Introduction
Budd-Chiari syndrome (BCS) is a heterogeneous
disorder that occurs due to an obstruction present between the
hepatic venules and the junction of the inferior vena cava,
resulting in significant mortality and morbidity (1). It is a complex disease with a wide
spectrum of etiologies and presentations (2). BCS remains rare with incidence rates of
0.2–0.8 million people affected per year (3,4)
Numerous studies have suggested that the clinical
features and etiology distribution of BCS vary according to the
geographical area (5–7). In western countries, pure hepatic
venule obstruction accounts for >50% of BCS cases, whereas
thrombosis is the predominant pathological lesion of BCS (1). The majority of cases are closely
associated with underlying inherited or acquired thrombotic risk
factors, with ~80% of BCS patients presenting at least one
thrombotic risk factor (8).
Myeloproliferative neoplasms (MPNs) are considered to be the
leading cause in the development of BCS, and have been reported in
~50% of BCS patients (9). In
developing countries, including Nepal and China, the most common
type of BCS is membranous obstruction of the inferior vena cava
(MOVC) (7,10), which has been shown to account for
62.7% of BCS cases in China (11).
Recent data from several centers have consistently shown that
underlying thrombotic disorders in Chinese BCS patients are rarely
detected (6). In addition, certain
risk factors that have been confirmed in western countries, such as
MPNs, do not seem to be etiological factors in Chinese BCS patients
(12,13). This observation suggests that the
etiological distribution of BCS may differ between western
countries and China. Accordingly, due to the potentially different
pathogenesis factors in BCS patients of different ethnicities
(14), the present study focused on
Chinese patients with BCS.
MicroRNAs (miRNAs or miR) are a class of naturally
small noncoding RNAs with a length of 21–23 nucleotides, which bind
to the 3′-untranslated regions of their target mRNAs and regulate
gene expression at the post-transcriptional level (15). Previous studies have revealed that
miRNAs serve a crucial role in numerous pathological and
physiological processes, including organ development, cell
differentiation, immune function and vascular diseases (16,17). In
addition, a number of miRNAs have been identified as important
modulators of vascular pathologies, including apoptosis,
angiogenesis, inflammation, hypertension and atherosclerosis
(18). Furthermore, the genetic
abnormality or dysregulation of miRNAs may contribute to the
pathogenesis of vascular diseases (19,20);
thus, these findings demonstrate that miRNAs play an important role
in vascular disorders.
Certain studies have demonstrated that the amount of
circulating miRNAs may be used as clinical biomarkers (21,22).
However, to the best of our knowledge, cell-free miRNAs in the
plasma of BCS patients with MOVC have not been previously
investigated. Therefore, the aim of the present study was to
identify a panel of plasma miRNAs that are differentially expressed
in patients with MOVC and to investigate the potential biological
function of these candidate miRNAs.
Materials and methods
Study population
The study was approved by the Ethics Committee of
the Affiliated Hospital of Xuzhou Medical College (Xuzhou, China),
and informed consent was obtained from each subject.
A total of 34 patients aged 27–75 years who were
diagnosed with incident MOVC at the Affiliated Hospital of Xuzhou
Medical College between February 2013 and September 2013 were
enrolled into the present study. Patients were diagnosed based on
the BCS characteristics, as previously described (REFold1). All
diagnoses were confirmed using radiographic imaging, including
Doppler ultrasound and magnetic resonance imaging. In addition,
venography images suggested the existence of a membrane in the
inferior vena cava. Patients with other coexisting diseases,
including hypertension, coronary heart disease, diabetes, blood
diseases and cancer, were excluded. Additionally, 30 healthy age-
and gender-matched subjects were recruited as the controls. The
microarray cohort of subjects included 9 MOVC patients and 5
healthy volunteers (MOVC-1/controls-1). The present study also
investigated a second group composed of 25 MOVC patients and 25
healthy controls (MOVC-2/controls-2) for independent validation
using reverse transcription-quantitative reverse transcription
(RT-qPCR) analysis. No statistically significant differences were
detected in the age and gender distribution of patients and
controls (Table I).
 | Table I.Characteristics of the study
samples. |
Table I.
Characteristics of the study
samples.
|
| miRNA
microarray | RT-qPCR |
|---|
|
|
|
|
|---|
| Characteristic | MOVC (n=9) | Control (n=5) | P-value | MOVC (n=25) | Control (n=25) | P-value |
|---|
| Male gender, n
(﹪) | 3 (33) | 2 (40) | 0.80 | 15 (60) | 15 (60) | 1.00 |
| Age,
yearsa | 42.6
(27.5–60.3) | 42.5
(28.1–1.7) | 0.65 | 48.1
(27.3–74.6) | 48.0
(26.9–74.8) | 0.98 |
Plasma collection and RNA
isolation
A 5-ml sample of venous blood was collected from
each patient or healthy volunteer before breakfast on the morning
after hospital admission. Blood samples were drawn into
EDTA-containing tubes and the plasma was immediately separated by a
two-step centrifugation protocol at room temperature
(centrifugation at 1,800 × g for 10 min, followed by centrifugation
at 15,000 × g for 10 min) (23). The
supernatant was then transferred into RNase-free microcentrifuge
tubes and stored at −80°C until use.
