Introduction
Colorectal cancer is the third most common type of
human cancer and a major global public health concern (1). The incidence rate of colorectal cancer
has been increasing in the Chinese population; in Beijing, the
annual incidence of CRC has increased from 16 per 100,000 to 24 per
100,000 in the past decade (2).
Chemoprevention, including the use of oxaliplatin, fluorouracil and
leucovorin, is considered to be the most promising strategy, since
other therapies fail to control gastrointestinal cancers (3). However, at present, no optimal adjuvant
chemotherapy exists; therefore, there is a constant requirement for
the development of rationally designed, novel adjuvant therapeutic
strategies for the treartment of colon cancer. In the past few
decades, traditional Chinese herbal medicines have become a widely
accepted treatment option for colorectal cancer and an increasing
number of studies have focused on the identification of new
bioactive pure compounds and herbs (4,5).
Danshen, also known as Radix Salviae miltiorrhiza, is widely
prescribed in traditional Chinese medicine for the treatment of
cardiovascular diseases (6,7). Tanshinone IIA (TSA;
C19H18O3) is extracted from
Danshen (8,9) and presents anti-inflammatory (10,11) and
antioxidant properties (12,13). Recently, TSA has been proven to also
harbor antitumor activities in various human malignant neoplasms
(14–17). Su et al reported that TSA may
inhibit cell growth and induce apoptosis in colon cancer (18); however, the molecular mechanisms of
TSA action remain unclear. In the present study, the influence and
molecular mechanism of TSA on colon cancer cell growth and
chemosensitivity of colon cancer cells to fluorouracil (5-FU) were
investigated.
Materials and methods
Cell culture and materials
HCT1116 and COLO205 colon cancer cells were
purchased from American Type Culture Collection (Manassas, VA,
USA). The cells were cultured in RPMI-1640 medium (Gibco-BRL,
Gasthersburg, MD, USA) supplemented with 10% heat-inactivated fetal
bovine serum (Gibco-BRL) and 2% penicillin/streptomycin
(penicillin, 10,000 U/ml; streptomycin, 10 mg/ml). The cells were
placed into tissue culture flasks (75 cm2, 250 ml) and
grown at 37°C in humidified atmosphere consisting of 5%
CO2 and 95% air. TSA was purchased from Dasherb Corp.
(Shenyang, China). Aprotinin and leupeptin were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Dimethyl sulfoxide was
purchased from EMD Millipore Corporation (Darmstadt, Germany). The
antibodies used in the present study were the following: Mouse
monoclonal anti-actin (1:1,000; cat. no. sc-8432; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA), rabbit polyclonal
anti-vascular endothelial growth factor (VEGF; 1:500; cat. no.
ab46154; Abcam, Cambridge, MA, USA), rabbit polyclonal anti-c-Myc
(1:1,000; cat. no. 9402S; Cell Signaling Technology, Inc., Denvers,
MA USA), mouse monoclonal anti-cyclooxygenase-2 (COX2; 1:1,000;
cat. no. sc-376861) and rabbit polyclonal anti-B-cell lymphoma-2
(Bcl-2; 1:2,000; cat. no. sc-492) (both from Santa Cruz
Biotechnology Inc.).
Cell viability assay
Cell viability was measured using a Cell Counting
kit (CCK)-8 kit (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). Briefly, cells were seeded in a 94-well plate at a density
of 2×104 cells per well in 200 µl culture medium. The
following day the cells were incubated with drug for 48 h. At the
end of the experiment, 20 µl CCK-8 solution was added to the cells.
The cells were then further incubated at 37°C for 2 h.
Subsequently, the optical density (OD) at 450 nm was measured using
a VICTOR™ × Multi-Label reader (PerkinElmer, Inc., Waltham, MA,
USA) and the percentage of cell viability was calculated as
ODdrug/ODcontrol × 100%.
