Introduction
The facial nerve is the longest peripheral nerve in
the human body. Its anatomical structure is unique and its
position, which is superficial and closely associated with the
parotid gland, leaves it vulnerable to damage during an operation
or as a result of trauma (1,2). Damage to the facial nerve can lead to
paralysis of the muscles that control facial expressions (3), which may seriously affect the patients'
quality of life and result in psychological stress for patients and
their families. Therefore it is unsurprising that facial nerve
defect repair is widely studied (4,5).
Current treatments for damaged facial nerves
following facial nerve injury include surgical repair, catheter
facial nerve repair and molecular repair (6). Most neurotrophic factors are capable of
inducing axonal growth and current research is predominantly
focused on three groups of neurotrophic factors which are involved
in the regulation of endogenous repair processes: Neurotrophic
factors, including insulin-like growth factor (IGF)-1, IGF-2 and
BDNF, transforming growth factor (TGF)-β, glial cell-derived
neurotrophic factor and IL-6 cytokines, including leukemia
inhibitory factor (7,8). Treatment with exogenous IGF-1 and IGF-2
improves the rate of axonal regeneration, whereas the application
of corresponding antibodies can block this effect (9). The underlying mechanism may involve
IGF-mediated activation of RAS/MAPK and PI3K/Akt signaling
pathways, thereby inhibiting the apoptosis of neurons and Schwann
cells and promoting the growth of axon.
TGF-β superfamily consists of pleiotropic
polypeptide growth factors with extensive biological activities, of
which TGF-β3 is a subtype. With the development of tissue
engineering, an increasing number of studies have investigated the
role of neural regulatory factors in the regeneration of injured
facial nerves (10). However, the
effects of TGF-β3 on the repair of injured facial nerves, and the
underlying mechanisms, are yet to be elucidated (11–16).
In the present study, a rabbit model was established
with trauma sustained to the bilateral superior buccal branches of
the facial nerve. In order to facilitate the interior
administration of TGF-β3 to investigate nerve regeneration, a nerve
growth chamber was constructed using a silicone tube. Dynamic,
continuous and comparative observations of the morphological
changes of the repaired nerve were performed at various time
points, utilizing various methods. Through a combination of neural
electrical physiological detection and image analysis, the effects
and possible mechanisms of TGF-β3 on facial nerve injury repair
were investigated.
Materials and methods
Reagents
Paraformaldehyde was obtained from Beijing Chemical
Reagent Company (Beijing, China). The JEOL 1230 transmission
electron microscope was purchased from JEOL, Ltd. (Tokyo, Japan).
The operating microscope and BX26 optical microscope were purchased
from Olympus Corporation (Tokyo, Japan). A Canon digital camera was
obtained from Canon Inc. (D70; Tokyo, Japan) and the EM UC6
ultramicrotome was purchased from Leica Microsystems (Wetzlar,
Germany).
Experimental animals and animal