Total plasma RNA was harvested with the RNeasy mini
kit (Qiagen, Hilden, Germany) according to the manufacturer's
instructions. Briefly, 200 µl plasma was diluted with 1 ml QIAzol
lysis reagent. After a 5-min incubation, 5 µl of 5 nmol/l synthetic
Caenorhabditis elegans miR-39 (Shanghai GenePharma Co.,
Ltd., Shanghai, China) was added to each sample as a spike control
(24). Next, phenol extraction and
various filter cartridge steps were performed according to the
manufacturer's instructions. A NanoDrop 2000 spectrophotometer
(NanoDrop Technologies; Thermo Fisher Scientific Inc., Wilmington,
DE, USA) was used to measure the quality and concentration of RNA
in each plasma sample.
miRNAs expression profiling
In order to assess the levels of specific miRNAs in
the patients with MOVC, miRNA expression profiling of the plasma
samples from 9 MOVC-1 patients and 5 healthy control-1 participants
was performed using miRNA microarray analysis. As BCS is a rare
disease, the maximum number of patients with MOVC were selected and
the number of controls were reduced accordingly due to economic
constraints. Microarray analysis and RT-qPCR are two common miRNA
detection methods (25). In order to
detect the miRNA expression levels of the samples, high-throughput
microarray experiments were performed by KangChen Bio-tech Inc.
(Shanghai, China), as this technique is highly sensitive and
time-efficient. However, as the detection results may contain
certain errors, it was necessary to conduct further validation
using RT-qPCR, as this technique is considered the gold standard
for detecting miRNAs (26).
Therefore, independent expanding samples were chosen for RT-qPCR
validation in order to make the results more reliable, as through
microarray analysis and subsequent RT-qPCR validation the
dysregulated miRNAs in patients with MOVC can be reflected with
increased accuracy.
Briefly, 3 µg RNA samples were labeled with the
Exiqon Hy3/Hy5 power labeling kit (Exiqon, Vedbaek, Denmark) and
hybridized on the miRCURY LNA™ array (version 18.0; Exiqon), which
contains 3,100 capture probes, covering all human miRNAs annotated
in the miRBase database (http://www.mirbase.org/). Subsequently, the slides
were scanned using a GenePix 4000B microarray laser scanner (Axon
Instruments; Molecular Devices, LLC, Sunnyvale, CA, USA) and
microarray images were analyzed using GenePix Pro 6.0 software. The
green signal intensity was calculated following background
subtraction, and the average of replicated spots on the same slide
was calculated to determine the median intensity. The median
normalization method was used to acquire normalized data
(foreground - background/median). The threshold value for
statistical significance used to define upregulated or
downregulated miRNAs was a fold change of >2, with a value of
P<0.05 calculated by the Student's t test.
RT-qPCR platform for relative
quantification of miRNAs
In order to confirm the miRNA array results,
stem-loop RT-qPCR was performed (Table
II). Briefly, 5 µl total RNA was reverse-transcribed into cDNA
in a volume of 15 µl with the TaqMan MicroRNAs Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific
Inc., Carlsbad, CA, USA). Subsequently, PCR was performed in 15 µl
reaction mixtures using the GeneAmp PCR system 9700 (Applied
Biosystems; Thermo Fisher Scientific Inc.). The samples were
subjected to thermal cycling parameters of 30 min at 16°C, 40 min
at 42°C, and 5 min at 85°C, and then kept at 4°C. qPCR reactions
were performed in triplicate using the TaqMan PCR Master Mix on an
ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific
Inc.). Each amplification reaction was performed in a volume of 20
µl containing 1.33 µl cDNA and 1 µl of gene-specific primers. The
cycle threshold (Cq) values were calculated with the SDS version
2.0.6 software (Applied Biosystems; Thermo Fisher Scientific Inc.),
and the fold change for each miRNA was calculated using the
comparative method (2−ΔΔCq) with cel-miR-39 as the
endogenous control (27).
 | Table II.Characterization of miRNAs selected
for reverse transcription-quantitative polymerase chain reaction
validation. |
Table II.
Characterization of miRNAs selected
for reverse transcription-quantitative polymerase chain reaction
validation.
| miRNA | Primer
sequence | miRBase accession
number |
|---|
|
hsa-miR-125a-5p |
UCCCUGAGACCCUUUAACCUGUGA | MIMAT0000443 |
| hsa-miR-423–5p |
UGAGGGGCAGAGAGCGAGACUUU | MIMAT0004748 |
| hsa-miR-133b |
UUUGGUCCCCUUCAACCAGCUA | MIMAT0000770 |
|
hsa-miR-1228–5p |
GUGGGCGGGGGCAGGUGUGUG | MIMAT0005582 |
| hsa-miR-1266 |
CCUCAGGGCUGUAGAACAGGGCU | MIMAT0005920 |
Prediction of target genes and
bioinformatics analysis of gene functions
In order to predict the target genes of
differentially expressed miRNAs that were significantly
dysregulated in the plasma of MOVC patients, the online databases
Miranda (http://www.microrna.org), miRDB
(http://mirdb.org/miRDB/) and TargetScan
(http://www.targetscan.org) were used. In
the present study, a predicted gene was considered as a putative
target candidate when it was predicted by at least two of the
aforementioned databases.
The predicted miRNA target genes were analyzed for
Gene Ontology (GO) annotation and Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway enrichment using the online DAVID
Bioinformatics Resources (http://david.abcc.ncifcrf.gov/). Fisher's exact test
and χ2 test were used to classify the GO category and
the pathway analysis. Only GO annotations or pathways with a
P-value of <0.01 and false discovery rate of <0.05 were
selected.
Statistical analysis
SPSS version 16.0 software (SPSS, Inc., Chicago, IL,
USA) was used to analyze the dataset from miRNA microarray
experiments. Descriptive statistics were applied to determine
differential individual characteristics between MOVC patients and
healthy controls by the Mann-Whitney U test for continuous
variables and the χ2 test or Fisher exact test
(two-tailed) for categorical variables. Statistical graph analysis
was performed using GraphPad Prism 5.0 (GraphPad Software Inc., La
Jolla, CA, USA). Differences with P<0.05 (two-tailed) were
considered to be statistically significant.