Preparation of nuclear extract
Following treatment, the cells were harvested and
washed twice with ice-cold phosphate-buffered saline (PBS). Next,
the cell samples were resuspended in 1 ml PBS and nuclear extracts
were prepared on ice as described in a previous study (19). Following centrifugation at 15,000 × g
for 10 min at 4°C, the cell pellet was suspended in an ice-cold
buffer (consisting of 10 mmol/l HEPES, 1.5 mmol/l MgCl2,
0.5 mmol/l dithiothreitol, 0.2 mmol/l phenylmethylsulphonylfluoride
and 0.2 mmol/l KCl), vortexed for 10 sec and centrifuged at 15,000
× g for 5 min at 4°C. Subsequently, the nuclear pellet was washed
in 1 ml buffer (consisting of 20 mmol/l HEPES, 25% glycerol, 0.2
mmol/l ethylenediaminetetraacetic acid, 1.5 mmol/l MgCl2
and 0.42 mol/l hypertonic saline), resuspended in 30 ml buffer (20
mmol/l HEPES, 25% glycerol, 0.42 mol/l NaCl, 1.5 mmol/l
MgCl2, 0.2 mmol/l EDTA), rotated for 30 min at 4°C and
centrifuged at 14,500 × g for 20 min at 4°C. Finally, the
supernatants were used as nuclear extracts.
Western blotting
Whole cell lysates or nuclear extracts were
separated using 12% SDS-PAGE, as described previously (20). Transfer buffer [25 mM Tris (pH 8.5),
20% methanol and 0.2 M glycine] was used to equilibrate the
separated proteins, which were then transferred onto a 0.4-µm
polyvinylidene fluoride membrane (EMD Millipore Corporation,
Bedford, MA, USA). The membranes were incubated with 5% non-fat dry
milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h,
followed by washing and then incubation with appropriate dilutions
of specific primary antibodies at 4°C overnight. Following
incubation with secondary anti-mouse peroxidase-conjugated antibody
(dilution, 1:15,000; Sigma-Aldrich), the immune-reactive bands were
visualized using an enhanced chemiluminescence detection kit (EMD
Millipore Corporation, Darmstadt, Germany). β-actin was used as an
internal control for western blotting.
Statistical analysis
The statistical analysis was performed using SPSS
17.0 software (SPSS Inc., Chicago, IL, USA) and are presented as
the mean ± standard deviation. One-way analysis of variance was
performed to compare numeric variables between groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of TSA on cell viability of
colon cancer cells
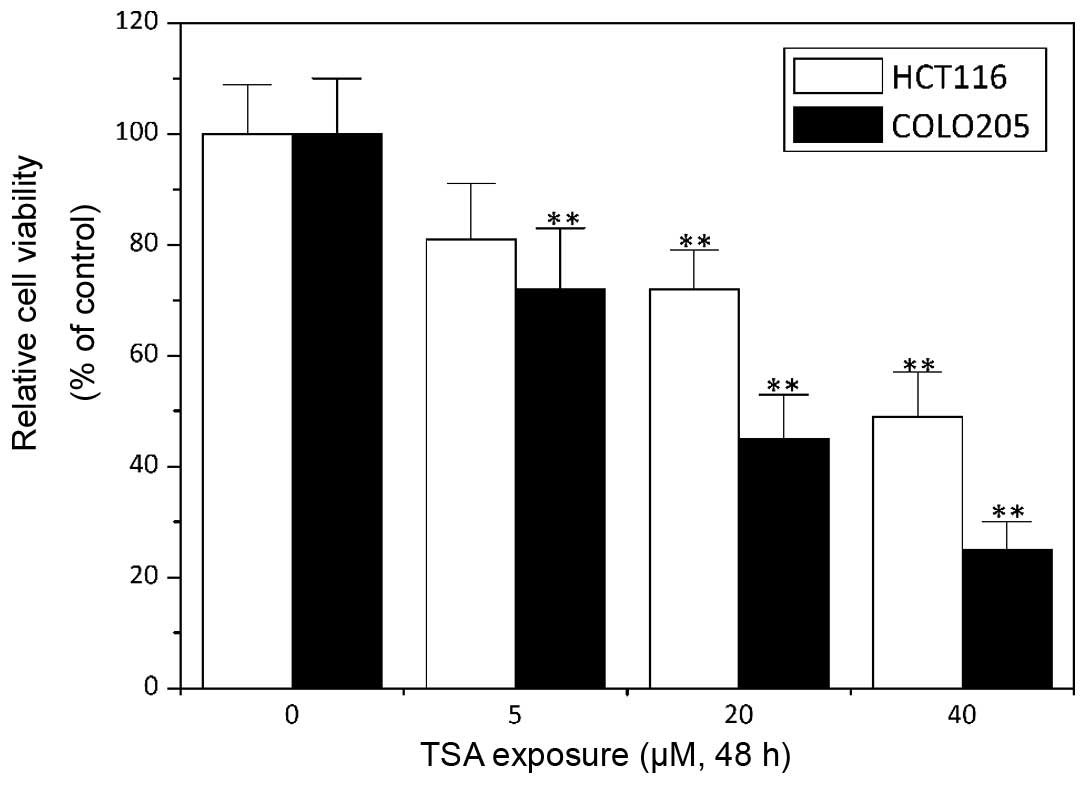
TSA has been demonstrated to exert an antitumor
activity in numerous types of human cancer, including breast, lung,
liver, prostate and ovarian cancer and leukemia (15,21–25). The
data of the present study showed that the exposure of the HCT1116
and COLO205 colon cancer cell lines to TSA greatly decreased their
cell viability in a concentration-dependent manner (P<0.05;
Fig. 1), which suggested an
antitumor effect of TSA in colon cancer.