modeling
A total of 20 adult rabbits, weighing 2.5–3.0 kg,
were provided by the Department of Experimental Animals of Xinjiang
Medical University (Urumchi, China). The rabbits were maintained
under standard conditions with ad libitum access to food and
water. The present study was approved by the Experimental Animal
Ethics Committee at Xinjiang Medical University.
Facial nerve trauma and repair models were
established in 10 rabbits. Rabbits were anesthetized with 30 mg/kg
pentobarbital via the ear vein and a transverse incision was
subsequently made in the bilateral cheek to expose ~2 cm of the
buccal branches of the facial nerve prior to the transection of ~5
mm of the superior buccal branches of facial nerve. A silicone
chamber, to be used as a nerve conduit, was created using a
surgical microscope (ASOM-4C; Chengdu Corder Optics &
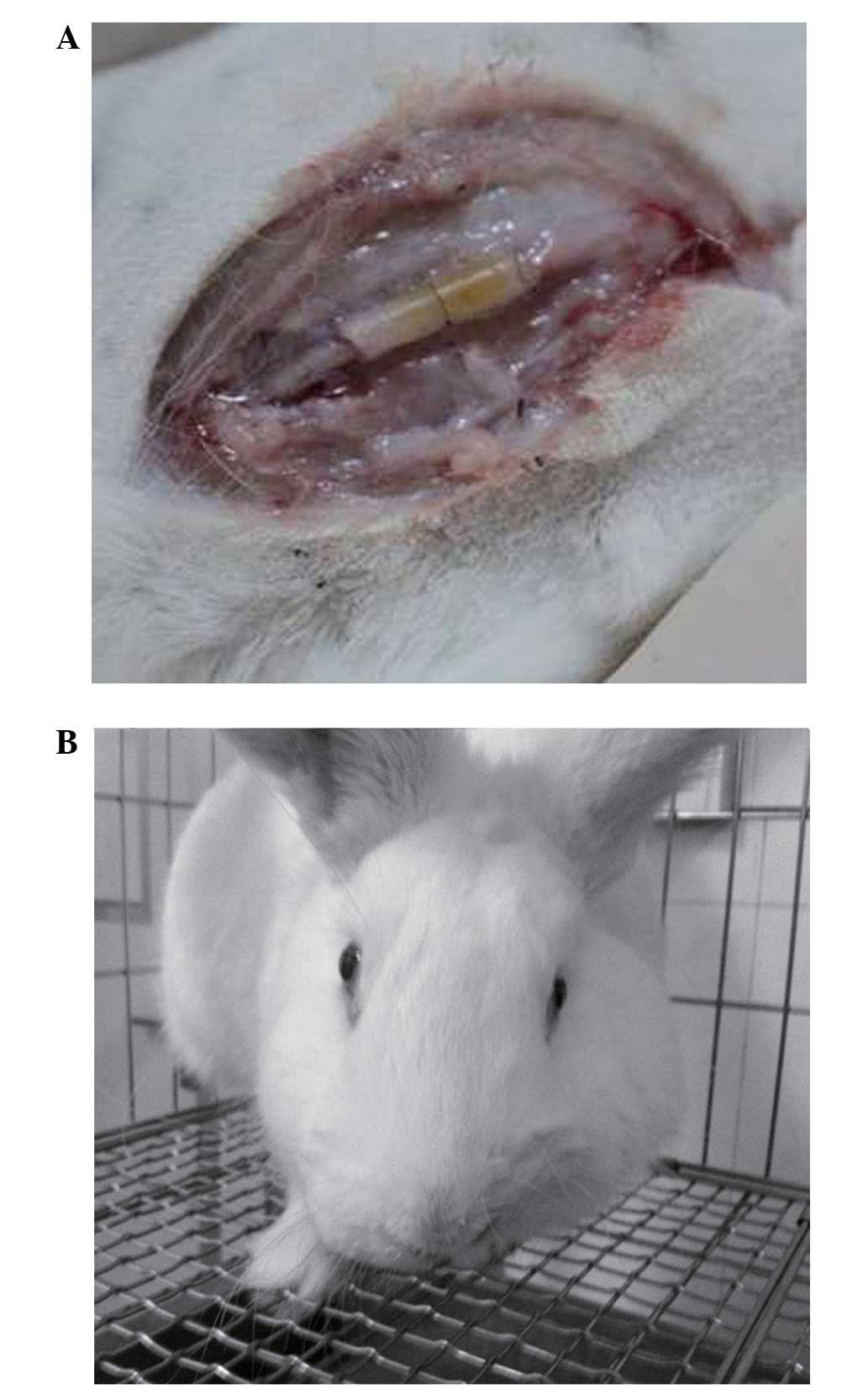
Electronics Co., Ltd., Chengdu, China). In order to form a nerve
regeneration chamber (Fig. 1A), the
broken ends of the facial nerve were embedded 1.5 mm into a sterile
silicon tube (outer diameter, 3 mm; inner diameter, 2 mm; length,
10 mm). Suture fixation of both ends of the epineurium to the
silica gel tube was performed using 10–0 thread. A collagen sponge
was used for support and as a vehicle for TGF-β3 implantation.
Following this, the right silicone chambers were loaded with 10 µl
TGF-β3 (50ng/µl) and were used as the TGF-β3 treatment group
(n=10); whereas the left chambers were filled with
phosphate-buffered saline and used as the surgical control group
(n=10). The wounds were subsequently sutured using 4–0 thread. A
total of 10 rabbits were randomly assigned to the normal control
group, and did not receive any treatment.
Examination of gross morphology
Preoperative and postoperative changes in the
vibrissal movements of the rabbits were observed, including
trembling symptoms. Gross morphology, anastomosis of neural
connections, stump neuroma and scars on the buccal branches of the
facial nerve were examined 12 weeks post-operation.
Hematoxylin and eosin (HE)
staining
Rabbits were anaesthetized using 3% pentobarbital
sodium and sacrificed via strangulation. The rabbits were perfused
with 4% paraformaldehyde, via the heart, for fixation and proximal
and distal allograft neural tissue samples (length, ~1 mm) were
subsequently collected from each group. Briefly, the buccal branch
of the facial nerve was exposed. Subsequently, the facial nerve was
dissected 5 mm from the distal end of the silicone tube and 5 mm
from the proximal end of the silicone tube. Following removal of
the silicone tube, the facial nerve specimens were harvested.
Specimens were fixed with 4% paraformaldehyde, embedded in
paraffin, sliced into sections and stained with HE. Subsequently,
>20 fields in each specimen were randomly selected for
observation of the regenerated nerve fibers. The number and
diameter of regenerating nerve fibers in the cross-section of the
regenerated nerve and the longitudinal section of the proximal
anastomosis were observed using light microscopy (SZM-45B1; Zhong
Tu Ao Si Micro-Optical Equipment Company, Shenzhen, China,
Shenzhen, China).