Results
MicroRNA microarray expression
profiling
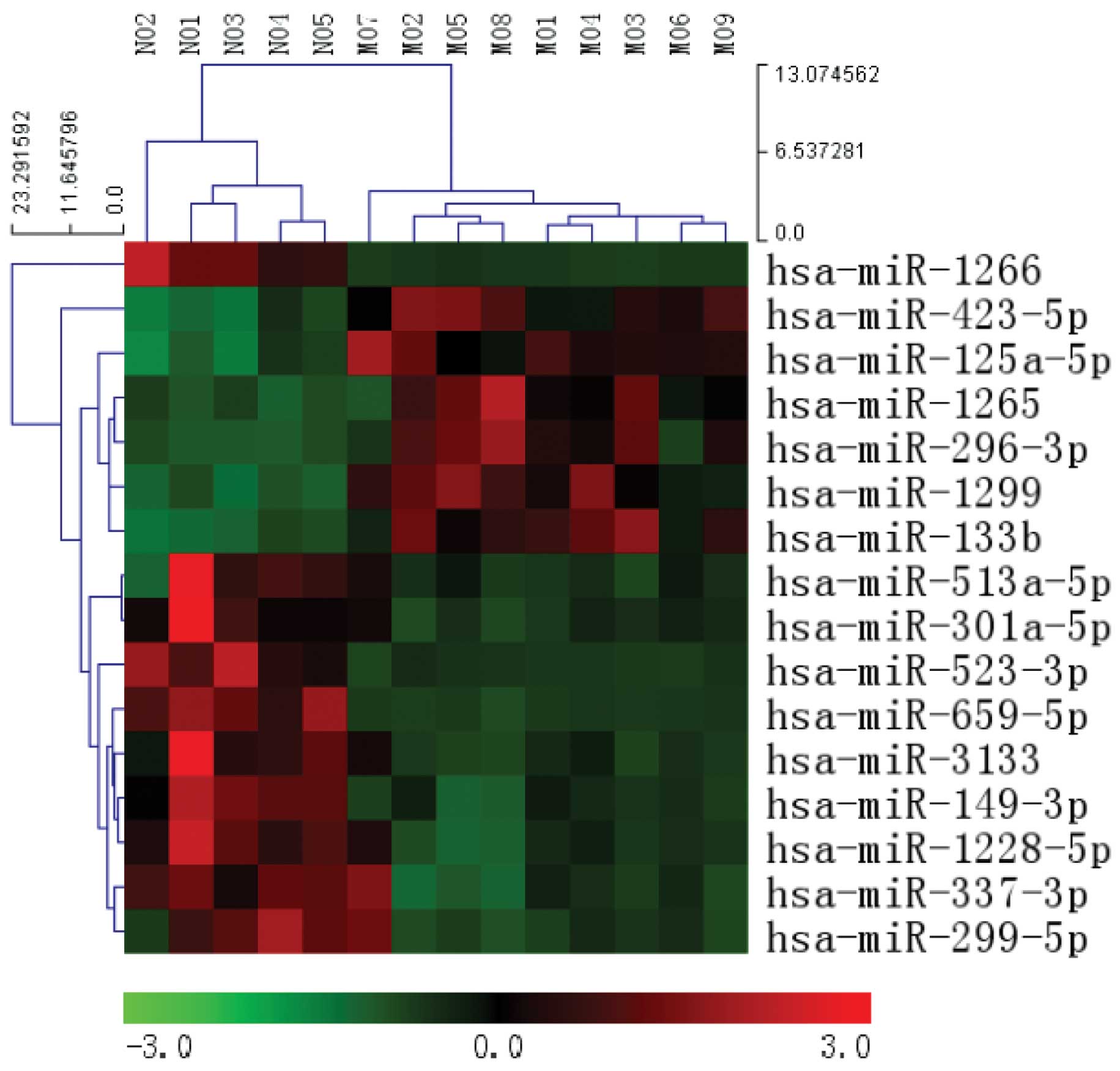
Following normalization of the raw data, a total of
16 microRNAs were found to be significantly differentially
expressed in the MOVC-1 patients, as compared with the controls-1
(>2-fold change; P<0.05). Among them, 6 upregulated miRNAs
were identified, while 10 downregulated miRNAs were identified
between the two groups (Table
III). Unsupervised hierarchical clustering analysis was
conducted using the expression levels of these 16 differentially
expressed miRNAs, resulting in clear differentiation of MOVC
samples from normal samples into two different clusters (Fig. 1).
 | Table III.Differentially expressed miRNAs in
MOVC-1 patients, as compared with healthy control-1
participants. |
Table III.
Differentially expressed miRNAs in
MOVC-1 patients, as compared with healthy control-1
participants.