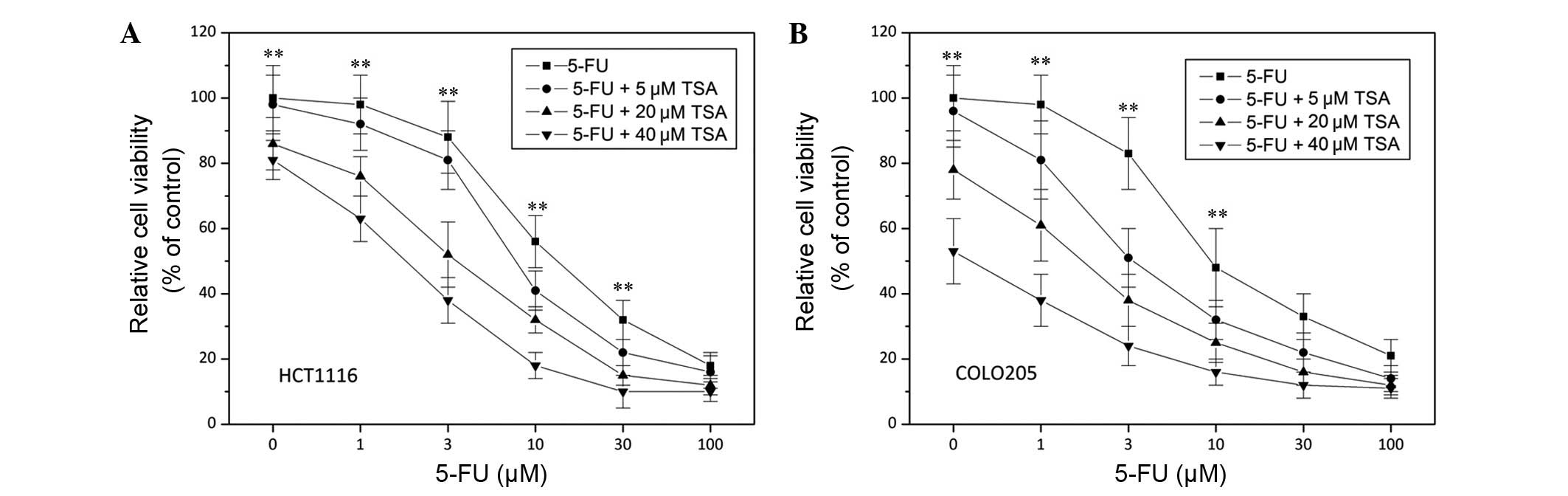
TSA enhances chemosensitivity of colon
cancer cells
Drug resistance is a clinical challenge that can
result in the failure of colon cancer therapy (26). In order to examine how TSA influences
the sensitivity of colon cancer cells to chemotherapy, 5-FU
chemotherapy was combined with TSA treatment. As shown in Fig. 2, 5-FU-induced cell death in HCT1116
and COLO205 cells was considerably enhanced by 20 µM TSA treatment
(P<0.05), which suggested that TSA was able to potentiate the
chemosensitivity of colon cancer cells.
