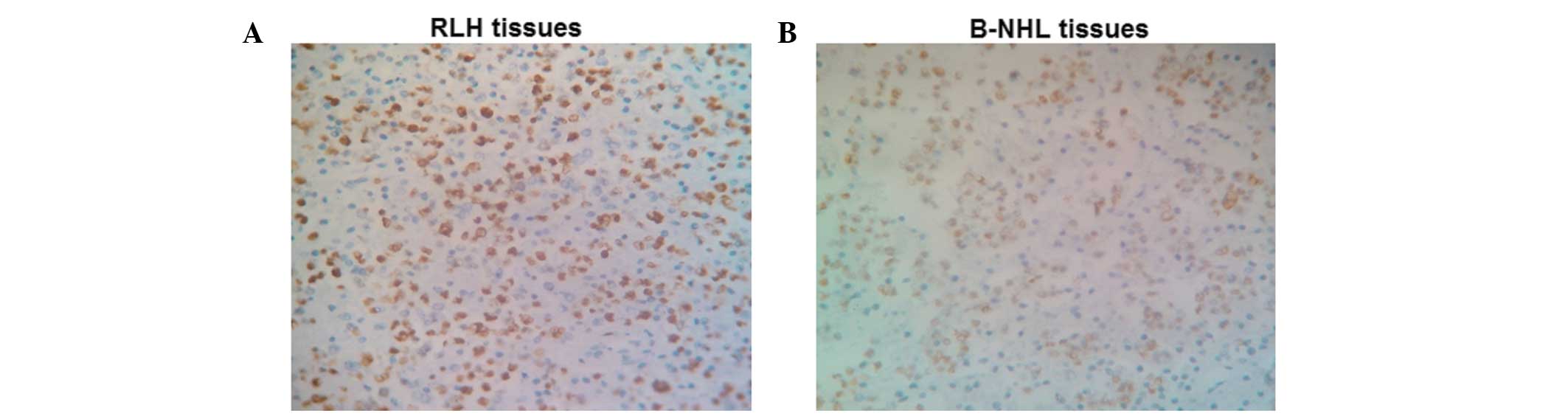
Representative immunohistochemical staining results are shown in
Fig. 2. Cells with brown staining
were considered to be KAI1-positive. In RLH tissues, there were 7
cases with weakly positive expression of KAI1 (26.9%) and 19 cases
with strongly positive expression of KAI1 (73.1%). A representative
image of strongly positive KAI1 expression in RLH tissues is shown
in Fig. 2A. However, in B-NHL
tissues, there were 37 cases with weakly positive KAI1 expression
(82.2%) and 8 cases with strongly positive KAI1 expression (17.8%).
A representative image of weakly positive KAI1 expression in B-NHL
tissues is shown in Fig. 2B. There
were no statistically significant differences in KAI1 expression
among different ages and genders (P>0.05).