| miRNAs | Fold change | Regulation | P-value |
|---|
| hsa-miR-423–5p | 4.22 | Up | 0.0032 |
| hsa-miR-133b | 3.42 | Up | 0.0012 |
|
hsa-miR-125a-5p | 2.89 | Up | 0.0140 |
| hsa-miR-1299 | 2.71 | Up | 0.0099 |
| hsa-miR-1265 | 2.56 | Up | 0.0380 |
| hsa-miR-296–3p | 2.01 | Up | 0.0370 |
| hsa-miR-1266 | 0.09 | Down | 0.0040 |
|
hsa-miR-1228–5p | 0.12 | Down | 0.0006 |
| hsa-miR-659–5p | 0.15 | Down | 0.0060 |
| hsa-miR-3133 | 0.22 | Down | 0.0002 |
| hsa-miR-523–3p | 0.32 | Down | 0.0065 |
|
hsa-miR-301a-5p | 0.37 | Down | 0.0080 |
| hsa-miR-299–5p | 0.44 | Down | 0.0110 |
|
hsa-miR-513a-5p | 0.45 | Down | 0.0280 |
| hsa-miR-149–3p | 0.45 | Down | 0.0240 |
| hsa-miR-337–3p | 0.47 | Down | 0.0030 |
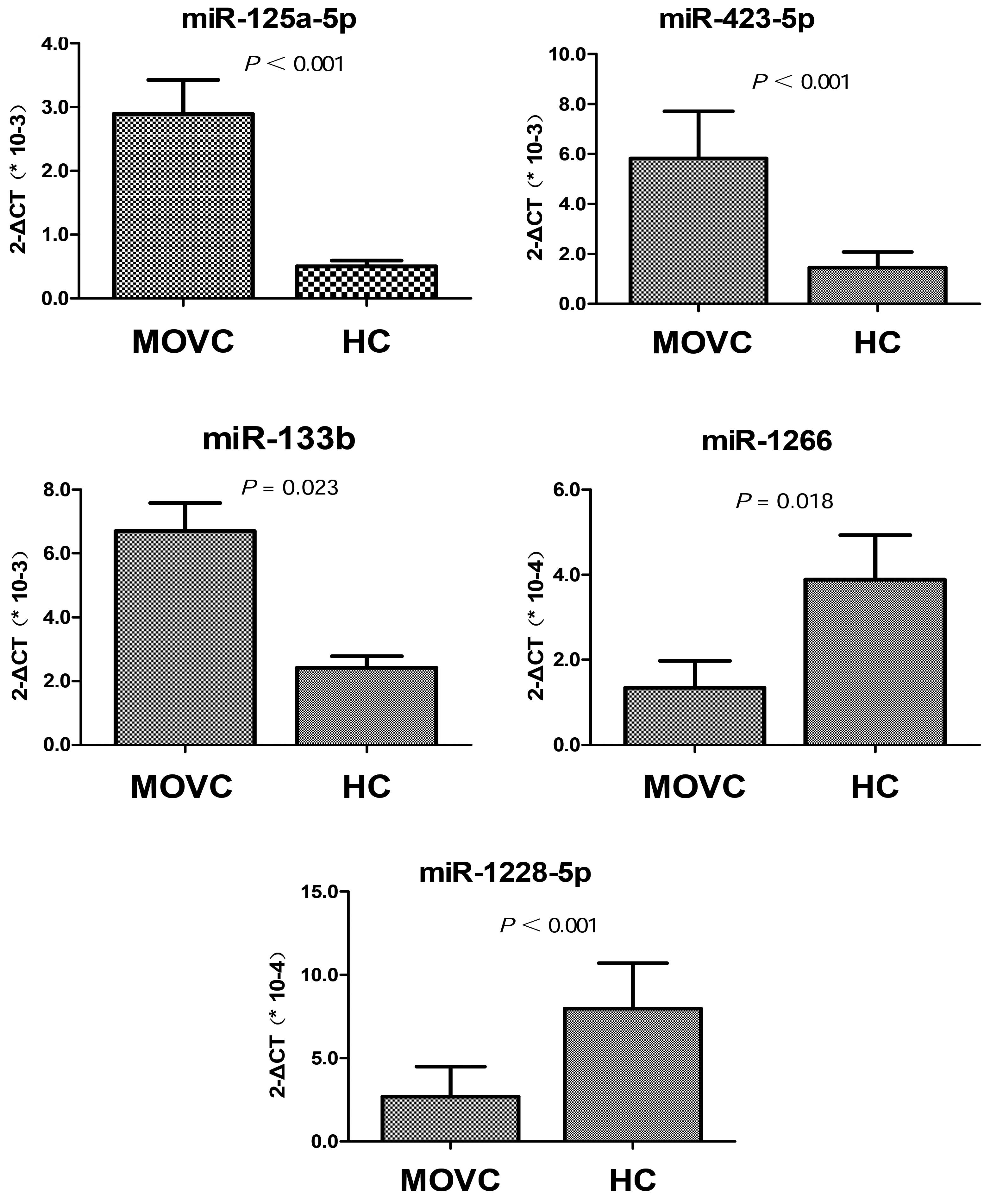
Validation of profiling data using
RT-qPCR
To verify the findings obtained through the miRNA
profile analysis, RT-qPCR was performed in the independent cohort
of 25 MOVC-2 patients and 25 healthy control-2 participants. The
investigation was focused on 5 candidate miRNAs (miR-125a-5p,
miR-133b, miR-423-5p, miR-1266 and miR-1228-5p), based on their
differential expression levels observed in the present microarray
analysis (2.89, 3.42, 4.22, 0.09 and 0.12-fold changes,
respectively) (Table III) and
their roles as crucial regulators of various biological processes
and vascular diseases (28–32). The RT-qPCR results were consistent
with the microarray data (Fig. 2).
Specifically, the expression levels of miR-125a-5p, miR-133b and
miR-423-5p were significantly increased, whereas those of
miR-1228-5p and miR-1266 were significantly decreased in the MOVC
patients. Therefore, the RT-qPCR results validated the observed
microarray data, suggesting that the data obtained from the miRNA
microarrays accurately reflected the miRNA expression levels.
