Introduction
With a rising incidence in recent years,
hepatocellular carcinoma (HCC) is now the fifth most common
malignancy worldwide (1). More
importantly, HCC has a poor prognosis due to its high rate of
recurrence and a lack of effective therapies (2). Hyperthermia is used as a cancer
treatment strategy and has few side-effects. It is a therapeutic
procedure that raises the body or local temperature >37°C and
the usual temperature ranges from 40–43°C (3). To date, improvements in thermometry,
heat-application technologies and control systems have made
hyperthermia a safer and more efficient treatment strategy
(4). Experimental and clinical
studies on the application of hyperthermia support its therapeutic
potential for the treatment of HCC (5).
Local, regional and whole-body hyperthermia
treatments have been used in the clinic and yield variable results
(6). A variety of techniques such as
radiotherapy, microwaves and lasers have gained popularity for the
treatment of HCC and have benefited patients who exhibited improved
local control and survival (7).
However, local recurrence remains an issue following hyperthermia
treatment, with local recurrence rates of 1.8–34% depending on the
location and size of the tumor (8).
Phenotypic changes in cells following hyperthermia treatment may
indicate a resistance to cancer thermotherapy and result in the
treatment failure or a high recurrence rate, a process known as
thermotolerance (9). Therefore, more
efficient strategies to sensitize tumor cells to heat are
required.
Heat shock proteins (HSPs) are well-known mediators
in response to thermal stress. Although HSPs are the predominantly
activated genes following heat shock, it has become apparent that
thermal stress also leads to the induction of a substantial number
of genes not traditionally considered to be HSPs (10,11).
Another major cellular response to thermal stress is the activation
of the phosphoinositide 3-kinase (PI3K)/protein kinase B
(Akt)/mammalian target of rapamycin (mTOR) signaling pathway
(12). As it serves as a central
point in numerous cellular signaling cascades, mTOR has an
important role in oncogenesis, DNA repair, tumor growth,
angiogenesis and migration (13).
Tumor relapse following thermotherapy is caused by thermal
tolerance in the residual tumor cells, which decreases the
therapeutic effect of heat. However, the activity of mTOR is
induced following exposure to hyperthermia (14). Therefore, it was hypothesized for the
purposes of the present study that mTOR may be a modulator for
thermosensitivity. The present study examined whether inhibition of
mTOR affected cellular responses to hyperthermia in SMMC-7721 human
HCC cells.
Materials and methods
Cell culture and hyperthermia
treatment
The SMMC-7721 human HCC cell line was obtained from
the Shanghai Institute of Cell Biology at the Chinese Academy of
Science (Shanghai, China). The present study was approved by the
Ethics committee of the Third Affiliated Hospital of Sun Yat-Sen
University (Guangzhou, China). The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Gibco; Thermo Scientific
Inc., Waltham, MA, USA). Cell cultures were incubated at 37°C in a
humidified atmosphere containing 5% CO2. When the cells
covered 70–80% of the bottle bottom, they were digested using 0.25%
trypsin (Gibco). The cells in the logarithmic phase of growth were
used for the following experiments. For hyperthermia treatment, the
cells were subjected to heat shock in an incubator (Forma Series II
3110; Thermo Fisher Scientific, Inc.) preheated to 40, 42 or 44°C
for 1 h. A control group was maintained at 37°C. The temperature
was monitored and maintained within 0.1°C during the treatment
period. Following heat shock treatment, the cells were then
re-incubated at 37°C for the indicated time periods.
Detection of cell viability
Cells were seeded at an initial density of 1×103
cells/well in 96-well plates. Following 24 h culture at 37°C in a
humidified atmosphere containing 5% CO2, the cells were
exposed to hyperthermic conditions (40, 42 or 44°C) separately for
1 h and recovered to 37°C. After a further 24 h, cell morphology
was observed using an inverted microscope (TE2000-U; Nikon
Corporation, Tokyo, Japan) and cell viability was measured using a
Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) according to the manufacturer's protocol. For
cells exposed to 42°C, cell viability was additionally detected at
12, 24, 48, 72 and 96 h following hyperthermia. The optical
absorbance at a wave length of 450 nm was measured using a
microplate reader (Model 680; Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The percentage of growth inhibition was calculated using
the following formula: 1 - (Absorbance hyperthermia/absorbance at
37°C) × 100%. The growth curves were constructed using average
absorbance at 450 nm (optical density [OD], 450 nm) from three
independent experiments.
Plasmid and transient
transfection
The antisense plasmid pEGFP-C1-mTOR was donated by
Dr Yu Guo (Liver Transplant Center, The Third Affiliated Hospital
of Sun Yat-Sen University). Transient transfection was conducted
using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
SMMC-7721 cells were seeded in 24-well plates (5×104
cells/well) one day prior to transfection until they reached 80%
confluence. A total of 1 µg plasmid DNA and 3 µl transfection
reagent were used to transfect the cells in each well in the
absence of serum. After 4 h, the medium was replaced with DMEM
supplemented with 10% FBS. The expression of green fluorescent
protein was observed using the TE2000-U inverted fluorescence
microscope.
Detection of mTOR mRNA expression
using reverse transcription-polymerase chain reaction (RT-PCR)
SMMC-7721 cells were seeded in 6-well plates (1×105
cells/well), and cells were collected 36 h post-transfection. Total
RNA was isolated using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RNA was
reverse transcribed and amplified using a one-step RT-PCR kit
(Beijing TransGen Biotech Co., Ltd., Beijing, China). Genomic DNA
was removed using phenol/chloroform extraction. Next, 1 µg isolated
RNA was included in a 20-µl reaction mixture together with 0.5 µl
Forward Primer, 0.5 µl Reverse Primer, 12 µl 2X One-Step Reaction
Mix and 0.5 µl EasyScript One-Step Enzyme Mix. RNA was reverse
transcribed and amplified using an ABI 2700 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc., Foster City,
CA, USA). The reaction was performed under the following
conditions: 94°C for 12 min, and then 30 cycles 94°C for 30 sec,
54°C for 30 sec and 72°C for 30 sec followed by a final extension
at 72°C for 5 min. The mRNA expression levels of mTOR were measured
using the following gene-specific primers: mTOR forward,
5′-CGCTGTCATCCCTTTATCG-3′ and reverse, 5′-ATGCTCAAACACCTCCACC-3′.
