Introduction
Acute pancreatitis (AP) is an acute disease of the
abdomen, which ranges from a mild, transient illness to a fatal
disease with a mortality rate of ~30%. In addition, 25–30% of
patients with severe AP (SAP) succumb to multiple organ system
failure and pulmonary complications (1–4), which
occur as a result of excessive inflammatory responses to pulmonary
and extra-pulmonary stimuli, including pneumonia, acid aspiration,
ischemia-reperfusion and sepsis (5).
Resulting from pronounced systemic inflammatory response with
increased endothelial and epithelial barrier permeability, gas
exchange and oxygenation are compromised with leakage of a
protein-rich exudate into the alveolar space and interstitial
tissues (6). Excessive infiltration
of polymorphonuclear leukocytes (PMNs) into the lungs has been
identified as a key event in the development of acute lung injury
(ALI) (7), and pulmonary
microvascular endothelial cells (PMVECs) have been associated with
the pathogenesis of ALI. Major events in the inflammatory response
include the migration of leukocytes from the blood, their adhesion
to the vascular endothelium, and their transmigration across the
endothelium. Various types of adhesion molecules on the surface of
leukocytes and endothelium have previously been associated with the
cell adhesion process (8,9). Intercellular cell adhesion molecule-1
(ICAM-1), which is a well-characterized adhesion molecule expressed
in PMVECs, can be stimulated by proinflammatory cytokines,
including tumor necrosis factor (TNF)-α, interleukin (IL)-6 and
interferon-γ (10,11), which act via signaling pathways,
including phosphoinositide 3-kinase, mitogen-activated protein
kinases (MAPKs), and the nuclear factor (NF)-κB pathway (12,13).
The Janus kinase/signal transducer and activator of
transcription (JAK/STAT) pathway (14) has been widely researched for its role
in tumorigenesis. IL-6, which is a proinflammatory cytokine that
preferentially activates STAT3, has been well recognized for its
role in initiating and amplifying inflammatory processes. During
inflammation, the adhesion molecules expressed on stimulated
endothelial cells (ECs) are essential for the recruitment and
transmigration of leukocytes into the subendothelial matrix
(15). The induction of ICAM-1 has
previously been associated with leukocyte adhesion and
transmigration, resulting in damage to ECs and amplification of
inflammatory responses. In addition, it has previously been
reported that the effects of cholinergic agonists on IL-6-mediated
ICAM-1 expression, and monocyte chemoattractant protein (MCP)-1
production by ECs, may be mediated via the JAK2/STAT3 signaling
pathway (16). Dexamethasone, a
synthetic adrenocortical steroid, is increasingly used in the
treatment of SAP (17).
Dexamethasone exerts anti-inflammatory, anti-toxicity and
anti-inflammatory effects in patients with SAP, in addition to
reducing the systemic inflammatory response to liver and lung
damage.
In the present study, the expression levels of
ICAM-1 in the pulmonary tissues of a rat model of SAP-ALI were
investigated, in order to elucidate the underlying molecular
mechanisms. ICAM-1 expression was shown to be induced by SAP via
the JAK2/STAT3 signaling pathway, but could be suppressed by
treatment with dexamethasone (DEX). In addition, DEX treatment was
able to attenuate the inflammatory responses in order to protect
the lungs against SAP-ALI.
Materials and methods
Rats
Male Sprague Dawley rats (body weight, 170–220 g)
were purchased from the Specific Pathogen Free Animal Laboratory of
Dalian Medical University (Dalian, China), and were housed in a
controlled environment. Prior to the experiments, the rats were
deprived of food; however, drinking water was available ad
libitum. The rats were anesthetized using intraperitoneal
administration of 10% chloral hydrate (3 ml/kg; Baier Di
Biotechnology Co., Ltd., Beijing, China), and the bile-pancreatic
duct was exposed. A rat model of SAP was induced via a standardized
pressure-controlled retrograde infusion of 1.5% deoxycholic acid
sodium salt (1 mg/kg; Baier Di Biotechnology Co., Ltd.) into the
bile-pancreatic duct, after which the bile-pancreatic duct near the
margin of the liver and duodenum was compressed with a clip for 5
min. Subsequently, the clip was removed and the abdomen was closed.
All rats received humane care in compliance with the Public Health
Service Policy on Humane Care, and use of the rats was approved by
the Institutional Animal Ethics Committee of Dalian Medical
University (Dalian, China).
Experimental groups
A total of 30 male Sprague Dawley rats were randomly
divided into three groups, as follows (n=10/group): i) Control
(CON) group, in which the abdomens of the rats were opened, the
pancreas was turned over and the abdomen was closed; ii) SAP group,
in which SAP was induced via retrograde infusion of 1.5%
deoxycholic acid sodium salts into the bile-pancreatic duct; and
iii) DEX group, in which SAP was induced and the rats were
subsequently treated with 1 ml/kg DEX (Henan Lingrui Pharmaceutical
Co., Ltd., Zhengzhou, China), which was administered via the vena
sublingualis, 5 min post-surgery. All rats were examined 24 h
post-surgery. The rats were drained of blood and lung tissue
samples were collected and stored at −80°C for subsequent
analysis.