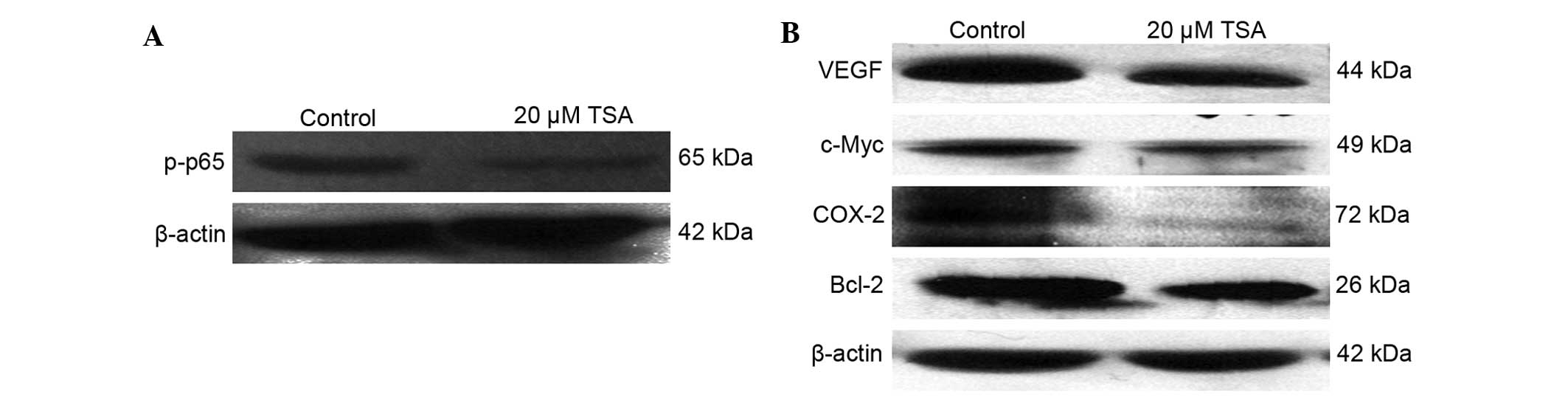
Involvement of nuclear factor-κB
(NF-κB) in TSA activity
As shown by the aforementioned data, TSA can
decrease cell viability and increase the chemosensitivity of colon
cancer cells; however, the molecular mechanisms of TSA action
remain to be elucidated. In order to further investigate the
mechanisms of TSA action, the influence of TSA on the activation of
NF-κB was determined, since it has been suggested that NF-κB is
involved in the development and progression of various malignant
neoplasms (27–33), which made it a target for tumor
therapeutics (34,35). The present data revealed that 20 µM
TSA treatment significantly decreased the level of phosphorylation
of p65, a subunit of NF-κB (Fig.
3A), which indicated the inhibitory effect of TSA on NF-κB
activation. In addition, the expression of NF-κB regulated genes
was examined. As illustrated in Fig.
3B, 20 µM TSA was able to considerably decrease VEGF, c-Myc,
COX-2 and Bcl-2 expression.
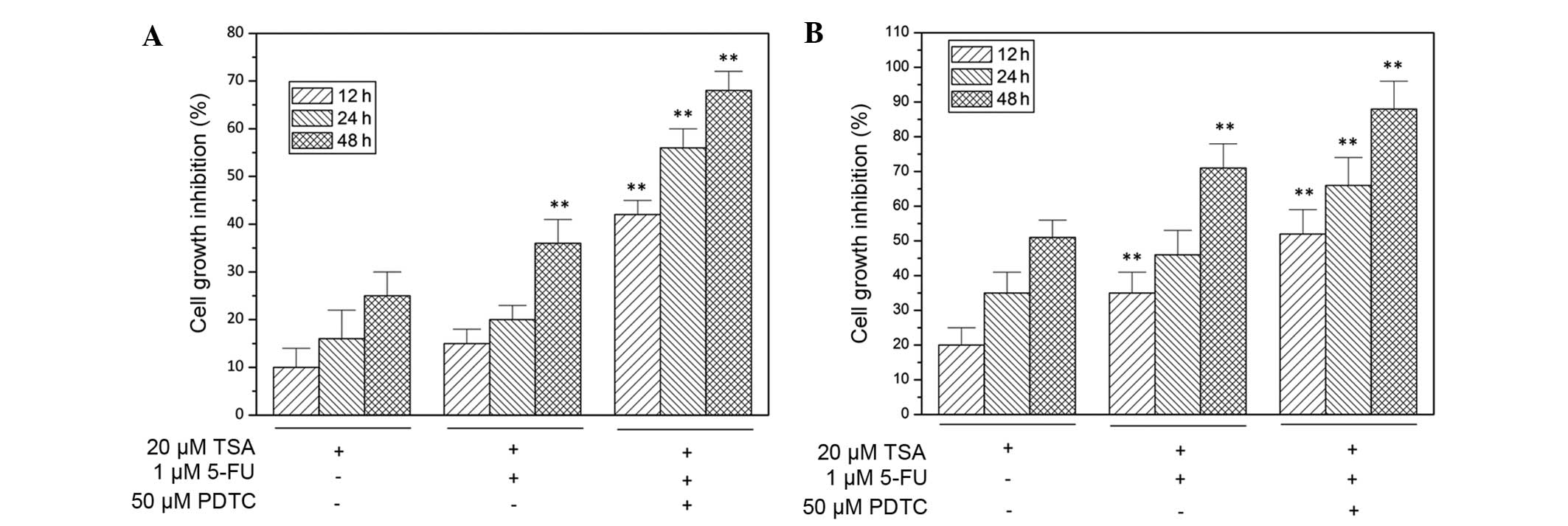
NF-κB inhibitor, pyrrolidine
dithiocarbamate (PDTC), enhances TSA activity
As indicated in Fig.
4, the inhibition of NF-κB signaling with specific inhibitor
PDTC significantly potentiated the suppression of TSA-induced cell
growth in HCT1116 and COLO205 cells. Furthermore, the
chemosensitizing effect of TSA on colon cancer cells was also
enhanced by 50 µM PDTC treatment (P<0.05), which is illustrated
as increased cell growth inhibition in Fig. 4.
Discussion
Due to drug resistance and the toxic effect of
current chemotherapy strategies, antitumor drug studies have been
attempting to identify natural chemical compounds, and Chinese
herbal medicine has been attracting increasing attention (36). TSA has been found to be an effective
chemical component extracted from Danshen (Radix Salviae
Miltiorrhizae) (37), which
harbors anti-inflammatory and anti-oxidative activities (10–13). It
has also been shown to have a cardioprotective effect by reducing
apoptosis (38–40). Recently, an increasing number of
studies has focused on the antitumor activity of TSA. For instance,
Jung et al (41) and Liu
et al (42) identified that
TSA induced apoptosis in leukemic cells in vitro, possibly