The relative mRNA expression levels of mTOR were normalized against
those of β-actin using the following gene-specific primers: β-actin
forward, 5′-GGACTTCGAGCAAGAGATGG-3′, and reverse,
5′-AGCACTGTGTTGGCGTACAG-3′. The primers were synthesized by
Shanghai Sangon Biological Engineering Technology and Services Co.,
Ltd. (Shanghai, China). The amplified PCR products were subjected
to electrophoresis in 1% agarose gel (Sangon Biotech Co. Ltd.,
Shanghai, China), then stained with ethidium bromide
(Sigma-Aldrich, St. Louis, MO, USA). All experiments were repeated
at least three times. The photo-density ratio of the RT-PCR product
for mTOR and β-actin was used to identify the expression levels of
the target gene. The optical density of each sample band was
captured using a UV gel imaging system (GDS-8000; Ultra-Violet
Products Ltd., Cambridge, UK) and analyzed using Quantity One Image
software (Bio-Rad Laboratories, Inc.). The density ratio of the
RT-PCR product for mTOR and β-actin was used to identify the
expression levels of the target gene.
Western blot analysis
Post-transfection, cells were harvested, washed
twice in ice-cold phosphate-buffered saline (PBS; Wuhan Boster
Biological Technology Ltd., Wuhan, China) and then lysed on ice for
30 min in RIPA lysis buffer containing 1 mM phenylmethylsulfonyl
fluoride (Beyotime Institute of Biotechnology, Shanghai, China).
After centrifugation at 10,000 × g for 5 min, the supernatant was
harvested to obtain the total cellular protein extract. The protein
concentrations were assessed using a bicinchoninic acid protein
assay kit (Beijing Comwin Biotech Co., Ltd., Beijing, China). After
the protein concentrations were assessed, 50 µg total protein was
separated using 10% SDS-PAGE (Beyotime Institute of Biotechnology)
and transferred onto nitrocellulose membranes (Beijing Comwin
Biotech Co., Ltd.). Following blocking in 5% non-fat dry milk in 25
mM Tris-buffered saline (150 mM NaCl) with 0.1% Tween 20 buffer
(Shanghai Sangon Biological Engineering Technology and Services
Co., Ltd., Shanghai, China), the membranes were incubated with
rabbit anti-human mTOR primary antibody (1:400; sc-8319) overnight
at 4°C, then with secondary horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G (1:5,000; sc-2004; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at room temperature for 1 h.
The immunoreactive bands were detected using an enhanced
chemiluminescence kit (PI-32209; Pierce Biotechnology, Inc.,
Rockford, IL, USA). β-actin was used as an internal control. The
protein concentrations were assessed using a bicinchoninic acid
protein assay kit (Beijing Comwin Biotech Co., Ltd.). BCA solution
(200 µl) was added to each well and allowed to stand at 37°C for 30
min. The OD value of each well was read at a wavelength of 562 nm
using a microplate reader (Model 680, Bio-Rad Laboratories Inc.). A
standard curve was drawn and the protein concentration was
calculated.
Cell proliferation
The CCK-8 kit was used to monitor cell
proliferation. Briefly, SMMC-7721 cells were plated at a density of
1×104 cells/well in 96-well plates. Three groups were established,
including an experimental group (transfection + hyperthermia), a
control group (hyperthermia) and a blank control group. In the
experimental group, the cells were subjected to heat shock in an
incubator at 42°C for 1 h post-transfection. Cells were cultured at
42°C for 1 h without transfection in the hyperthermia group. The
blank control group cells were incubated at 37°C without any
treatment. The absorbance of the cells was determined at a
wavelength of 450 nm according to the manufacturer's protocol.
Experiments were conducted in triplicate and the average results
were calculated.
Wound-healing assay
SMMC-7721 cells were seeded at an initial density of
5×104 cells/well on 24-well plates and cultured to 80% confluence.
Following treatment, the cells were wounded by manual scraping with
a sterile 10-µl pipette tip. The culture medium was then replaced
with fresh, FBS-free DMEM. Wound closure was monitored at various
time points by observation under an inverted microscope, and images
were captured at regular time intervals (TE2000-U; Nikon
Corporation). Wound distances were measured at each time point and
expressed as the average percentage of wound closure compared with
that at 0 h.
Colony forming assay
SMMC-7721 cells were divided into three groups as
described above. Cells were collected following hyperthermia
treatment and reseeded in 6-well plates (1×103 cells/well), each
group contained three wells. Following incubation at 37°C for 14
days, the cells were washed twice with PBS, fixed with 4%
paraformaldehyde for 15 min and stained with 2 ml Giemsa reagent
for 30 min (Hematology Laboratory, the Third Affiliated Hospital of
Sun Yat-Sen University). The number of colonies containing 50 cells
was counted manually under low magnification. Clone formation
efficiency was calculated using the following formula: Clone
formation efficiency = (number of colonies/number of cells
inoculated) × 100%.