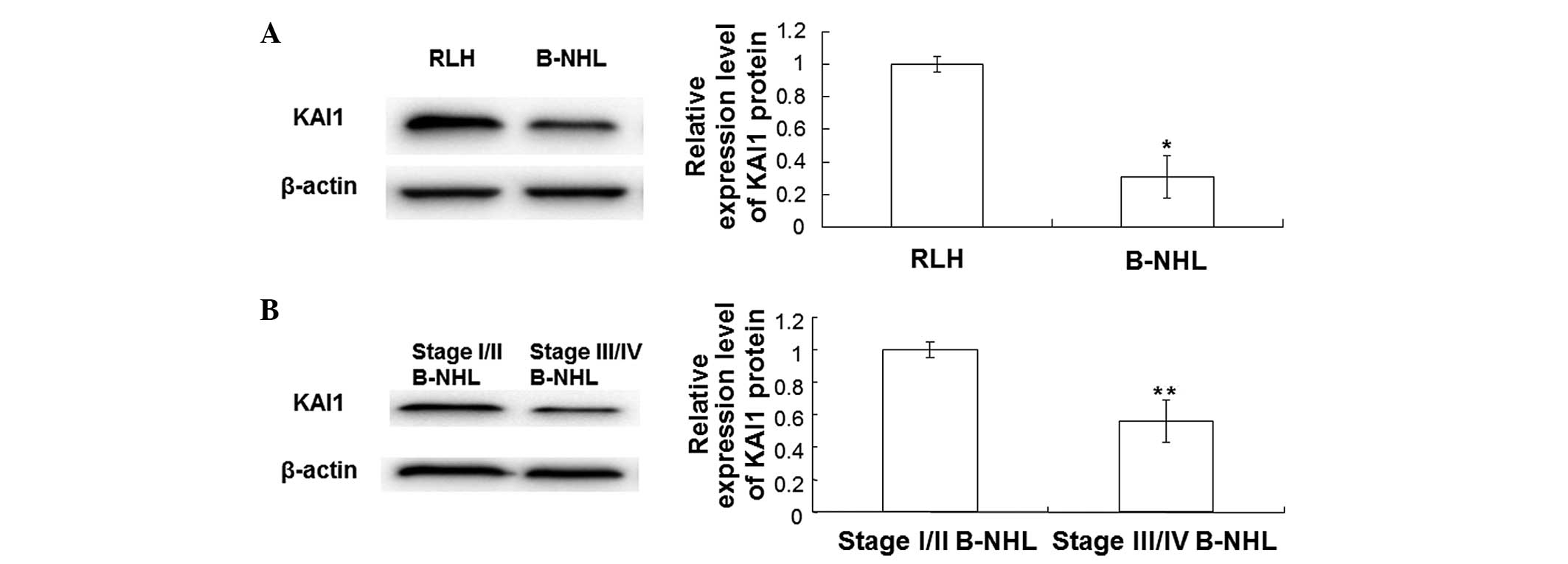
To further verify the expression of KAI1 protein,
western blotting was performed in B-NHL and RLH tissues. As shown
in Fig. 3, the expression of KAI1
protein was significantly decreased in B-NHL tissues when compared
with that in RLH tissues (P<0.05). The expression levels of KAI1
in B-NHL tissues of patients with stage I/II and stage III/IV
disease were also detected by western blotting. As shown in
Fig. 3B, KAI1 expression in stage
III/IV B-NHL tissues was significantly lower compared with that in
stage I/II B-NHL tissues (P<0.05). These results indicate that
the expression levels of KAI1 are decreased in B-NHL tissues and
that KAI1 expression is lower in stage III/IV B-NHL tissues.
Expression levels of miR-503 are
increased, whereas those of KAI1 mRNA are decreased in the
peripheral blood
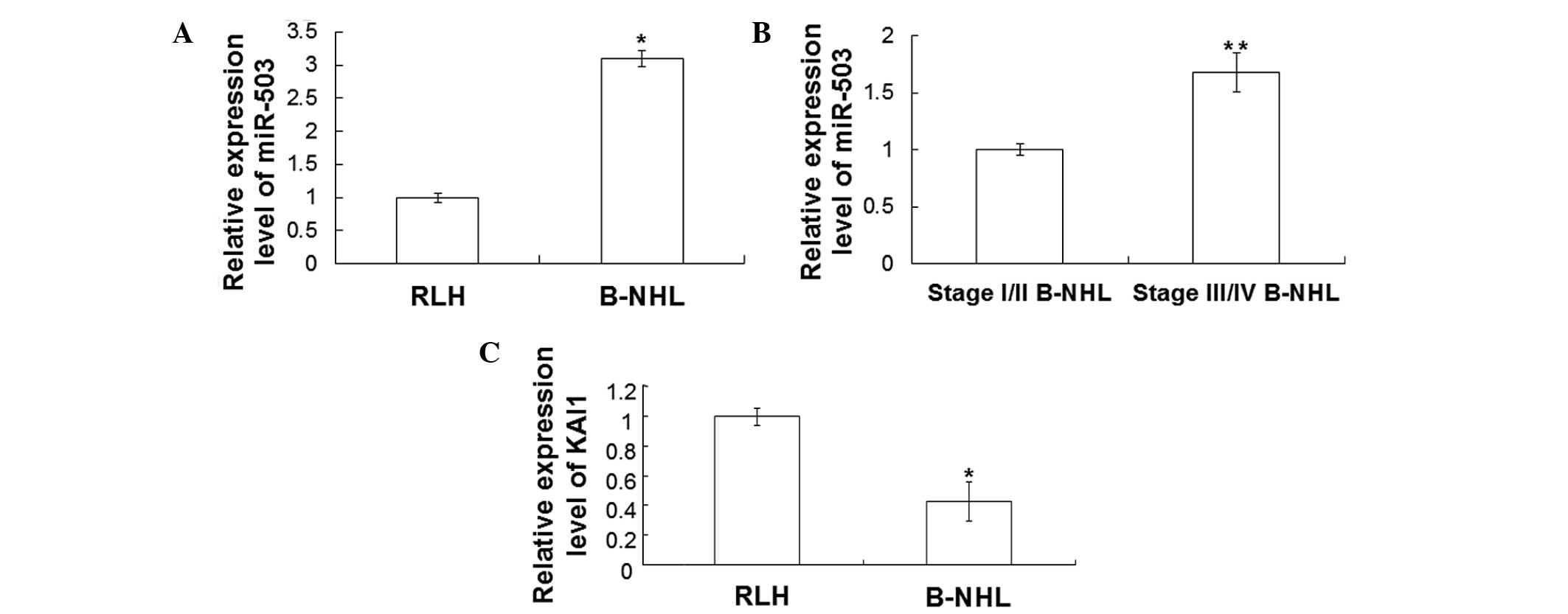
RT-qPCR was performed to detect the expression
levels of miR-503 and KAI1 mRNA in peripheral blood. The RT-qPCR
results are shown in Fig. 4. As
shown in Fig. 4A, miR-503 expression
levels in the peripheral blood of B-HNL patients were significantly
increased when compared with those of RLH patients (P<0.05). In
addition, miR-503 expression levels in the peripheral blood of
patients with stage III/IV B-NHL were significantly increased when
compared with those in patients with stage I/II B-NHL (P<0.05;
Fig. 4B). Furthermore, as shown in
Fig. 4C, KAI1 expression levels were
significantly decreased in the peripheral blood of B-NHL patients
(P<0.05). However, no statistically significant differences in
the KAI1 mRNA expression levels in the peripheral blood were
detected between B-NHL patients with stage I/II and stage III/IV
disease (data not shown). These results indicate that the
expression levels of miR-503 and KAI1 in the peripheral blood of
B-NHL patients are consistent with those in B-NHL tissues.
Discussion
Recently, the prognosis of B-NHL patients has
improved significantly due to better treatments, including the