through the JAK/STAT3/5 and SHP1/2 pathways. In addition, Fu et
al (25) found that TSA blocked
the epithelial-mesenchymal transition through the downregulation of
hypoxia-inducible factor-1α, reversing hypoxia-induced chemotherapy
resistance in breast cancer cell lines. In addition, increasing
evidence indicated that TSA can inhibit tumor cell growth and
induce apoptosis (15,17,43);
however, despite the fact that in certain type of cancer evidence
showed that intrinsic apoptosis pathways were involved (14,44), the
underlying mechanisms remain unclear. With regard to colon cancer,
in 2008, Su et al identified that TSA inhibited cell growth
and induced apoptosis in colon cancer cells (18). Shan et al (45) also indicated that TSA was able to
inhibit colon cancer cell migration and invasion. Furthermore, in
2012 Su et al (46) found
that TSA potentiated the efficacy of 5-FU in colon cancer cells
in vivo through the downregulation of P-glycoprotein and
LC3-II. The results of the present study confirmed the antitumor
activity and chemosensitizing effect of TSA in colon cancer cells
and provided new evidence that TSA can sensitize colon cancer cells
to 5-FU through the suppression of NF-κB activation.
NF-κB is constitutively activated in numerous human
cancer types (30–32,35). The
suppression of NF-κB may induce apoptosis and sensitize cancer
cells to chemotherapy (33,34,47,48). As
an eukaryotic transcription factor, NF-κB regulates numerous genes
that are differentially expressed and implicated in tumorigenesis,
including c-Myc, COX-2 and matrix metalloproteinase-9 (49). In colon cancer, NF-κB activation
participates in the promotion and progression of colon cancer
(49); therefore, the aim of the
present study was to investigate the effect of TSA on NF-κB
activation and on NF-κB-regulated gene products. The results showed
that TSA treatment decreased the level of the phosphorylated p65 in
the nucleus of colon cancer cells and the NF-κB-regulated gene
expression levels of VEGF, COX-2, c-Myc and Bcl-2. In addition, the
inhibition of NF-κB with specific inhibitor PDTC may further
enhance the induction of cell death and the chemosensitizing effect
of TSA in colon cancer cells.