Flow cytometry
SMMC-7721 cells at an initial density of 5×104
cells/well were incubated in 24-well plates and treated as
mentioned previously. Cells with different treatments were
collected separately and resuspended with PBS. Subsequently, cells
(5×105) were centrifuged at 500 × g for 5 min and the
supernatant solutions were discarded. An Annexin V-PE/7-AAD
Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ, USA)
was used according to the manufacturer's protocol. After adding 50
µl binding buffer and 5 µl 7-AAD, cells were incubated at room
temperature for 15 min in the dark. After that, cells were
resuspended with 450 µl binding buffer and 1 µl Annexin V-PE was
added. The fluorescence of Annexin V-PE and 7-AAD was measured by
flow cytometry (FACSCalibur; BD Biosciences). For cell cycle
analysis, cells were collected, washed twice with PBS and incubated
with ice-cold 70% ethanol overnight. Subsequently, the fixed cells
were resuspended in PBS and stained with 50 µg/ml propidium iodide
(Sigma-Aldrich) at room temperature in the dark for 30 min. Flow
cytometry analysis was performed using the FACSCalibur
cytometer.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). All results were presented
as means ± standard deviation. One-way analysis of variance was
used to analyze the statistical differences between multiple
groups. P<0.05 was considered to indicate a statistically
significant result.
Results
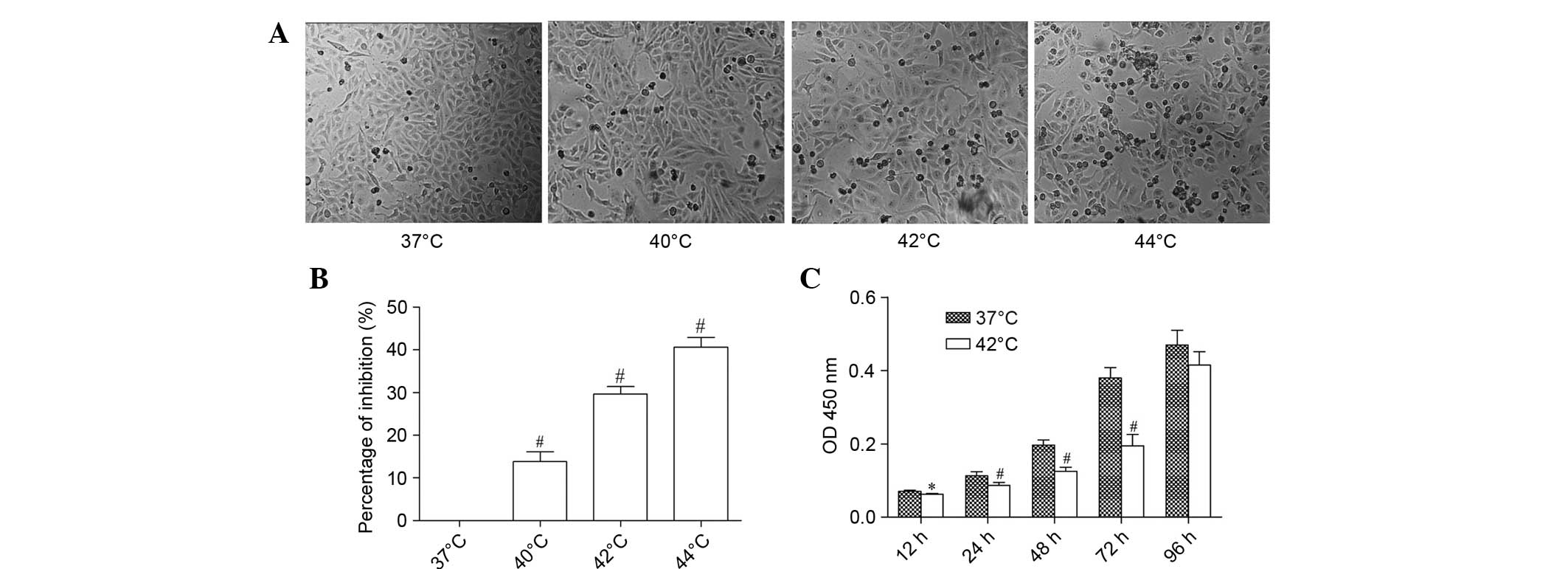
Hyperthermia inhibits SMMC-7721 cell
viability
It has been reported that cancer cells are
susceptible to hyperthermia (15).
To detect the effects of hyperthermia on SMMC-7721 cells, the cells
were exposed to heat treatment at various temperatures. Following
hyperthermia, folding of the cell membrane was observed and
numerous vacuoles formed in the cytoplasm. As the temperature
increased, the number of necrotic cells also increased (Fig. 1A). Hyperthermia significantly
inhibited the growth of SMMC-7721 cells compared with cells at 37°C
(P<0.01). The inhibitory rate of cells at 44°C was increased by
40.65%. Hyperthermia reduced cell proliferation in a
temperature-dependent manner (Fig.
1B). SMMC-7721 cell viability was significantly decreased at
42°C compared with cells at 37°C after 96 h treatment (Fig. 1C).