application of radiotherapy, chemotherapy and immunotherapy;
however, factors such as tumor cell metastasis, drug resistance and
relapse affect the prognosis of patients with B-NHL (22). It has been that several factors, such
as miRNA (23), CHFR prophase
checkpoint gene (24) and human
phosphatidylethanolamine-binding protein 4 (25), may be associated with B-NHL
occurrence and development, the molecular mechanisms of which
require further study.
Previous studies have reported that altered miRNA
expression is associated with the development of lymphoma (26,27). In
the present study, immunohistochemical staining, western blotting
and RT-qPCR assay were performed to investigate the expression
levels of miR-503 and KAI1 in the tissues and peripheral blood of
B-NHL patients. The results demonstrated that the expression levels
of miR-503 were significantly increased in B-NHL tissues when
compared with those in RLH tissues. In addition, the miR-503
expression levels in B-NHL tissues of patients with stage III/IV
disease were increased when compared with those with stage I/II
disease. The expression levels of miR-503 in the peripheral blood
of B-NHL patients were detected using RT-qPCR, which revealed that
the levels were significantly increased compared with those in the
peripheral blood of RLH patients. Furthermore, miR-503 expression
levels in the peripheral blood of patients with stage III/IV B-NHL
were significantly increased when compared with those in stage I/II
B-NHL patients. However, previous studies have indicated that
miR-503 expression levels were downregulated in endometrioid
endometrial cancer (6) and gastric
cancer (28). This difference in the
expression profile of miR-503 between our results and the results
of previous studies may be caused by the difference in the cancer
type. In addition, the expression levels of miR-503 in B-NHL
tissues and peripheral blood changed with the pathological stage of
B-NHL, which indicates that miR-503 may be used as a molecular
marker for the prognosis of B-NHL.