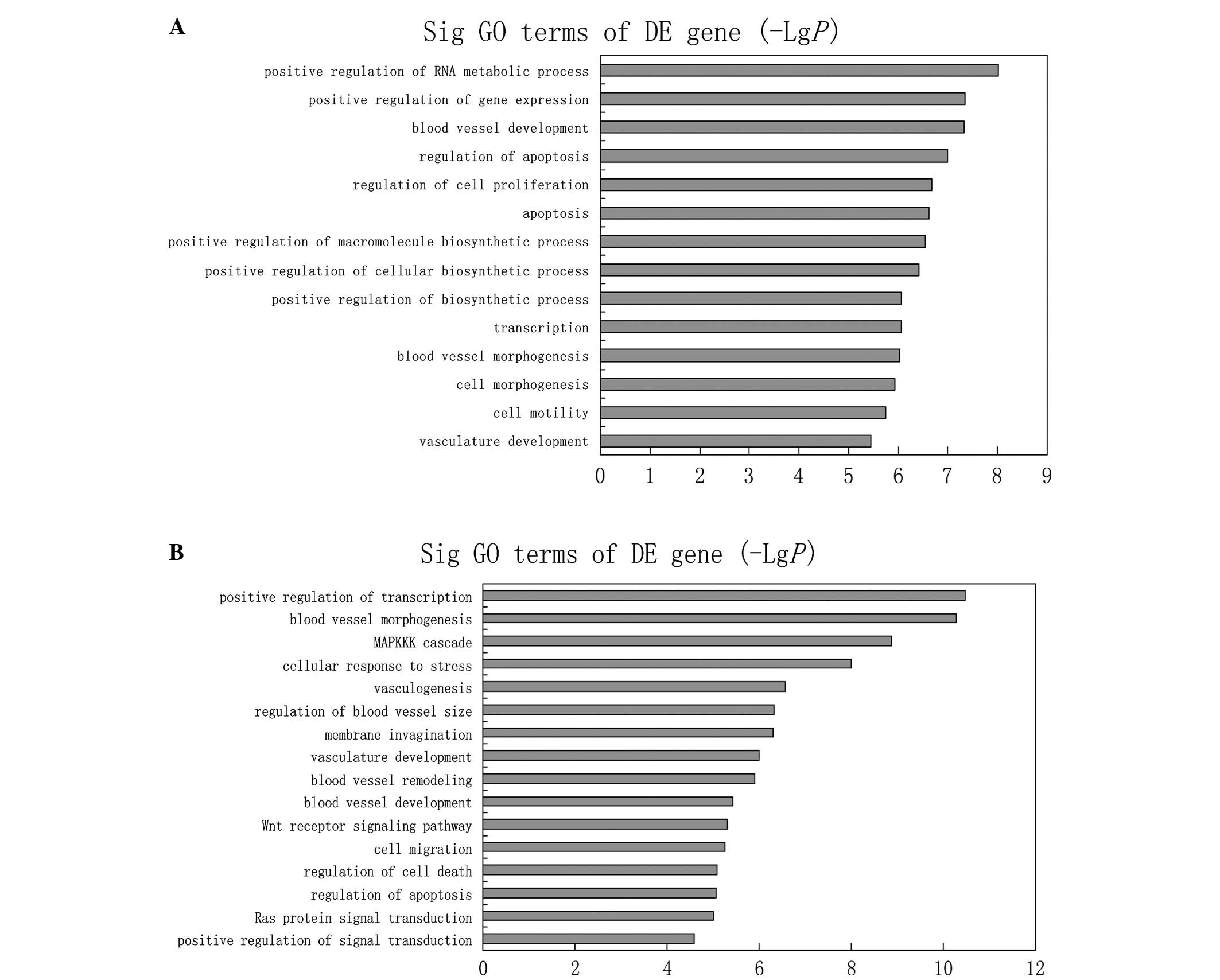
Bioinformatics analysis of miRNAs
Three available databases (Miranda, miRBase and
TargetScan) were used to predict targets genes of the 16 miRNAs
that were differentially expressed in the MOVC-1 and control-1
groups. In order to reduce the possibility of a false positive,
only targets estimated by at least two of these databases were
considered as putative candidates. To assess the potential
biological impact of the differentially expressed miRNAs, the
present study performed GO annotations and KEGG pathway enrichment
analysis for the predicted target genes. Employing that criterion,
20,009 target genes were predicted for the upregulated miRNAs,
including Janus kinase 2 and vascular endothelial growth factor B
(VEGFB); whereas 25,831 target genes were predicted for the
downregulated miRNAs, including mitogen-activated protein kinase
(MAPK) and patelet-derived growth factor. The primary GO terms
targeted by upregulated miRNAs were the response to blood vessel
development and morphogenesis, apoptosis and cell motility. By
contrast, significant GO terms corresponding to downregulated
miRNAs appeared to be the regulation of blood vessel morphogenesis,
the MAPK kinase kinase cascade, blood vessel remodeling and Wnt
receptor signaling pathway (Fig.
3).
KEGG pathway analysis showed that the target genes
for upregulated miRNAs were associated with pathways in cancer,
axon guidance, focal adhesion and the ErbB and Wnt signaling
pathways. The target genes for downregulated miRNAs were associated
with pathways in cancer, as well as with the ErbB, Wnt, p53 and T
cell receptor signaling pathways. Notably, certain signaling
pathways, such as pathways in cancer and the ErbB, Wnt, MAPK and
VEGF signaling pathways, were targeted by the upregulated and
downregulated miRNAs, suggesting that extensive miRNA regulation of
these pathways is involved in MOVC (Table IV).
 | Table IV.KEGG pathway analysis of putative
target genes regulated by differentially expressed miRNAs. |
Table IV.