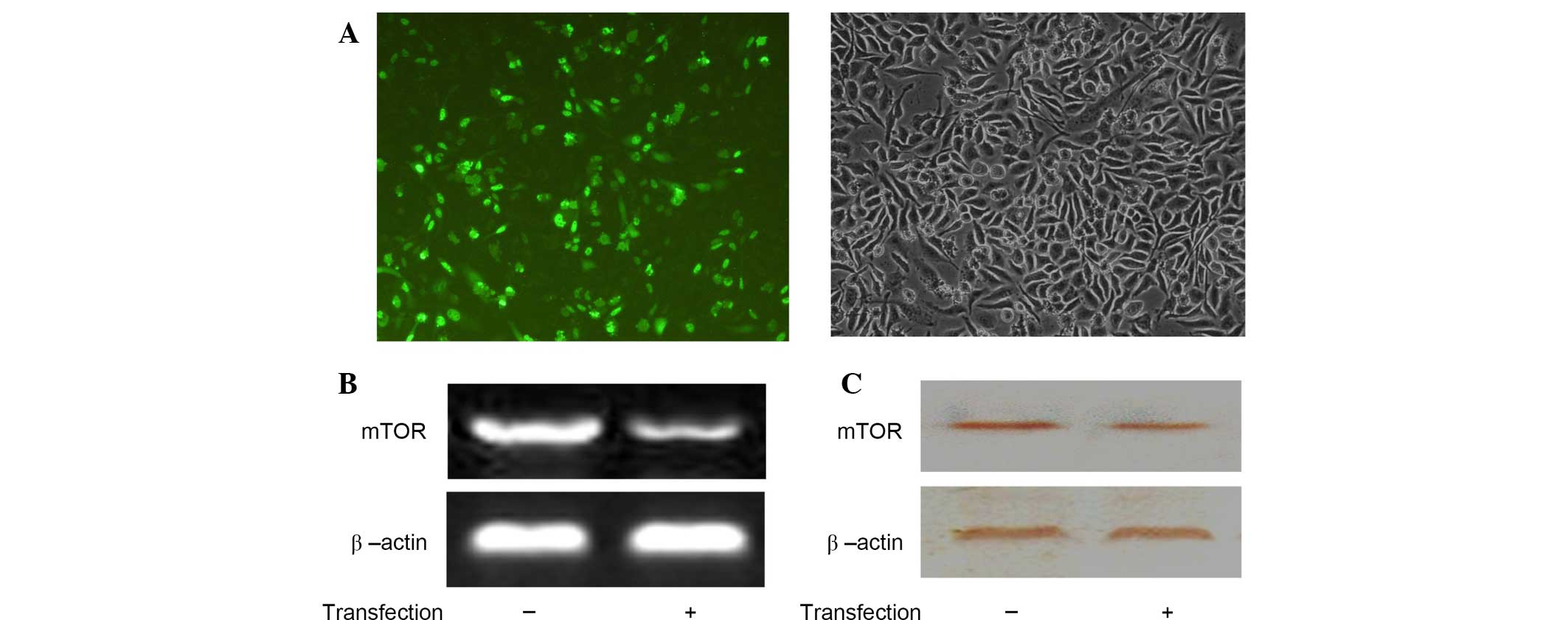
Expression of mTOR
Following antisense plasmid pEGFP-C1-mTOR
transfection into the SMMC-7721 cells, the expression of green
fluorescent protein was observed using inverted fluorescent
microscopy (Fig. 2A). The results
from the RT-PCR analysis demonstrated that the expression of mTOR
mRNA was significantly reduced post-transfection (P<0.01). The
relative values of the two PCR assays were 0.74±0.04 and 0.23±0.01
(Fig. 2B). The protein expression
levels of mTOR were detected using western blotting and
demonstrated similar results. The relative values of mTOR protein
expression were 0.65±0.03 and 0.31±0.02, respectively. These
results indicated that mTOR protein expression was also
significantly decreased post-transfection (P<0.05; Fig. 2C).
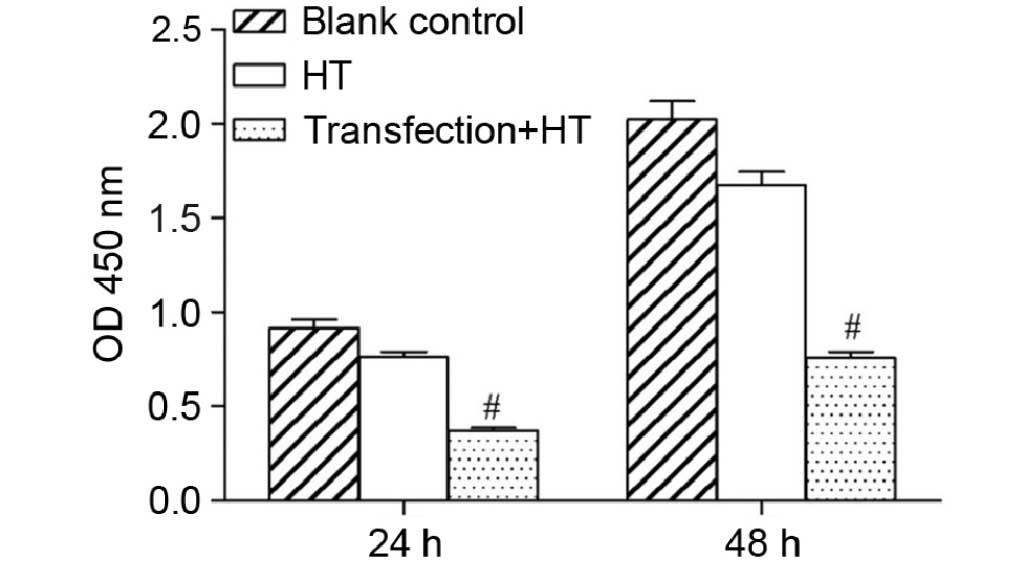
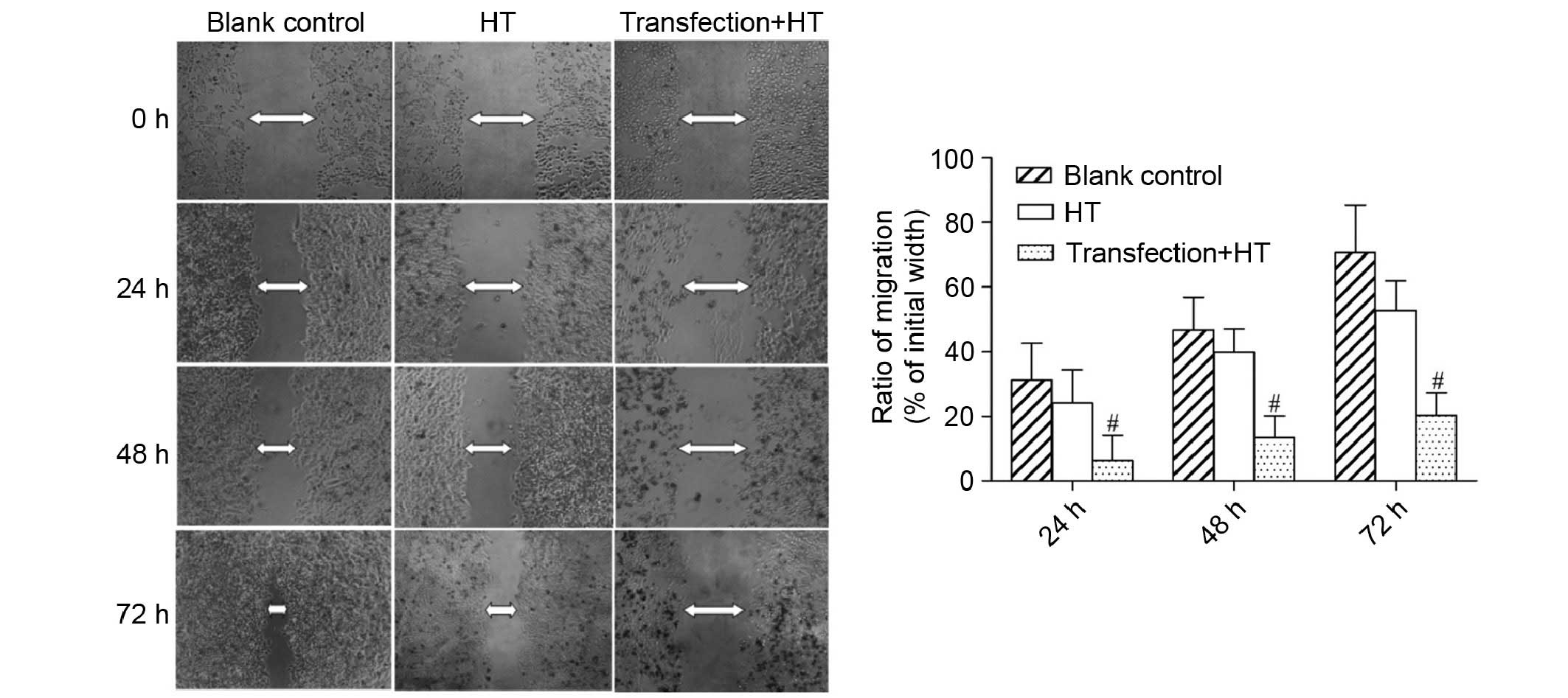
Effects of hyperthermia on cell