Light microscopy and transmission
electron microscopy examination
The neural tissue samples were fixed with 2.5%
glutaraldehyde at 4°C for 6 h prior to immobilization with 1% osmic
acid and subsequent gradient acetone dehydration. Following this,
the tissues were washed with 0.1 M phosphate buffer solution,
embedded overnight with anhydrous Epoxy resin 618 (Wuxi Resin
Factory, Wuxi, China) and incubated in the dryer for pure embedding
at 37°C for 2 h and 60°C for 48 h, until the resin had totally
polymerized. Part of the embedded tissue was sliced into semi-thin
sections and stained with toluidine blue (Shanghai Mai Ke Lin
Biochemical Company, Shanghai, China). Ten sections of each
specimen were used for examination of the regenerated nerve using
light microscopy. Part of the embedded tissue was sliced into
ultrathin sections, saturated with uranyl acetate for 30 min and
stained with citric acid for 15 min. Subsequently >20 fields in
each specimen were randomly selected for observation of regenerated
nerve fibers. The ultrathin sections of the regenerated nerves were
observed under a transmission electron microscope.
Electrophysiological examination
The compound muscle action potentials of the facial
nerve were detected using an electromyogram evoked potential
instrument (A3101-b; Shanghai Hai Shen Medical Electronic
Instrument Co., Ltd., Shanghai, China), 12 weeks post-operation.
The latency period and amplitude values of the facial nerve action
potentials were recorded and analyzed using SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA).
Statistical analysis
All data were analyzed by SPSS 16.0 software. Paired
t-tests were performed to analyze the difference between the
treatment and control groups. Data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Changes in gross morphology
In order to determine whether TGF-β3 had an effect
on nerve regeneration, the general condition and gross morphology
of the rabbits in the various groups were observed. The general
condition of the rabbits was observed immediately after surgery,
various facial paralysis symptoms were detected in all of the
rabbits with facial nerve trauma, including: Varying degrees of
photophobia, a pendulous ear on the operative side, 1–2 cm eyelid
distance, a drooping upper lip, and a failure to completely close
eyelids, even following stimulation (Fig. 1B).
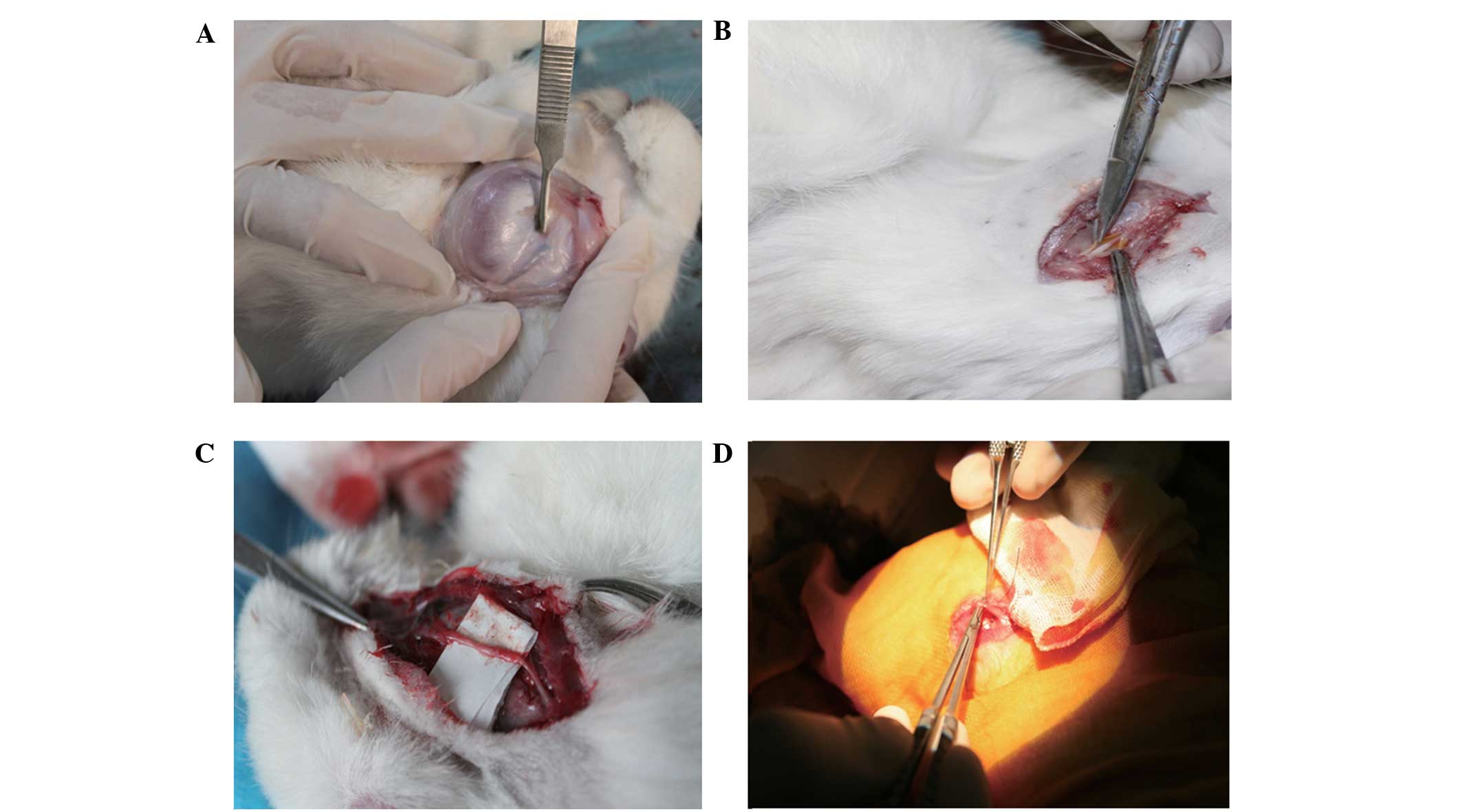
A total of 12 weeks after the operation, the
original incision was reopened under general anesthesia, in order
to assess the repair of the nerve injury. The silicone tube was