KEGG pathway analysis of putative
target genes regulated by differentially expressed miRNAs.
| A, Upregulated
miRNAs |
|
|---|
|
|
|---|
| Analysis for
predicted target genes | P-value |
|---|
| Pathways in
cancer |
2.55×10−10 |
| Axon guidance |
6.90×10−8 |
| ErbB signaling
pathway |
6.52×10−6 |
| Insulin signaling
pathway |
1.15×10−5 |
| Focal adhesion |
3.41×10−5 |
| Wnt signaling
pathway |
1.15×10−4 |
| MAPK signaling
pathway |
1.30×10−4 |
| VEGF signaling
pathway |
3.39×10−4 |
| p53 signaling
pathway |
1.23×10−3 |
|
| B, Downregulated
miRNAs |
|
|
|
| Analysis for
predicted target genes | P-value |
|
| Pathways in
cancer |
2.96×10−9 |
| ErbB signaling
pathway |
1.45×10−6 |
| Wnt signaling
pathway |
1.67×10−5 |
| p53 signaling
pathway |
2.93×10−5 |
| T cell receptor
signaling pathway |
5.47×10−5 |
| Apoptosis |
1.70×10−4 |
| Vascular smooth
muscle contraction |
4.99×10−4 |
| MAPK signaling
pathway |
7.90×10−4 |
| VEGF signaling
pathway |
3.69×10−3 |
Discussion
The levels of circulating miRNAs potentially reflect
altered pathological and physiological conditions (33). As previously discussed, numerous
studies have shown that miRNAs serve a crucial role in vascular
disorders (19,20). However, the gene regulation of miRNAs
in the pathogenesis of MOVC has not been extensively investigated.
Thus, the present study aimed to assess the miRNA expression
profiles in the plasma of patients with MOVC in comparison with
those in age- and gender-matched healthy individuals.
The profiling data in the present study demonstrated
a distinct miRNA expression profile in MOVC patients, including 6
miRNAs that were significantly upregulated and 10 miRNAs that were
significantly downregulated. Furthermore, 5 significantly altered
miRNAs (miR-125a-5p, miR-133b, miR-423-5p, miR-1266 and
miR-1228-5p) were verified in a second cohort of patients using
RT-qPCR, and the results were found to be in line with those
obtained by the miRNA microarray analysis, thus suggesting that the
profiling results were reliable. Among the dysregulated miRNAs
(Table III), miR-125a-5p has
previously been reported to have a higher expression level in
vascular endothelial cells (VECs) and brain endothelial cells
(34,35), and thus potentially contributing
various vascular diseases, including atherosclerosis, hypertension
and stroke. It has been reported that the upregulation of
miR-125a-5p may be involved in angiogenesis defects in mature
endothelial cells by targeting the associated transcriptional
enhancer factor-1 (36).
Additionally, Li et al (34)
identified that miR-125a-5p was able to inhibit endothelin-1 (ET-1)
expression in VECs, while Hao et al (37) found that miR-125a-5p was able to
suppress the expression of ET-1 in the coronary arteries. ET-1, as
a potent vasoconstrictive peptide, plays critical roles in the
progression of atherosclerosis, vascular inflammation and
remodeling (38,39).
miR-133b, a myogenic miRNA, has been shown to have a
close association with multiple tumors and is generally considered
to have tumor-suppressive functions (40,41). It
is also overproduced in early myocardial injury subsequent to heart
transplantation (42), suggesting
its association with cardiovascular system disorders. Furthermore,
miR-423-5p, which has been reported to be upregulated in human
failing myocardium, may be a novel blood-based biomarker for heart
failure (43). Thus far, studies
concerning miR-1266 and miR-1228-5p are limited, with only a small
number of reports investigating their role in cancer (44,45).
Combining the aforementioned findings and our results, these
dysregulated miRNAs (miR-125a, miR-133b, miR-423-5p, miR-1266 and
miR-1228-5p) are suggested to be closely associated with the
pathogenesis of MOVC; however, the detailed underlying mechanism
requires further investigation.
To further clarify the role of miRNAs in the
pathogenesis of MOVC, GO annotations and KEGG pathway analysis were
performed on the target genes known to be regulated by the
differentially expressed miRNAs. KEGG pathway analysis showed
significant changes associated with the ErbB, Wnt, MAPK and VEGF
signaling pathways in the MOVC patients, as compared with the
healthy controls. ErbB signaling is implicated in the regulation of
the normal function of the embryonic and adult heart (46). The Wnt signaling pathway plays an
important role in morphogenesis, cell survival, differentiation and
proliferation (47). Additionally,
the MAPK and VEGF signaling pathways are closely associated with
the function of VECs. Siddiqui et al (48) found that MAPK had a considerable
effect on the endothelial monolayer integrity by signaling
proliferation and survival of endothelial cells. VEGF, which is
crucial in angiogenesis, is an endothelial cell-specific mitogen
(49) that regulates endothelial
cell proliferation, migration, vascular permeability, secretion and
other endothelial functions (50).
Bioinformatics analysis performed in future studies may improve the
understanding on the pathogenesis of MOVC.
To the best of our knowledge, the present study is
the first to evaluate the plasma miRNA expression pattern in MOVC
patients by microarray-based miRNA analysis. In conclusion, a total
of 16 differentially expressed miRNAs were identified in the plasma
between MOVC patients and healthy controls. In total, 5 of these
miRNAs were verified by RT-qPCR, which provided results in line
with those obtained by the miRNA microarray analysis. Functional
bioinformatics analysis demonstrated that the target genes
regulated by these miRNAs were involved in several biological
processes and signaling pathways. The study of these miRNAs may
provide a clearer understanding on the pathogenesis of MOVC and
help identify novel methods for the diagnosis, treatment and
prevention of MOVC. However, further investigation using more
samples is required to verify the differential expression of the
miRNAs observed in the present study, and their cellular origin and
function in MOVC should be analyzed.