proliferation, colony-forming ability and motility
post-transfection
CCK-8 analysis demonstrated a significant reduction
in cell viability of the experimental group at 24 and 48 h compared
with the other groups (Fig. 3). The
wound-healing assay demonstrated that numerous cells migrated into
the scratch wound area in the control and blank control groups,
whereas there were fewer cells in the experimental group (Fig. 4). Furthermore, a larger number of
necrotic cells was also observed in the experimental group. These
results indicated that cell migration was reduced following
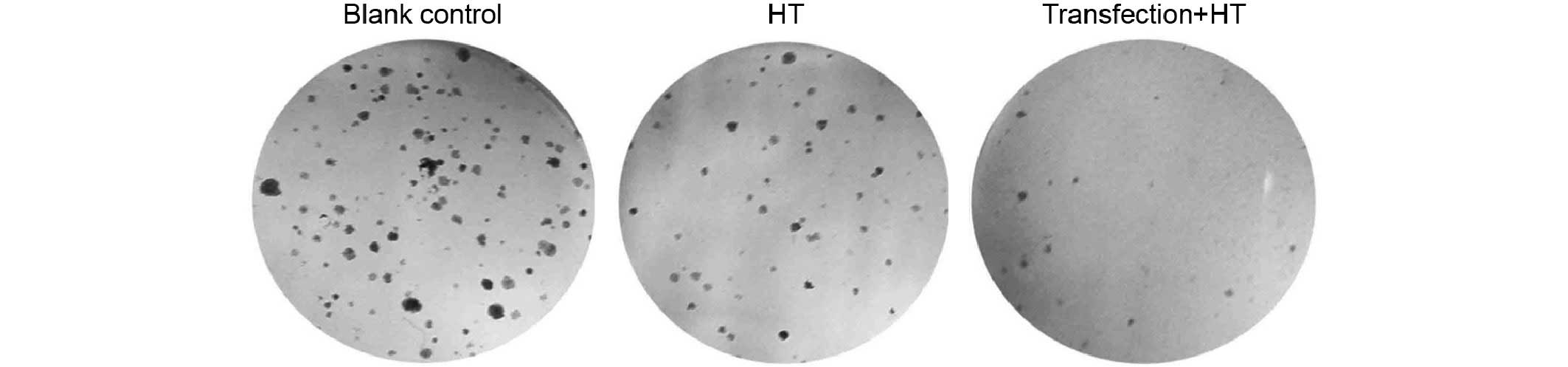
treatment. The colony forming assay determined that the clone
formation efficiencies for the control and blank control groups
were 9.05±1.97 and 14.5±2.45%, respectively. The number of cell
colonies was markedly decreased in the experimental group, and the
clone formation efficiency was 1.98±0.61% (Fig. 5).