Maurer et al (29) reported that KAI1 expression was
decreased in advanced-stage colon cancer. In addition, Guo et
al (30) identified that KAI1
expression was increased in early pancreatic cancer and decreased
in the presence of metastases. However, Yang et al (31) reported that KAI1 expression was
reduced in non-metastatic human colorectal cancer, but was regained
in metastatic human colorectal cancer. Through the analysis of the
KEGG database that was performed in the present study, we
hypothesized that KAI1 may be regulated by miR-503 in B-NHL
tissues. As revealed by immunohistochemical staining, KAI1
expression was weakly positive in B-NHL tissues and strongly
positive in RHL tissues. Similarly, the expression levels of KAI1
protein were significantly decreased in B-NHL tissues. In addition,
KAI1 expression levels in the tissues of B-NHL patients with stage
III/IV disease were decreased when compared with those of patients
with stage I/II disease. Furthermore, KAI1 mRNA expression levels
were significantly decreased in the peripheral blood of B-NHL
patients. However, no statistically significant differences in the
KAI1 mRNA expression levels were detected between patients with
stage I/II B-NHL and patients with stage III/IV B-NHL. These
results were consistent with the findings of Maurer et al
(29) and Guo et al (30), which indicated that the expression
levels of KAI1 are decreased in B-NHL tissues and the peripheral
blood of patients with advanced-stage B-NHL. The expression levels
of miR-503 and KAI1 detected in B-NHL patients suggest that miR-503
may be involved in the occurrence and development of B-NHL through
the regulation of KAI1 expression levels.
In conclusion, in the present study, the expression
levels of miR-503 were found to be increased in the tissues and
peripheral blood of B-NHL patients. However, the expression levels
of KAI1 were decreased in B-NHL tissues and peripheral blood. Since
miR-503 and KAI1 are associated with B-NHL, these findings may have
a clinical significance in predicting recurrence and metastasis of
B-NHL, based on miR-503 expression. Thus, miR-503 may have an
application as a novel therapeutic and diagnostic marker in B-NHL
patients.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of China (grant no. 81201793).
References
|
1
|
Nair VS, Pritchard CC, Tewari M and
Ioannidis JP: Design and analysis for studying microRNAs in human
disease: A primer on -omic technologies. Am J Epidemiol.
180:140–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arora S, Swaminathan SK, Kirtane A,
Srivastava SK, Bhardwaj A, Singh S, Panyam J and Singh AP:
Synthesis, characterization and evaluation of poly
(D,L-lactide-co-glycolide-based nanoformulation of miRNA-150:
Potential implications for pancreatic cancer therapy. Int J
Nanomedicine. 9:2933–2942. 2014.PubMed/NCBI
|
|
3
|
Chen WX, Cai YQ, Lv MM, Chen L, Zhong SL,
Ma TF, Zhao JH and Tang JH: Exosomes from docetaxel-resistant
breast cancer cells alter chemosensitivity by delivering microRNAs.
Tumour Biol. 35:9649–9659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo J, Liu X and Wang M: miR-503
suppresses tumor cell proliferation and metastasis by directly
targeting RNF31 in prostate cancer. Biochem Biophys Res Commun.