encapsulated by fibrous connective tissue in both the TGF-β3
treatment and surgical control groups (Fig. 2A and B), as observed with the naked
eye. Furthermore, when the connective tissue was dissected from the
translucent silicone tubes, both ends of the nerve had successfully
crossed the defect area and connected; however, the wall of the
silica gel tube had no adhesion with the regenerated nerve and it
was easy to separate them. In the TGF-β3 treatment group, the
diameters of the regenerated nerves were similar to those of the
nerve stems in the distal and proximal ends. Furthermore, no
neuroma was detected and the newly formed epineurium comprised of a
complex network of blood vessels gray in color, and was firm and
tough, as demonstrated by the visible tension caused by pulling
with tweezers without snapping (Fig.
2C). In the surgical control group, the end of the regenerated
nerve was slightly thicker and the nerve appeared delicate, with
poor elasticity; therefore, it was easy to break (Fig. 2D). The results suggest that the
general condition and gross morphology of the damaged facial nerves
was improved following treatment with TGF-β3.
Observation of the regenerated facial
nerve using a light microscope
In order to analyze the effects of TGF-β3 on the
regeneration of facial nerves, the number and diameter of the
regenerated nerve fibers were measured at 12 weeks post-operation.
As outlined in Table I, the total
number and diameter of the regenerated nerve fibers was
significantly increased (P<0.01) in the TGF-β3 treatment group
(1052.00±144.34 root and 6.16±0.45 cm), as compared with those in
the surgical control group (555.30±86.74 root and 3.59±0.61
cm).
 | Table I.Comparison of the total number and
diameter of the regenerated nerve fibers at 12 weeks post-operation
(n=10). |
Table I.
Comparison of the total number and
diameter of the regenerated nerve fibers at 12 weeks post-operation
(n=10).
| Group | Total number
(root) | Diameter (µm) |
|---|
| TGF-β3 treatment | 1052.00±144.34 | 6.16±0.45 |
| Surgical control | 555.30±86.74 | 3.59±0.61 |
| T-value | 9.327 | 10.689 |
| P-value | <0.001 | <0.001 |
In the TGF-β3 treatment group, the epineurium of the
facial nerve was intact and the nerve fibers were morphologically
intact and arranged in neat rows with visible myelin swelling.
However, in the surgical control group, the epineurium was
incomplete, atrophy was detected in the nerve fiber, the axons had
partially disappeared, and the myelin was of uneven thickness with
fuzzy borders.