Effects of hyperthermia on apoptosis
and cell cycle post-transfection
The present study examined whether the change in
cell proliferation was associated with the apoptosis induced by
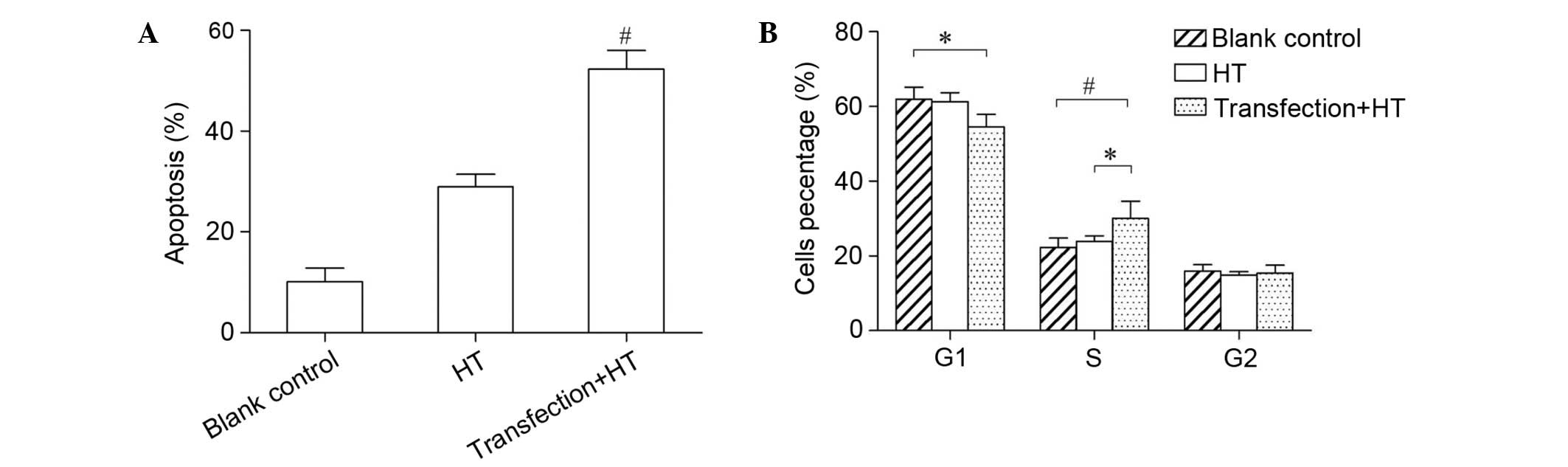
heat. As shown in Fig. 6A, the rate
of apoptosis for the control and blank control groups was
10.14±2.66 and 28.93±2.51%, respectively. The apoptotic rate in the
experimental group was significantly lower compared with that in
the other two groups (52.27±3.72%; P<0.01). This result suggests
that mTOR expression protects SMMC-7721 cells from heat-induced
apoptosis, and thus increases cell survival following exposure to
heat. To determine whether the cell cycle was among the mechanisms
underlying the change in cell proliferation, flow cytometric
analysis was performed. Compared with the other two groups, the
number of cells in the G1 phase was decreased in the
experimental group. However the proportion of cells in S phase was
increased (Fig. 6B). These results
suggest that the transfected cells were arrested in S phase
following hyperthermia.
Discussion
Hyperthermia has been demonstrated in randomized
clinical trials to be an effective treatment strategy in oncology
(16). Successful thermotherapies
rely on the balance between eliminating cancerous cells and
protecting normal cells exposed to high temperatures (17). Hyperthermia leads to cell death
depending on the temperature and duration of exposure (3). Tumor heterogeneity may contribute to
differences in therapy response, due to the fact that cell lines
exhibit different thermosensitivities (18).
The sensitivity of SMMC-7721 cells to hyperthermia
was investigated in the present study. Following hyperthermia at
various temperatures, the results demonstrated that SMMC-7721 cells
were sensitive to heat treatment. Heat shock destroyed the
membranes of the cells and changed the morphology of the cells.
Cell proliferation was significantly inhibited following heat
treatment. The degree of cell death either by apoptosis or necrosis
depends on the tumor cell type and the treatment conditions, with
the usual break-point temperature at which cells underwent
significant cell death being 42°C for 1 h (3). Therefore, 42°C was selected as the
temperature to be used in the subsequent experiments, and heat
induced a significant growth arrest in the cells within 72 h. The
possible mechanisms underlying cell growth arrest may include
direct and indirect processes. Direct cytotoxic effects would mean
that heat shock destroyed the membrane structure of the tumor cell
directly and induced cell apoptosis. In the case of an indirect
effect, heat shock would destroy the tumor cell through the
induction of reactive oxygen species and free radicals (19,20).
The detailed mechanisms underlying heat induction of
cell death remain to be elucidated, although several signaling
pathways have been demonstrated to be mediators of this response
including the PI3K/Akt/mTOR, mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated kinase and p38/MAPK signaling
pathways (21). The PI3K/Akt/mTOR
pathway is a survival signaling pathway that is constitutively
activated in numerous types of cancers (22). mTOR has recently been recognized as
potential therapeutic target for cancer therapy (13). The potential applications of mTOR
inhibitors for treating various types of cancer, including renal,
prostate, breast, pancreatic and lung cancers have been studied
preclinically and clinically (23–25).
Therapy resistance is a common clinical problem in
HCC. It has been demonstrated that the mTOR signaling pathway is
aberrantly activated in HCC (26).
Furthermore, activated mTOR confers resistance to numerous types of
cancer therapies, and contributes to the observed poor prognosis in
HCC (27). The results of the
present study were concordant with those of previous studies that
reported that inhibition of mTOR may serves as an HCC treatment by
enhancing the chemosensitivity of HCC cells (28,29). In
addition, inhibition of the mTOR signaling pathway enhanced heat
sensitivity in human breast and lung cancers; however, few studies
have evaluated heat sensitivity in HCC (30,31).
Following these observations, we aimed to determine whether
thermosensitization could be achieved by inhibiting mTOR. The
presence of green fluorescent protein in the cells indicated that
the antisense pEGFP-C1-mTOR was successfully transfected into the
SMMC-7721 cells. The expression levels of mTOR were decreased
post-transfection, which made it possible to study the response to
thermal treatment in SMMC-7721 cells following knockdown of mTOR
expression.