In conclusion, the present study revealed that TSA
was able to induce cell death in colon cancer cells and sensitize
colon cancer cells to 5-FU therapy by inhibiting NF-κB activation;
therefore, TSA appears to be a good option of adjuvant chemotherapy
for colon cancer.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin P, Wu ZT, Li SR, Li SJ, Wang JH, Wang
ZH, Lu JG, Cui XJ, Han Y, Rao J and Sheng JQ: Colorectal cancer
screening with fecal occult blood test: A 22-year cohort study.
Oncol Lett. 6:576–582. 2013.PubMed/NCBI
|
|
3
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verhoef MJ, Balneaves LG, Boon HS and
Vroegindewey A: Reasons for and characteristics associated with
complementary and alternative medicine use among adult cancer
patients: A systematic review. Integr Cancer Ther. 4:274–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boon H and Wong J: Botanical medicine and
cancer: A review of the safety and efficacy. Expert Opin
Pharmacother. 5:2485–2501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fish JM, Welchons DR, Kim YS, Lee SH, Ho
WK and Antzelevitch C: Dimethyl lithospermate b, an extract of
danshen, suppresses arrhythmogenesis associated with the brugada
syndrome. Circulation. 113:1393–1400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang PN, Mao JC, Huang SH, Ning L, Wang
ZJ, On T, Duan W and Zhu YZ: Analysis of cardioprotective effects
using purified Salvia miltiorrhiza extract on isolated rat
hearts. J Pharmacol Sci. 101:245–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Che AJ, Zhang JY, Li CH, Chen XF, Hu ZD
and Chen XG: Separation and determination of active components in
Radix Salviae miltiorrhizae and its medicinal preparations
by nonaqueous capillary electrophoresis. J Sep Sci. 27:569–575.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou L, Zuo Z and Chow MS: Danshen: An
overview of its chemistry, pharmacology, pharmacokinetics and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jang SI, Kim HJ, Kim YJ, Jeong SI and You
YO: Tanshinone IIA inhibits LPS-induced NF-kappaB activation in raw
264.7 cells: Possible involvement of the NIK-IKK, ERK1/2, P38 and
JNK pathways. Eur J Pharmacol. 542:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Li J, Ashok M, Wu R, Chen D, Yang L,
Yang H, Tracey KJ, Wang P, Sama AE and Wang H: A cardiovascular
drug rescues mice from lethal sepsis by selectively attenuating a
late-acting proinflammatory mediator, high mobility group box 1. J
Immunol. 178:3856–3864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin R, Wang WR, Liu JT, Yang GD and Han
CJ: Protective effect of tanshinone IIA on human umbilical vein
endothelial cell injured by hydrogen peroxide and its mechanism. J
Ethnopharmacol. 108:217–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang AM, Sha SH, Lesniak W and Schacht J:
Tanshinone (Salviae miltiorrhizae extract) preparations
attenuate aminoglycoside-induced free radical formation in vitro
and ototoxicity in vivo. Antimicrob Agents Chemother. 47:1836–1841.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian HL, Yu T, Xu NN, Feng C, Zhou LY, Luo
HW, Chang DC, Le XF and Luo KQ: A novel compound modified from
tanshinone inhibits tumor growth in vivo via activation of the
intrinsic apoptotic pathway. Cancer Lett. 297:18–30. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Wang J, Jiang JY, Liu SD, Fu K
and Liu HY: Tanshinone IIA induces cytochrome c-mediated caspase
cascade apoptosis in A549 human lung cancer cells via the JNK
pathway. Int J Oncol. 45:683–690. 2014.PubMed/NCBI
|
|
16
|
Yun SM, Jung JH, Jeong SJ, Sohn EJ, Kim B
and Kim SH: Tanshinone IIA induces autophagic cell death via
activation of AMPK and ERK and inhibition of mTOR and p70 S6k in
KBM-5 leukemia cells. Phytother Res. 28:458–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang L, Guo H, Dong L, Wang L, Liu C and
Wang X: Tanshinone IIA inhibits the growth, attenuates the stemness
and induces the apoptosis of human glioma stem cells. Oncol Rep.