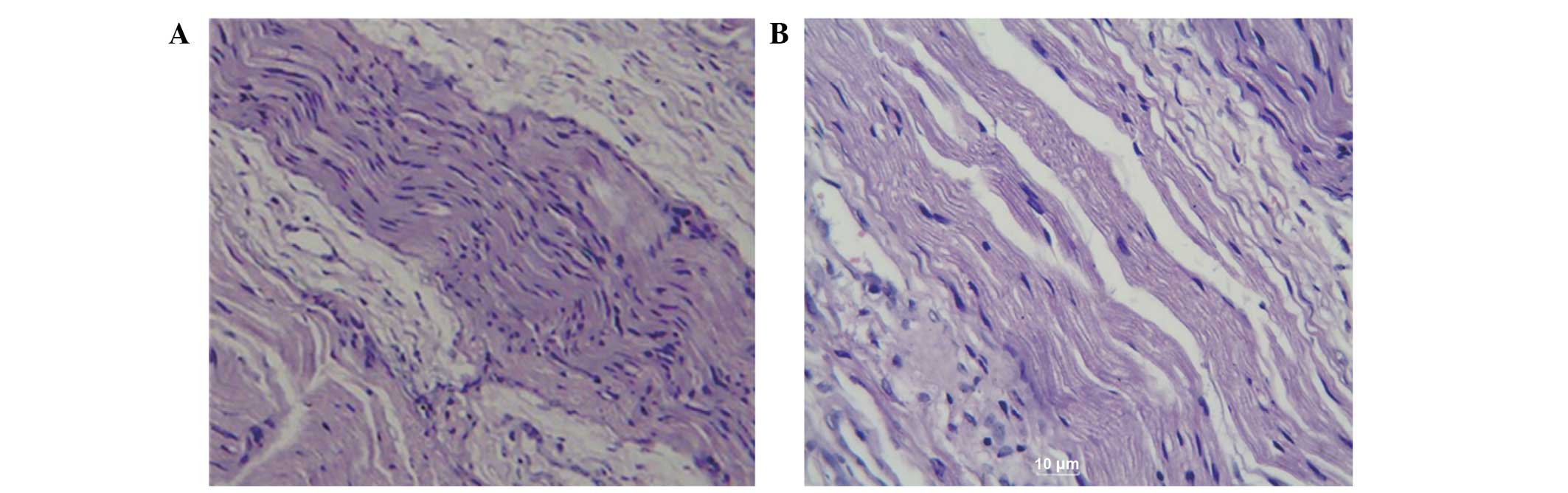
In order to observe any pathological changes in the
newly regenerated nerve fibers, the facial nerves were subsequently
stained with HE and examined. As demonstrated in Fig. 3A, numerous regenerated nerve fibers
were observed in the cross sections from the TGF-β3 treatment
group, and the majority of these fibers formed into bundles
containing blood vessels. Furthermore, hypertrophy and hyperplasia
of the Schwann cells was visible, the size of the regenerated
fibers was not uniform, the nuclei were thick, and in the
longitudinal sections, the regenerated nerve fibers twisted in
parallel across the distal anastomosis. In the cross sections
obtained from the surgical control group (Fig.3B), minimal newborn nerve fibers and
few myelin fibers were detected, in addition to fat cell
infiltration and considerable scar tissue. In the longitudinal
sections, the nerve fibers were arranged in a disordered and
discontinuous manner and some were partially dissolved. The
regenerated nerve fibers with obvious distortions presented as
spirals with substantial scar tissue. In addition, the axons were
unevenly distributed and their numbers were significantly reduced,
with no obvious myelin sheath. Collectively, these results suggest
that TGF-β3 may effectively promote the repair of transected facial
nerves.
Observation of the facial nerve under
a transmission electron microscope
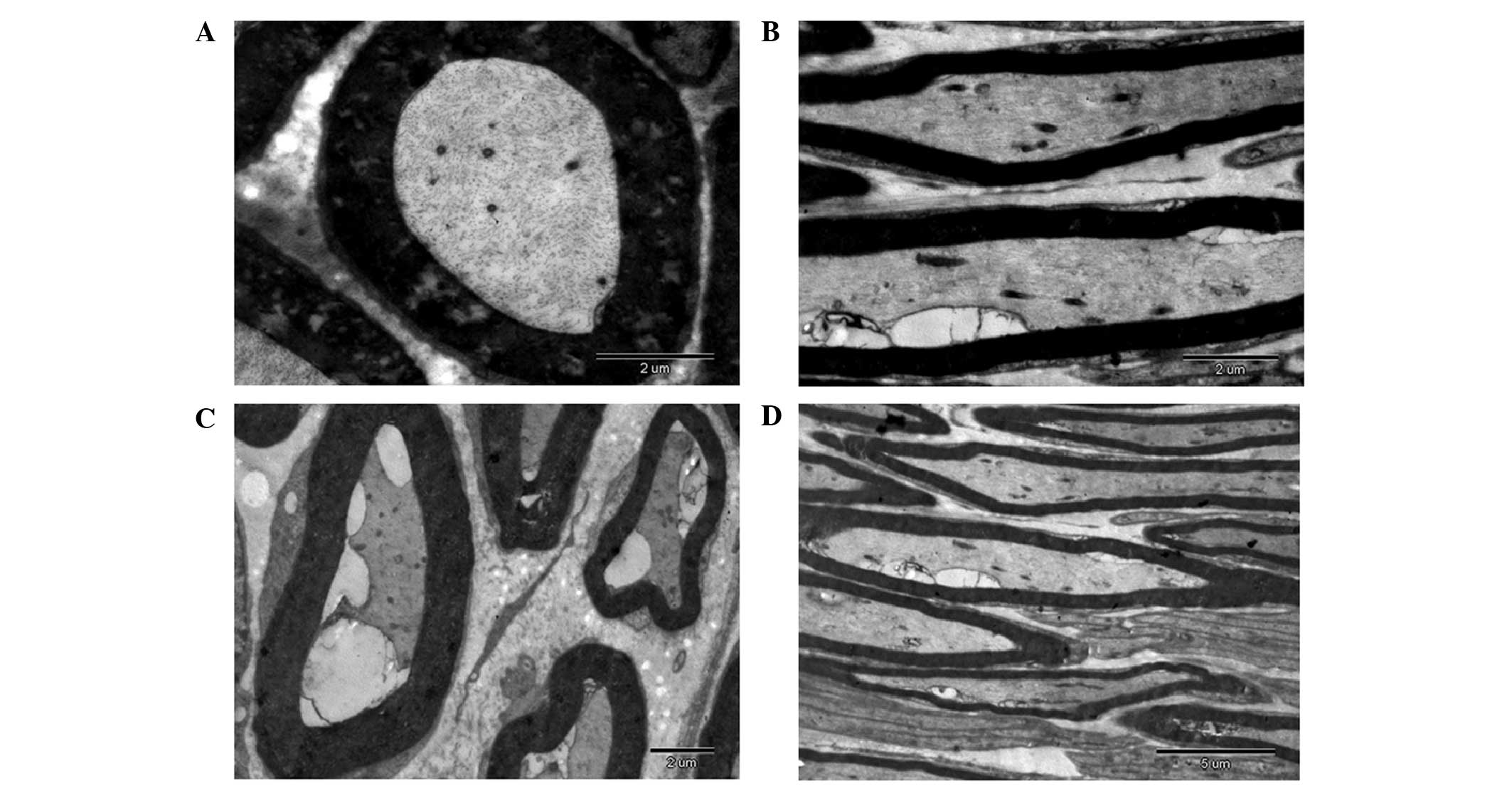
In order to analyze the structure of the regenerated
nerve fiber, the regenerated facial nerve was observed under a
transmission electron microscope. As outlined in Fig. 4A and B, the regenerated facial nerves
in the TGF-β3 treatment group were predominantly myelinated nerves
with regular form. Furthermore, the layered structure of the myelin
sheath was clear with distinct dark and light banding and there
were abundant organelles in the axoplasm. Notably, most of the
regenerated fibers formed into bundles with rich blood vessels;
whereas in the surgical control group, nerve regeneration and