64:1302–3028. 2015. View Article : Google Scholar
|
|
5
|
Li B, Liu L, Li X and Wu L: miR-503
suppresses metastasis of hepatocellular carcinoma cell by targeting
PRMT1. Biochem Biophys Res Commun. 64:982–987. 2015. View Article : Google Scholar
|
|
6
|
Xu YY, Wu HJ, Ma HD, Xu LP, Huo Y and Yin
LR: MicroRNA-503 suppresses proliferation and cell-cycle
progression of endometrioid endometrial cancer by negatively
regulating cyclin D1. FEBS J. 280:3768–3779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu YC, Chen YJ, Wang HM, Tsai CY, Chen WH,
Huang YC, Fan KH, Tsai CN, Huang SF, Kang CJ, et al: Oncogenic
function and early detection potential of miRNA-10b in oral cancer
as identified by microRNA profiling. Cancer Prev Res (Phila).
5:665–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou J, Tao Y, Peng C, Gu P and Wang W:
miR-503 regulates metastatic function through Rho guanine
nucleotide exchanger factor 19 in hepatocellular carcinoma. J Surg
Res. 188:129–136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao F, Zhang W, Chen L, Chen F, Xie H,
Xing C, Yu X, Ding S, Chen K, Guo H, et al: MicroRNA-503 inhibits
the G1/S transition by downregulating cyclin D3 and E2F3 in
hepatocellular carcinoma. J Transl Med. 11:1952013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukui T, Fukumoto K, Okasaka T, Kawaguchi
K, Nakamura S, Hakiri S, Ozeki N, Hirakawa A, Tateyama H and Yokoi
K: Clinical evaluation of a new tumour-node-metastasis staging
system for thymic malignancies proposed by the International
Association for the Study of Lung Cancer Staging and Prognostic
Factors Committee and the International Thymic Malignancy Interest
Group. Eur J Cardiothorac Surg: Nov. 7:2015.(Epub ahead of
print).
|
|
11
|
Yang Y, Liu L, Zhang Y, Guan H, Wu J, Zhu
X, Yuan J and Li M: MiR-503 targets PI3K p85 and IKK-β and
suppresses progression of non-small cell lung cancer. Int J Cancer.
135:1531–1542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou L, Wu SW, Yu L, Song WQ, Cheng ZN and
Wang DN: The expression of KAI1 in gastric adenocarcinoma and
relationship with angiogenesis/lymphangiogenesis. Sichuan Da Xue
Xue Bao Yi Xue Ban. 45:43–48. 2014.(In Chinese). PubMed/NCBI
|
|
13
|
Liu X, Guo XZ, Li HY, Chen J, Ren LN and
Wu CY: KAI1 inhibits lymphangiogenesis and lymphatic metastasis of
pancreatic cancer in vivo. Hepatobiliary Pancreat Dis Int.
13:87–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiu B, Lin Y, Grote DM, Ziesmer SC,
Gustafson MP, Maas ML, Zhang Z, Dietz AB, Porrata LF, Novak AJ, et
al: IL-10 induces the development of immunosuppressive
CD14(+)HLA-DR(low/-) monocytes in B-cell non-Hodgkin lymphoma.
Blood Cancer J. 5:e3282015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hua Q, Zhu Y and Liu H: Severe and fatal
adverse events risk associated with rituximab addition to B-cell
non-Hodgkin's lymphoma (B-NHL) chemotherapy: a meta-analysis. J
Chemother. 2015.Apr 15: 1973947815Y0000000025. [Epub ahead of
print]. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghorbian S, Jahanzad I, Javadi GR and
Sakhinia E: Evaluation diagnostic usefulness of immunoglobulin
light chains (Igκ, Igλ) and incomplete IGH D-J clonal gene
rearrangements in patients with B-cell non-Hodgkin lymphomas using
BIOMED-2 protocol. Clin Transl Oncol. 16:1006–1011. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scwab M: Ann Arbor Staging System.