32:1303–1311. 2014.PubMed/NCBI
|
|
18
|
Su CC, Chen GW, Kang JC and Chan MH:
Growth inhibition and apoptosis induction by tanshinone IIA in
human colon adenocarcinoma cells. Planta Med. 74:1357–1362. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo P, Tan Z, Zhang Z, Li H and Mo Z:
Inhibitory effects of salvianolic acid B on the high
glucose-induced mesangial proliferation via NF-kappaB-dependent
pathway. Biol Pharm Bull. 31:1381–1386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lui VW, Boehm AL, Koppikar P, Leeman RJ,
Johnson D, Ogagan M, Childs E, Freilino M and Grandis JR:
Antiproliferative mechanisms of a transcription factor decoy
targeting signal transducer and activator of transcription (stat)
3. The role of stat1. Mol Pharmacol. 71:1435–1443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen SJ: A potential target of Tanshinone
IIA for acute promyelocytic leukemia revealed by inverse docking
and drug repurposing. Asian Pac J Cancer Prev. 15:4301–4305. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiu SC, Huang SY, Chen SP, Su CC, Chiu TL
and Pang CY: Tanshinone IIA inhibits human prostate cancer cells
growth by induction of endoplasmic reticulum stress in vitro and in
vivo. Prostate Cancer Prostatic Dis. 16:315–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Won SH, Lee HJ, Jeong SJ, Lü J and Kim SH:
Activation of p53 signaling and inhibition of androgen receptor
mediate tanshinone IIA induced G1 arrest in LNCaP prostate cancer
cells. Phytother Res. 26:669–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kan S, Cheung WM, Zhou Y and Ho WS:
Enhancement of doxorubicin cytotoxicity by tanshinone IIA in HepG2
human hepatoma cells. Planta Med. 80:70–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu P, Du F, Chen W, Yao M, Lv K and Liu Y:
Tanshinone IIA blocks epithelial-mesenchymal transition through
HIF-1α downregulation, reversing hypoxia-induced chemotherapy
resistance in breast cancer cell lines. Oncol Rep. 31:2561–2568.
2014.PubMed/NCBI
|
|
26
|
Housman G, Byler S, Heerboth S, Lapinska
K, Longacre M, Snyder N and Sarkar S: Drug resistance in cancer: An
overview. Cancers (Basel). 6:1769–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garg A and Aggarwal BB: Nuclear
transcription factor-kappaB as a target for cancer drug
development. Leukemia. 16:1053–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu HG, Yu LL, Yang Y, Luo HS, Yu JP, Meier
JJ, Schrader H, Bastian A, Schmidt WE and Schmitz F: Increased
expression of RelA/nuclear factor-kappa B protein correlates with
colorectal tumorigenesis. Oncology. 65:37–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liptay S, Weber CK, Ludwig L, Wagner M,
Adler G and Schmid RM: Mitogenic and antiapoptotic role of
constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J
Cancer. 105:735–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nair A, Venkatraman M, Maliekal TT, Nair B
and Karunagaran D: Nf-kappaB is constitutively activated in
high-grade squamous intraepithelial lesions and squamous cell
carcinomas of the human uterine cervix. Oncogene. 22:50–58. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W, Tan D, Zenali MJ and Brown RE:
Constitutive activation of nuclear factor-kappa B (NF-κB) signaling
pathway in fibrolamellar hepatocellular carcinoma. Int J Clin Exp
Pathol. 3:238–243. 2010.
|
|
32
|
Nagel D, Vincendeau M, Eitelhuber AC and
Krappmann D: Mechanisms and consequences of constitutive nf-kappab
activation in B-cell lymphoid malignancies. Oncogene. 11:5655–5565.
2014. View Article : Google Scholar
|
|
33
|
Shishodia S, Amin HM, Lai R and Aggarwal
BB: Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB
activation, induces G1/s arrest, suppresses proliferation and
induces apoptosis in mantle cell lymphoma. Biochem Pharmacol.