repair only occurred in five injured facial nerves. The remaining
facial nerve injuries demonstrated scar tissue and when the
ultrathin sections were observed under a transmission electron
microscope the regenerated nerve fibers were irregular in shape. In
addition, the axoplasmic organelles were unclear, the lamellar
structures were disordered, and myelin dysplasia and degeneration
of the nerve fiber was observed (Fig. 4C
and D). These results suggest that 12 weeks post-operation, the
regenerated facial nerve fibers of rabbits in the experimental
group were predominantly myelinated nerve fibers with a clear
lamellar structure and rich axoplasmic organelles, as compared with
the control group.
Electrophysiological examination
To evaluate the functional recovery of the facial
nerves, an electrophysiological examination was performed 12 weeks
post-operation. In order to detect the compound muscle action
potentials of the facial nerve, latency period and amplitude values
were recorded and analyzed using an electromyogram evoked potential
instrument. As shown in Table II,
the action potential latency periods were increased in the surgical
control (3.42±0.56 msec) and TGF-β3 treatment groups (2.35±0.41
msec), as compared with the normal control group (1.47±0.42 msec).
The latency periods of the action potentials in the TGF-β3
treatment group were significantly decreased (P<0.01), as
compared with the surgical control group. As compared with the
normal control group (11.32±5.36 mV), the amplitudes of the action
potentials in the surgical control group (4.62±0.77 mV) were
significantly decreased (P<0.01); whereas a significant increase
(P<0.01) was detected in the TGF-β3 treatment group (8.60±1.87
mV). These results suggest that administration of TGF-β3 into the
regeneration chamber following the transection of the superior
buccal branches of the facial nerve may significantly promote the
conduction velocity of the facial nerve.
 | Table II.Comparison of the latency period and
amplitude of action potentials at 12 weeks post-operation
(n=10). |
Table II.