Encyclopedia of Cancer (2nd). (Berlin, Heidelberg). Springer Berlin
Heidelberg. 169. 2009. View Article : Google Scholar
|
|
18
|
Swerdlow SH, Campo E, Harris NL, et al:
WHO Classification of Tumours of Haematopoietic and Lymphoid
Tissues. 2:(4th). Lyon, France: IARC Press. 2008.
|
|
19
|
Yamashita Y, Hasegawa M, Deng Z, Maeda H,
Kondo S, Kyuna A, Matayoshi S, Agena S, Uehara T, Kouzaki H, et al:
Human papillomavirus infection and immunohistochemical expression
of cell cycle proteins pRb, p53, and p16(INK4a) in sinonasal
diseases. Infect Agent Cancer. 10:232015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
You J, Madigan MC, Rowe A, Sajinovic M,
Russell PJ and Jackson P: An inverse relationship between KAI1
expression, invasive ability, and MMP-2 expression and activity in
bladder cancer cell lines. Urol Oncol. 30:502–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Altman T, Travers M, Kothari A, Caspi R
and Karp PD: A systematic comparison of the MetaCyc and KEGG
pathway databases. BMC Bioinformatics. 14:1122013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sekimzu M, Mori T, Kikuchi A, Mitsui T,
Sunami S, Kobayashi R, Fujita N, Inada H, Takimoto T, Saito AM, et
al: Prognostic impact of cytogenetic abnormalities in children and
adolescents with mature B-cell non-Hodgkin lymphoma: A report from
the Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG).
Pediatr Blood Cancer. 62:1294–1296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bruni R, Marcantonio C, Pulsoni A, Tataseo
P, De Angelis F, Spada E, Marcucci F, Panfilio S, Bianco P,
Riminucci M, et al: microRNA levels in paraffin-embedded indolent
B-cell non-Hodgkin lymphoma tissues from patients chronically
infected with hepatitis B or C virus. BMC Infect Dis 14 Suppl.
5:S62014. View Article : Google Scholar
|
|
24
|
Song A, Ye J, Zhang K, Yu H, Gao Y, Wang
H, Sun L, Xing X, Yang K and Zhao M: Aberrant expression of the
CHFR prophase checkpoint gene in human B-cell non-Hodgkin lymphoma.
Leuk Res. 39:536–543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang K, Jiang Y, Zheng W, Liu Z, Li H, Lou
J, Gu M and Wang X: Silencing of Human
Phosphatidylethanolamine-Binding Protein 4 Enhances
Rituximab-Induced Death and Chemosensitization in B-Cell Lymphoma.
PLoS One. 8:e568292013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Di Lisio L, Sánchez-Beato M, Gómez-López
G, Rodríguez ME, Montes-Moreno S, Mollejo M, Menárguez J, Martínez
MA, Alves FJ, Pisano DG, et al: MicroRNA signatures in B-cell
lymphomas. Blood Cancer J. 2:e572012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tagawa H, Ikeda S and Sawada K: Role of
microRNA in the pathogenesis of malignant lymphoma. Cancer Sci.
104:801–809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng Y, Liu YM, Li LC, Wang LL and Wu XL:
microRNA-503 inhibits gastric cancer cell growth and
epithelial-to-mesenchymal transition. Oncol Lett. 7:1233–1238.
2014.PubMed/NCBI
|
|
29
|
Maurer CA, Graber HU, Friess H, Beyermann
B, Willi D, Netzer P, Zimmermann A and Büchler MW: Reduced
expression of the metastasis suppressor gene KAI1 in advanced colon
cancer and its metastases. Surgery. 126:869–880. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo X, Friess H, Graber HU, Kashiwagi M,
Zimmermann A, Korc M and Büchler MW: KAI1 expression is
up-regulated in early pancreatic cancer and decreased in the
presence of metastases. Cancer Res. 56:4876–4880. 1996.PubMed/NCBI
|
|
31
|
Yang JL, Jackson P, Yu Y, Russell PJ,
Markovic B and Crowe PJ: Expression of the KAI1 metastasis
suppressor gene in non-metastatic vs. metastatic human colorectal
cancer. Anticancer Res. 22:3337–3342. 2002.PubMed/NCBI
|