70:700–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen HM and Tergaonkar V: NFkappaB
signaling in carcinogenesis and as a potential molecular target for
cancer therapy. Apoptosis. 14:348–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Yang G, Li X, Zhang Y, Yang J, Chang
J, Sun X, Zhou X, Guo Y, Xu Y, et al: Traditional Chinese medicine
in cancer care: A review of controlled clinical studies published
in chinese. PloS One. 8:e603382013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shang Q, Xu H and Huang L: Tanshinone IIA.
A promising natural cardioprotective agent. Evid Based Complement
Alternat Med. 2012:7164592012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang FL, Leo S, Wang XG, Li H, Gong LY,
Kuang Y and Xu XF: Effect of tanshinone IIA on cardiomyocyte
hypertrophy and apoptosis in spontaneously hypertensive rats. Exp
Ther Med. 6:1517–1521. 2013.PubMed/NCBI
|
|
39
|
Jin HJ and Li CG: Tanshinone IIA and
cryptotanshinone prevent mitochondrial dysfunction in
hypoxia-induced H9c2 cells: Association to mitochondrial ROS,
intracellular nitric oxide and calcium levels. Evid Based
Complement Alternat Med. 2013:6106942013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin HJ, Xie XL, Ye JM and Li CG:
Tanshinoneiia and cryptotanshinone protect against hypoxia-induced
mitochondrial apoptosis in H9c2 cells. PLoS One. 8:e517202013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jung JH, Kwon TR, Jeong SJ, Kim EO, Sohn
EJ, Yun M and Kim SH: Apoptosis induced by tanshinone IIA and
cryptotanshinone is mediated by distinct JAK/STAT3/5 and SHP1/2
signaling in chronic myeloid leukemia K562 cells. Evid Based
Complement Alternat Med. 2013:8056392013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu JJ, Lin DJ, Liu PQ, Huang M, Li XD and
Huang RW: Induction of apoptosis and inhibition of cell adhesive
and invasive effects by tanshinone IIA in acute promyelocytic
leukemia cells in vitro. J Biomed Sci. 13:813–823. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang JF, Feng JG, Han J, Zhang BB and Mao
WM: The molecular mechanisms of tanshinone IIA on the apoptosis and
arrest of human esophageal carcinoma cells. Biomed Res Int.
2014:5827302014.PubMed/NCBI
|
|
44
|
Tseng PY, Lu WC, Hsieh MJ, Chien SY and
Chen MK: Tanshinone IIA induces apoptosis in human oral cancer KB
cells through a mitochondria-dependent pathway. Biomed Res Int.
2014:5405162014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shan YF, Shen X, Xie YK, Chen JC, Shi HQ,
Yu ZP, Song QT, Zhou MT and Zhang QY: Inhibitory effects of
tanshinone II-a on invasion and metastasis of human colon carcinoma
cells. Acta Pharmacol Sin. 30:1537–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Su CC: Tanshinone IIA potentiates the
efficacy of 5-FU in colo205 colon cancer cells in vivo through
downregulation of P-gp and LC3-II. Exp Ther Med. 3:555–559.
2012.PubMed/NCBI
|
|
47
|
Hernandez-Flores G, Ortiz-Lazareno PC,
Lerma-Diaz JM, Dominguez-Rodriguez JR, Jave-Suarez LF,
Aguilar-Lemarroy Adel C, de Celis-Carrillo R, del Toro-Arreola S,
Castellanos-Esparza YC and Bravo-Cuellar A: Pentoxifylline
sensitizes human cervical tumor cells to cisplatin-induced
apoptosis by suppressing nf-kappa B and decreased cell senescence.
BMC Cancer. 11:4832011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu T, Liu D, Liu J, Song JT, Gao SL, Li
H, Hu LH and Liu BR: Effect of NF-κB inhibitors on the
chemotherapy-induced apoptosis of the colon cancer cell line HT-29.
Exp Ther Med. 4:716–722. 2012.PubMed/NCBI
|
|
49
|
Wang S, Liu Z, Wang L and Zhang X:
NF-kappaB signaling pathway, inflammation and colorectal cancer.
Cell Mol Immunol. 6:327–334. 2009. View Article : Google Scholar : PubMed/NCBI
|