Acknowledgements
The present study was supported by grants from the
Priority Academic Program Development of Jiangsu Higher Education
Institutions (grant no. 2012SJB190015) and the National Natural
Science Foundation of China (grant no. 81172604).
References
|
1
|
Valla DC: Primary Budd-Chiari syndrome. J
Hepatol. 50:195–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hefaiedh R, Cheikh M, Marsaoui L, Ennaifer
R, Romdhane H, Ben Nejma H, Bel Hadj N, Arfa N and Khalfallah MT:
The Budd-Chiari syndrome. Tunis Med. 91:376–381. 2013.PubMed/NCBI
|
|
3
|
Leebeek FW, Smalberg JH and Janssen HL:
Prothrombotic disorders in abdominal vein thrombosis. Neth J Med.
70:400–405. 2012.PubMed/NCBI
|
|
4
|
Plessier A, Rautou PE and Valla DC:
Management of hepatic vascular diseases. J Hepatol. 56:S25–S38.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Plessier A and Valla DC: Budd-Chiari
syndrome. Semin Liver Dis. 28:259–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi X, Han G, Wu F, Ren W, He C, Yin Z, Niu
J, Bai M, Yang Z, Wu K and Fan D: Thrombotic risk factors in
Chinese Budd-Chiari syndrome patients. An observational study with
a systematic review of the literature. Thromb Haemost. 109:878–884.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng D, Xu H, Lu ZJ, Hua R, Qiu H, Du H,
Xu X and Zhang J: Clinical features and etiology of Budd-Chiari
syndrome in Chinese patients: A single. center study. J
Gastroenterol Hepatol. 28:1061–1067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murad Darwish S, Plessier A,
Hernandez-Guerra M, Fabris F, Eapen CE, Bahr MJ, Trebicka J, Morard
I, Lasser L, Heller J, et al: EN-Vie (European Network for Vascular
Disorders of the Liver): Etiology, management and outcome of the
Budd-Chiari syndrome. Ann Intern Med. 151:167–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smalberg JH, Arends LR, Valla DC,
Kiladjian JJ, Janssen HL and Leebeek FW: Myeloproliferative
neoplasms in Budd-Chiari syndrome and portal vein thrombosis: A
meta-analysis. Blood. 120:4921–4928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okuda K: Membranous obstruction of the
inferior vena cava (obliterative hepatocavopathy, Okuda). J
Gastroenterol Hepatol. 16:1179–1183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang ZG, Zhang FJ, Yi MQ and Qiang LX:
Evolution of management for Budd-Chiari syndrome: A team's view
from 2564 patients. ANZ J Surg. 75:55–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi X, Zhang C, Han G, Zhang W, He C, Yin
Z, Liu Z, Bai W, Li R, Bai M, et al: Prevalence of the JAK2V617F
mutation in Chinese patients with Budd-Chiari syndrome and portal
vein thrombosis: A prospective study. J Gastroenterol Hepatol.
27:1036–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Sun G, Zhang P, Zhang J, Gui E, Zu
M, Jia E, Xu H, Xu L, Zhang J and Lu Z: JAK2 V617F mutation and
46/1 haplotype in Chinese Budd-Chiari syndrome patients. J
Gastroenterol Hepatol. 29:208–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valla DC: Hepatic venous outflow tract
obstruction etiopathogenesis: Asia versus the West. J Gastroenterol
Hepatol. 19:S204–S211. 2004. View Article : Google Scholar
|
|
15
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Urbich C, Kuehbacher A and Dimmeler S:
Role of microRNAs in vascular diseases, inflammation and
angiogenesis. Cardiovasc Res. 79:581–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Small EM and Olson EN: Pervasive roles of
microRNAs in cardiovascular biology. Nature. 469:336–342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yue J, Guan J, Wang X, Zhang L, Yang Z, Ao
Q, Deng Y, Zhu P and Wang G: MicroRNA-206 is involved in
hypoxia-induced pulmonary hypertension through targeting of the
HIF-1α/Fhl-1 pathway. Lab Invest. 93:748–759. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Icli B, Wara AK, Moslehi J, Sun X, Plovie
E, Cahill M, Marchini JF, Schissler A, Padera RF, Shi J, et al:
MicroRNA-26a regulates pathological and physiological angiogenesis
by targeting BMP/SMAD1 signaling. Circ Res. 113:1231–1241. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Z, Zhao Q, Warrick J, Lockwood CM,
Woodworth A, Moley KH and Gronowski AM: Circulating microRNA
miR-323-3p as a biomarker of ectopic pregnancy. Clin Chem.
58:896–905. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan L, Yu JT, Liu QY, Tan MS, Zhang W, Hu
N, Wang YL, Sun L and Jiang T: Circulating miR-125b as a biomarker
of Alzheimer's disease. J Neurol Sci. 336:52–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El-Hefnawy T, Raja S, Kelly L, Bigbee WL,
Kirkwood JM, Luketich JD and Godfrey TE: Characterization of
amplifiable, circulating RNA in plasma and its potential as a tool
for cancer diagnostics. Clin Chem. 50:564–573. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ach RA, Wang H and Curry B: Measuring
microRNAs: Comparisons of microarray and quantitative PCR
measurements, and of different total RNA prep methods. BMC
Biotechno. 8:692008. View Article : Google Scholar
|
|
26
|
Kroh EM, Parkin RK, Mitchell PS and Tewari
M: Analysis of circulating microRNA biomarkers in plasma and serum
using quantitative reverse transcription-PCR (qRT-PCR). Methods.