Comparison of the latency period and
amplitude of action potentials at 12 weeks post-operation
(n=10).
| Group | Latency period
(ms) | Amplitude (mV) |
|---|
| TGF-β3 treatment |
2.35±0.41a,b |
8.60±1.87a,b |
| Surgical control |
3.42±0.56a |
4.62±0.77a |
| Normal control | 1.47±0.42 | 11.32±5.36 |
| T-value | 8.596 | 23.731 |
Discussion
Regeneration of an injured nerve is a complex
process and it is a comprehensive reflection of the reconstruction
of neural pathways, recovery of metabolism and functional repair
(17,18). Commonly used methods for the repair
of nerves include: Autologous nerve transplantation, nerve
allograft transplantation, end-to-end anastomosis, facial nerve
transplantation, vascularized nerve graft and non-nervous tissue
transplantation (19–21). End-to-end anastomosis cannot be
performed when there is a large defect in the facial nerve,
therefore the most effective method for the repair of facial nerves
with larger defects is an autologous nerve transplantation
(22,23). However, for peripheral nerve defects
involving thick and long segments this method is not appropriate,
as the autologous nerve graft source is difficult to acquire and
the following problems may occur: Neuroma formation, scarring in
the donor area, sensorimotor disorder and errors in the neural
network (24,25). It has been suggested that nerve
conduit technology may be an effective method to solve these
problems; Lundborg et al (26) demonstrated that silicone tubes could
be used for nerve defect repair and this promoted the development
of nerve catheter technology. Previous studies (27,28) have
used nerve substitutes and various materials in the repair of
facial nerve injury and have demonstrated that nerve catheter
orientation and the nutrition of the regenerative microenvironment
have a vital role in regeneration of the damaged nerve. Therefore,
the use of nerve regeneration chambers to alter the regenerative
microenvironment and the addition of nerve regeneration factors
into the chamber is now widely used in neural regeneration
(29,30).
In the present study, a silicone tube was used as a
nerve conduit to simulate the epineurium, and a collagen sponge
with a 3D network structure was used for support and as a vehicle
for TGF-β3 implantation. The nerve conduit provided a combination
of nutrition, support and contact guidance as the chamber prevented
the formation of scar tissue and the invasion of connective tissue,
in addition to maintaining the stability of the microenvironment
for nerve regeneration.
The results of the present study demonstrated that
administration of TGF-β3 into the regeneration chamber may
effectively promote the growth and maturity of regenerating facial
nerves. The repair effects demonstrated by the TGF-β3 treatment
group were significantly improved, as compared with the surgical
control group. Therefore, the local application of TGF-β3 may
significantly increase the diameter of axons and nerve conduction
velocity, and accelerate facial nerve fiber regeneration and
myelination, thus promoting the overall repair of facial
nerves.
In conclusion, TGF-β3 may promote the regeneration
of facial nerves following trauma; however, the process of facial
nerve repair is rather complicated. Therefore, whether TGF-β3 can
be used clinically remains unknown and requires further
investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 30960423).
References
|
1
|
Malik TH, Kelly G, Ahmed A, et al: A
comparison of surgical techniques used in dynamic reanimation of
the paralyzed face. 0tol Neurotol. 26:284–291. 2005. View Article : Google Scholar
|
|
2
|
Guntinas-Lichius O, Streppel M and
Stennert E: Postoperative functional evaluation of different
reanimation techniques for facial nerve repair. Am J Surg.
191:61–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yetiser S and Karapinar U:
Hypoglossal-facial nerve anastomosis:a meta-analytic study [J].
Annals of Otology Rhinology & Laryngolog. 116:542–549. 2007.
View Article : Google Scholar
|
|
4
|
Lloyd BM, Luginbuhl RD and Brenner MJ: Use
of motor nerve material in peripheral nerve repair with conduits.
Microsurgery. 27:138–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
lgnatiadis IA, Yiannakopoulos CK and
Barbitsioti AD: Diverse types of epineural conduits for bridging
short nerve defects = An experimental study in the rabbit.
Microsurgery. 27:98–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Serpe CJ, Byram SC, Sanders VM and Jones
KJ: Brain-derived neurotrophic factor supports facial motoneuron
survival after facial nerve transection in immunodeficient mice.
Brain, Behavior, and Immunity. 19:946–949. 2005. View Article : Google Scholar
|
|
7
|
Indrawattana N, Chen G, Tadokoro M, Shann
LH, Ohgushi H, Tateishi T, Tanaka J and Bunyaratvej A: Growth
factor combination for chondrogenic induction from human
mesenchymal stem cell. Biochem Biophys Res Commun. 320:914–919.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saber SE: Tissue engineering in
endodontics. J Oral Sci. 51:495–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen SY, Li YX, Ma Xin, Chen JS, Zhou GY
and Gong ZX: Experimental study on the effect of extrinsic
insulin-like growth factor-2 on the healing of skeletal muscle
contusion. Zhong Guo Yun Dong Yi Xue Za Zhi. 3:23–26. 2002.
|
|
10
|
Markou N, Pepelassi E and Kotsovilis S:
The use of platelet-rich plasma combined with demineralized
freeze-dried bone al ograft in the treatment of periodontal
endosseous defects:a report of two clinical cases. Journal of the
American Dental Association. 141:967–978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baardsnes J, Hinck CS, Hinck AP and
O'Connor-McCourt MD: TbetaR-II discriminates the high-and
low-affinity TGF-beta isoforms via two hydrogen-bonded ion pairs.