To understand the role of mTOR in modulating
cellular responses to thermal stress, transfected SMMC-7721 cells
were subjected to heat treatment. The potential antitumoral effect
of heat treatment was also investigated in SMMC-7721 cells. A CCK-8
assay was used to assess cell viability and a colony-forming assay
to assess cell proliferation. These assays demonstrated that
hyperthermia inhibits the proliferation of SMMC-7721 cells in
vitro. In our previous study, it was observed that treatment of
cells with antisense pEGFP-C1-mTOR could inhibit the growth of HCC
(28). In the present study, the
results demonstrated that the inhibitory effects of hyperthermia on
the transfected cells was even more significant. The effects of
hyperthermia on cell migration were also examined. A previous study
showed that mTOR has a critical role in the regulation of tumor
cell invasion and cancer metastasis (32). The wound healing assay demonstrated
that hyperthermia decreased the ability of SMMC-7721 cells to
migrate and repair wounds. In addition, the effect was more
apparent when mTOR expression was decreased. These results
demonstrated that inhibition of mTOR expression increased the
sensitivity of SMMC-7721 cells to hyperthermia.
The results of the Annexin V-PE/7-AAD staining and
flow cytometric analyses indicated that the inhibition of mTOR
significantly increased the rate of heat-induced apoptosis compared
with the controls. This was consistent with the data obtained on
the anti-proliferative effects of heat in SMMC-7721 cells and
suggested that inhibition of mTOR expression enhanced heat-induced
apoptosis.
Finally, the cell cycle was analyzed, and the data
indicated that there was no significant difference in the
proportion of cells in G2 phase. However, the proportion
of cells in G1 phase was reduced, whereas the proportion
of cells in S phase was increased. Therefore, the
S-to-G2 phase transition was blocked and the cells were
arrested in S phase. Hyperthermia usually blocks cells in
G1 phase, and few have reported cells blocked in other
phases (33). Since DNA synthesis
occurs in S phase and the basic principle of hyperthermia is to
change the microenvironment of the cells, the altered
microenvironment causes chromosome aberrations and multinucleated
cells to form, which may induce apoptosis and necrosis.
Furthermore, cells in S phase are sensitive to heat. The mTOR gene
is involved in regulating cyclins D1/A and
cyclin-dependent kinases (34). It
has been reported that mTOR inhibition induced G1 phase
cell cycle arrest (35). Therefore,
the interaction between mTOR inhibition and hyperthermia in the
cell cycle requires further investigation.
In summary, the present study demonstrated that
SMMC-7721 cells were sensitive to heat treatment, and cell
viability was significantly inhibited following hyperthermia.
Inhibition of mTOR expression increased the thermosensitivity of
SMMC-7721 cells by increasing cell apoptosis and S phase cell cycle
arrest. Therefore, mTOR may be involved in mediating heat-induced
apoptosis and survival in SMMC-7721 cells. Further understanding of
this important signaling pathway may facilitate the development of
novel therapeutic strategies to improve the outcomes for patients
with HCC. However, the molecular mechanisms underlying mTOR
regulation of SMMC-7721 cell response to hyperthermia requires
further investigation.
Acknowledgements
The present study was supported by a grant from the
Science and Technology Program of Guangzhou City (grant no.
1563000226).
References
|
1
|
Waly RS, Yangde Z and Yuxiang C:
Hepatocellular carcinoma: focus on different aspects of management.
ISRN Oncol. 2012:4216732012.PubMed/NCBI
|
|
2
|
Yang JD and Roberts LR: Epidemiology and
management of hepatocellular carcinoma. Infect Dis Clin North Am.
24:899–919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roti Roti JL: Cellular responses to
hyperthermia (40-46 degrees C): Cell killing and molecular events.
Int J Hyperthermia. 24:3–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Habash RW, Bansal R, Krewski D and Alhafid
HT: Thermal therapy, part. 2 hyperthermia techniques. Crit Rev
Biomed Eng. 34:491–542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mayrhauser U, Stiegler P, Stadlbauer V,
Koestenbauer S, Leber B, Konrad K, Iberer F, Portugaller RH and
Tscheliessnigg K: Effect of hyperthermia on liver cell lines:
Important findings for thermal therapy in hepatocellular carcinoma.
Anticancer Res. 31:1583–1588. 2011.PubMed/NCBI
|
|
6
|
Ahmed K and Zaidi SF: Treating cancer with
heat: Hyperthermia as promising strategy to enhance apoptosis. J
Pak Med Assoc. 63:504–508. 2013.PubMed/NCBI
|
|
7
|
Jansen MC, van Hillegersberg R, Chamuleau
RA, van Delden OM, Gouma DJ and van Gulik TM: Outcome of regional
and local ablative therapies for hepatocellular carcinoma: A
collective review. Eur J Surg Oncol. 31:331–347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Decadt B and Siriwardena AK:
Radiofrequency ablation of liver tumours: systematic review. Lancet
Oncol. 5:550–560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Griffin RJ, Dings RP, Jamshidi-Parsian A
and Song CW: Mild temperature hyperthermia and radiation therapy:
Role of tumour vascular thermotolerance and relevant physiological
factors. Int J Hyperthermia. 26:256–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sonna LA, Fujita J, Gaffin SL and Lilly
CM: Invited review: Effects of heat and cold stress on mammalian
gene expression. J Appl Physiol (1985). 92:1725–1742. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng H, Ravikumar TS and Yang WL: Bone
morphogenetic protein-4 inhibits heat-induced apoptosis by
modulating MAPK pathways in human colon cancer HCT116 cells. Cancer
Lett. 256:207–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma N, Szmitko P, Brade A, Chu I, Lo A,
Woodgett J, Klamut H and Liu FF: Kinase-dead PKB gene therapy
combined with hyperthermia for human breast cancer. Cancer Gene
Ther. 11:52–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buitrago-Molina LE and Vogel A: mTor as a
potential target for the prevention and treatment of hepatocellular
carcinoma. Curr Cancer Drug Targets. 12:1045–1061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shaw M, Cohen P and Alessi DR: The
activation of protein kinase B by H2O2 or heat shock is mediated by
phosphoinositide 3-kinase and not by mitogen-activated protein
kinase-activated protein kinase-2. Biochem J. 336(Pt 1): 241–246.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soares PI, Ferreira IM, Igreja RA, Novo CM
and Borges JP: Application of hyperthermia for cancer treatment:
recent patents review. Recent Pat Anticancer Drug Discov. 7:64–73.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kouloulias V, Plataniotis G, Kouvaris J,
Dardoufas C, Gennatas C, Uzunoglu N, Papavasiliou C and Vlahos L:
Chemoradiotherapy combined with intracavitary hyperthermia for anal
cancer: Feasibility and long-term results from a phase II
randomized trial. Am J Clin Oncol. 28:91–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Palazzi M, Maluta S, Dall'Oglio S and
Romano M: The role of hyperthermia in the battle against cancer.