50:298–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li D, Yang P, Xiong Q, Song X, Yang X, Liu
L, Yuan W and Rui YC: MicroRNA-125a/b-5p inhibits endothelin-1
expression in vascular endothelial cells. J Hypertens.
28:1646–1654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang E, Nie Y, Zhao Q, Wang W, Huang J,
Liao Z, Zhang H, Hu S and Zheng Z: Circulating miRNAs reflect early
myocardial injury and recovery after heart transplantation. J
Cardiothorac Surg. 8:1652013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tijsen AJ, Creemers EE, Moerland PD, de
Windt LJ, van der Wal AC, Kok WE and Pinto YM: MiR423-5p as a
circulating biomarker for heart failure. Circ Res. 106:1035–1039.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen L, Lu MH, Zhang D, Hao NB, Fan YH, Wu
YY, Wang SM, Xie R, Fang DC, Zhang H, et al: miR-1207-5p and
miR-1266 suppress gastric cancer growth and invasion by targeting
telomerase reverse transcriptase. Cell Death Dis. 5:e10342014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia L, Wu J, Zhang L, Chen J, Zhong D, Xu
S, Xie C and Cai J: Restoration of miR-1228* expression suppresses
epithelial-mesenchymal transition in gastric cancer. PLoS One.
8:e586372013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu Y, Yi Z, Wu X, Li J and Xu F:
Circulating microRNAs in patients with active pulmonary
tuberculosis. J Clin Microbiol. 49:4246–4251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li D, Yang P, Xiong Q, Song X, Yang X, Liu
L, Yuan W and Rui YC: MicroRNA-125a/b-5p inhibits endothelin-1
expression in vascular endothelial cells. J Hypertens.
28:1646–1654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reijerkerk A, Lopez-Ramirez MA, van Het
Hof B, Drexhage JA, Kamphuis WW, Kooij G, Vos JB, van der Pouw
Kraan TC, van Zonneveld AJ, Horrevoets AJ, et al: MicroRNAs
regulate human brain endothelial cell-barrier function in
inflammation: Implications for multiple sclerosis. J Neurosci.
33:6857–6863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Che P, Liu J, Shan Z, Wu R, Yao C, Cui J,
Zhu X and Wang J, Burnett MS, Wang S and Wang J: MiR-125a-5p
impairs endothelial cell angiogenesis in aging mice via RTEF-1
downregulation. Aging Cell. 13:926–934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hao L, Wang XG, Cheng JD, You SZ, Ma SH,
Zhong X, Quan L and Luo B: The up-regulation of endothelin-1 and
down-regulation of miRNA-125a-5p, −155 and −199a/b-3p in human
atherosclerotic coronary artery. Cardiovasc Pathol. 23:217–223.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dashwood MR and Tsui JC: Endothelin-1 and
atherosclerosis: Potential complications associated with
endothelin-receptor blockade. Atherosclerosis. 160:297–304. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kowala MC: The role of endothelin in the
pathogenesis of atherosclerosis. Adv Pharmacol. 37:299–318. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao H, Li M, Li L, Yang X, Lan G and
Zhang Y: MiR-133b is down-regulated in human osteosarcoma and
inhibits osteosarcoma cells proliferation, migration and invasion
and promotes apoptosis. PLoS One. 8:e835712013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao Y, Huang J, Zhang L, Qu Y, Li J, Yu
B, Yan M, Yu Y, Liu B and Zhu Z: MiR-133b is frequently decreased
in gastric cancer and its overexpression reduces the metastatic
potential of gastric cancer cells. BMC Cancer. 14:342014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang E, Nie Y, Zhao Q, Wang W, Huang J,
Liao Z, Zhang H, Hu S and Zheng Z: Circulating miRNAs reflect early
myocardial injury and recovery after heart transplantation. J
Cardiothorac Surg. 8:1652013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tijsen AJ, Creemers EE, Moerland PD, de
Windt LJ, van der Wal AC, Kok WE and Pinto YM: MiR423-5p as a
circulating biomarker for heart failure. Circ Res. 106:1035–1039.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen L, Lü MH, Zhang D, Hao NB, Fan YH, Wu
YY, Wang SM, Xie R, Fang DC, Zhang H, et al: MiR-1207-5p and
miR-1266 suppress gastric cancer growth and invasion by targeting
telomerase reverse transcriptase. Cell Death Dis. 5:e10342014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jia L, Wu J, Zhang L, Chen J, Zhong D, Xu
S, Xie C and Cai J: Restoration of miR-1228* expression suppresses
epithelial-mesenchymal transition in gastric cancer. PLoS One.
8:e586372013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pentassuglia L and Sawyer DB:
ErbB/integrin signaling interactions in regulation of myocardial
cell-cell and cell-matrix interactions. Biochim Biophys Acta.
1833:909–916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Koziński K and Dobrzyń A: Wnt signaling
pathway-its role in regulation of cell metabolism. Postepy Hig Med
Dosw (Online). 67:1098–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Siddiqui SS, Siddiqui ZK, Uddin S,
Minshall RD and Malik AB: P38 MAPK activation coupled to
endocytosis is a determinant of endothelial monolayer integrity. Am
J Physiol Lung Cell Mol Physiol. 292:L114–L124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liang X, Xu F, Li X, Ma C, Zhang Y and Xu
W: VEGF signal system: The application of antiangiogenesis. Curr
Med Chem. 21:894–910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shibuya M: Vascular endothelial growth
factor and its receptor system: Physiological functions in
angiogenesis and pathological roles in various diseases. J Biochem.
153:13–19. 2013. View Article : Google Scholar : PubMed/NCBI
|