Biochemistry. 48:2146–2155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boillée S, Cadusseau J, Coulpier M,
Grannec G and Junier P: Transforming growth factor alpha: A
promoter of motoneuron survival of potential biological relevance.
J Neurosci. 21:7079–7088. 2001.PubMed/NCBI
|
|
13
|
Caron PL, Fréchette-Frigon G, Shooner C,
Leblanc V and Asselin E: Transforming growth factor beta isoforms
regulation of Akt activity and XIAP levels in rat endometrium
during estrous cycle, in a model of pseudopregnancy and in cultured
decidual cells. Reprod Biol Endxocrinol. 7:802009. View Article : Google Scholar
|
|
14
|
Memon MA, Anway MD, Covert TR, Uzumcu M
and Skinner MK: Transforming growth factor beta (TGFbeta1, TGFbeta2
and TGFbeta3) null-mutant phenotypes in embryonic gonadal
development. Mol Cell Endocrinol. 294:70–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin EJ, Lee SY, Jung JC, Bang OS and Kang
SS: TGF-beta3 inhibits chondrogenesis of cultured chick leg bud
mesenchymal cells via downregulation of connexin 43 and integrin
beta4. J Cell Physiol. 214:345–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasaki R, Aoki S, Yamato M, Uchiyama H,
Wada K, Okano T and Ogiuchi H: Neurosphere generation from dental
pulp of adult rat incisor. Eur J Neurosci. 27:538–548. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shan ZQ, Wang XB, Zhao L and Yu GS:
Anatomy and application of extracranial section of rat facial
nerve. Zhong Guo Jie Pou Xue Za Zhi. 28:82–83. 2005.(In
Chinese).
|
|
18
|
Gallo D, Zannoni GF, Apollonio P,
Martinelli E, Ferlini C, Passetti G, Riva A, Morazzoni P,
Bombardelli E and Scambia G: Characterization of the pharmacologic
profile of a standardized soy extract in the ovariectomized rat
model of menopause: Effects on bone, uterus, and lipid profile.
Menopause. 12:589–600. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao JM, Xu DC and Zhong SZ: Research
progress on repair donors of the facial nerve defects. Lin Chuang
Jie Pou Xue Za Zhi. 22:222–223. 2004.(In Chinese).
|
|
20
|
Koshima I, Nanba Y, Tsutsui T, Takahashi Y
and Itoh S: New one-stage nerve pedicle grafting technique using
the great auricular nerve for reconstruction of facial nerve
defects. J Reconstr Microsurg. 20:357–361. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin H and Hou LJ: Study on traumatic
facial nerve injury: Recent progress. Di Er Jun Yi Da Xue Bao.
29:1248–1250. 2008.(In Chinese).
|
|
22
|
Wang WH, Xu B, Zhu J and Xia B:
Expressions of BCL-2 and P53 in neurons following facial nerve
injury. Xian Dai Kou Qiang Yi Xue Za Zhi. 23:1552009.(In
Chinese).
|
|
23
|
Donzelli R, Maiuri F, Peca C, Cavallo LM,
Motta G and de Divitiis E: Microsurgical repair of the facial
nerve. Zentralbl Neurochir. 66:63–69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malik TH, Kelly G, Ahmed A, Saeed SR and
Ramsden RT: A comparison of surgical techniques used in dynamic
reanimation of the paralyzed face. Otol Neurotol. 26:284–291. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guntinas-Lichius O, Streppel M and
Stennert E: Postoperative functional evaluation of different
reanimation techniques for facial nerve repair. Am J Surg.
191:61–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lundborg G and Hansson HA: Nerve
regeneration through preformed pseudosynovial tubes. A preliminary
report of a new experimental model for studying the regeneration
and reorganization capacity of peripheral nerve tissue. J Hand Surg
Am. 5:35–38. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang XW, Tao ZZ, Cui YH and Zhang P: The
study on facial nerve regeneration guided by chitin guidance
channel. Lin Chuang Kou Qiang Yi Xua Za Zhi. 19:26–27. 2003.(In
Chinese).
|
|
28
|
Zhou CY and Luo WL: Preliminary
experimental study on the facial nerve regeneration in silicone
chamber: the influence of hepatocyte growth factor. Chong Qing Yi
Xue Za Zhi. 32:299–300. 2003.(In Chinese).
|
|
29
|
Luo WL and Zhang H: Study on role of
thyroid hormone in repair of rabbit facial nerve injury. Chong Qing
Yi Xue Za Zhi. 36:1121–1123. 2007.(In Chinese).
|
|
30
|
Guo BF, Dong MM and Ren XH: Effect of
neural stem cell transplantation on rabbit facial nerve defect
repair. Zhong Guo Er Bi Yan Hou Tou Jing Wai Ke. 9:6–8. 2005.(In
Chinese).
|