Tumori. 96:902–910. 2010.PubMed/NCBI
|
|
18
|
Armour EP, McEachern D, Wang Z, Corry PM
and Martinez A: Sensitivity of human cells to mild hyperthermia.
Cancer Res. 53:2740–2744. 1993.PubMed/NCBI
|
|
19
|
Hildebrandt B, Wust P, Ahlers O, Dieing A,
Sreenivasa G, Kerner T, Felix R and Riess H: The cellular and
molecular basis of hyperthermia. Crit Rev Oncol Hematol. 43:33–56.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wust P, Hildebrandt B, Sreenivasa G, Rau
B, Gellermann J, Riess H, Felix R and Schlag PM: Hyperthermia in
combined treatment of cancer. Lancet Oncol. 3:487–497. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gourgou E, Aggeli IK, Beis I and Gaitanaki
C: Hyperthermia-induced Hsp70 and MT20 transcriptional upregulation
are mediated by p38-MAPK and JNKs in Mytilus galloprovincialis
(Lamarck); a pro-survival response. J Exp Biol. 213:347–357. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Georgakis GV and Younes A: From Rapa Nui
to rapamycin: Targeting PI3K/Akt/mTOR for cancer therapy. Expert
Rev Anticancer Ther. 6:131–140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dudkin L, Dilling MB, Cheshire PJ, Harwood
FC, Hollingshead M, Arbuck SG, Travis R, Sausville EA and Houghton
PJ: Biochemical correlates of mTOR inhibition by the rapamycin
ester CCI-779 and tumor growth inhibition. Clin Cancer Res.
7:1758–1764. 2001.PubMed/NCBI
|
|
24
|
Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL
and Reddy SA: The rapamycin analog CCI-779 is a potent inhibitor of
pancreatic cancer cell proliferation. Biochem Biophys Res Commun.
331:295–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Atkins MB, Hidalgo M, Stadler WM, Logan
TF, Dutcher JP, Hudes GR, Park Y, Liou SH, Marshall B, Boni JP, et
al: Randomized phase II study of multiple dose levels of CCI-779, a
novel mammalian target of rapamycin kinase inhibitor, in patients
with advanced refractory renal cell carcinoma. J Clin Oncol.
22:909–918. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parent R, Kolippakkam D, Booth G and
Beretta L: Mammalian target of rapamycin activation impairs
hepatocytic differentiation and targets genes moderating lipid
homeostasis and hepatocellular growth. Cancer Res. 67:4337–4345.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmitz KJ, Wohlschlaeger J, Lang H,
Sotiropoulos GC, Malago M, Steveling K, Reis H, Cicinnati VR,
Schmid KW and Baba HA: Activation of the ERK and AKT signalling
pathway predicts poor prognosis in hepatocellular carcinoma and ERK
activation in cancer tissue is associated with hepatitis C virus
infection. J Hepatol. 48:83–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo Y, Liang X, Lu M, Weng T, Liu Y and Ye
X: Mammalian target of rapamycin as a novel target in the treatment
of hepatocellular carcinoma. Hepatogastroenterology. 57:913–918.
2010.PubMed/NCBI
|
|
29
|
Hui IC, Tung EK, Sze KM, Ching YP and Ng
IO: Rapamycin and CCI-779 inhibit the mammalian target of rapamycin
signalling in hepatocellular carcinoma. Liver Int. 30:65–75. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma N, Jin J, Lu F, Woodgett J and Liu FF:
The role of protein kinase B (PKB) in modulating heat sensitivity
in a human breast cancer cell line. Int J Radiat Oncol Biol Phys.
50:1041–1050. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohnishi K, Yasumoto J, Takahashi A and
Ohnishi T: LY294002, an inhibitor of PI-3 K, enhances heat
sensitivity independently of p53 status in human lung cancer cells.
Int J Oncol. 29:249–253. 2006.PubMed/NCBI
|
|
32
|
Chin YR and Toker A: Function of Akt/PKB
signaling to cell motility, invasion and the tumor stroma in
cancer. Cell Signal. 21:470–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zölzer F and Streffer C: Quiescence in
S-phase and G1 arrest induced by irradiation and/or hyperthermia in
six human tumour cell lines of different p53 status. Int J Radiat
Biol. 76:717–725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ekim B, Magnuson B, Acosta-Jaquez HA,
Keller JA, Feener EP and Fingar DC: mTOR kinase domain
phosphorylation promotes mTORC1 signaling, cell growth, and cell
cycle progression. Mol Cell Biol. 31:2787–2801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Masuda M, Shimomura M, Kobayashi K, Kojima
S and Nakatsura T: Growth inhibition by NVP-BEZ235, a dual
PI3K/mTOR inhibitor, in hepatocellular carcinoma cell lines. Oncol
Rep. 26:1273–1279. 2011.PubMed/NCBI
|