Determination of ICAM-1, JAK2, STAT3
and NF-κB mRNA expression levels
Total cellular RNA was extracted from the cells 24 h
post-surgery using RNAiso Plus (Takara Bio, Inc., Otsu, Japan)
according to the manufacturer's protocol. The mRNA expression
levels of ICAM-1, JAK2, STAT3 and NF-κB were quantified using
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), in which the Maxima SYBR-Green/ROX qPCR Master Mix (2X)
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used. RNA
was solubilized in ribonuclease-free water and quantified by
measuring the absorbance at 260 nm using an Ultrospec 2100 Pro
spectrophotometer (GE Healthcare, Buckinghamshire, UK). The
concentration was 220–280 ng/µl [the purity of RNA was confirmed by
examining the optical density (OD) 260/280 as 1.6/1.9]. cDNA was
synthesized from 1 µl RNA using the Bio-Rad iScript™ cDNA Synthesis
kit (Bio-Rad Laboratories, Inc. Hercules, CA, USA). Amplification
and detection of 500 ng cDNA was conducted using the Rotor Gene
3000™ sequence detection system (Qiagen, Inc., Valencia, CA,
USA).
The primers and probes (Takara Bio, Dalian, China)
used were as follows: ICAM-1 forward, 5′-GCTTCTGCCACCATCACTGTGTA-3′
and reverse, 5′-ATGAGGTTCTTGCCCACCTG-3′; JAK2 forward,
5′-TTTGAAGACAGGGACCCTACACAG −3′ and reverse,
5′-TCATAGCGGCACATCTCCACA −3′; STAT3 forward,
5′-CACCCATAGTGAGCCCTTGGA −3′ and reverse,
5′-TGAGTGCAGTGACCAGGACAGA-3′; NF-κB forward,
5′-GATGGGACGACACCTCTACACATA−3′ and reverse,
5′-CCCAAGAGTCGTCCAGGTCA-3′; and β-actin forward,
5′-GGAGATTACTGCCCTGGCTCCTA-3′ and reverse,
5′-GACTCATCGTACTCCTGCTTGCTG-3′. β-actin was used as an internal
control. The PCR cycling conditions included 40 cycles of 95°C for
5 sec and 60°C for 31 sec, as per manufacturers protocol. This was
followed by an annealing step at 95°C for 15 sec followed by a
cycle at 60°C for 60 sec and a final step at 95°C for 15 sec. For
relative quantification, the copy ratios of ICAM-1/β-actin,
JAK2/β-actin, STAT3/β-actin and NF-κB/β-actin, were calculated and
used as an indication of the relative expression levels.
Western blot analysis
Frozen lung tissue was mechanically homogenized in 1
ml ice-cold extraction buffer, containing 50 mmol/l Tris-His (pH
7.4), 1% NP-40.0, 25% sodium deoxycholate, 150 mmol/l NaCl, 1
mmol/l ethylene diamine tetraacetic acid, 1 mmol/l
phenylmethylsulfonyl fluoride, 0.1% sodium dodecylsulfate, and 1
µg/ml each of aprotinin and leupeptin. Following incubation on ice
for 30 min, the homogenate was centrifuged at 12,000 × g for 10 min
at 4°C, after which the supernatant was stored at −80°C prior to
analysis. The protein concentration was determined using the
Bradford Protein Assay (Thermo Fisher Scientific, Inc.), with
bovine serum albumin as a standard. Equal volumes of each sample
(10 µg) were separated by 12% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis, and transferred to a polyvinylidene difluoride
membrane (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China).
Subsequently, the membrane was incubated with 200 µg/ml goat
polyclonal anti-rat ICAM-1 antibodies (1:500; sc-1511; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C, prior to
washing with Tris-buffered saline (TBS) containing 0.05% Tween-20.
The membrane was then incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rat (500 µg/ml; 1:10,000; ab6721; Abcam,
Cambridge, UK) for 1 h at room temperature. The antibody-antigen
complexes were detected using an enhanced chemiluminescence reagent
(Immobilon Western HRP Substrate; WBKLS0100; EMD Millipore,
Billerica, MA, USA), and exposed to X-OMAT BT Film (Eastman Kodak,
Rochester, NY, USA). Films were scanned on a UniScan C800 (Tsinghua
UniSplendour Co., Ltd., Beijing, China) using Photoshop software
(Adobe Systems, Inc., San Jose, CA, USA), and the OD of each band
was determined using Gel-Pro Analyzer 4.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Enzyme-linked immunosorbent assay
(ELISA)
An ELISA was performed in order to quantify the
concentrations of IL-6, IL-8 and TNF-α in the serum samples.
Commercially available kits were used (R&D Systems, Inc.,
Minneapolis, MN, USA), following the manufacturers
instructions.
Immunohistochemical staining and
immunofluorescence
The rat lungs were infused with 10% buffered
formalin for 24 h and embedded in paraffin. Subsequently, 5 µm
tissue sections were prepared, and continuous sections of the
paraffin-embedded tissue were taken for pathological examination
using hematoxylin-eosin staining (G1120–100; Beijing Solarbio
Science & Technology Co., Ltd. Beijing, China). After
deparaffinization, the tissue sections were treated with sodium
citrate and boiled for 2 min in a pressure cooker for restoration.
After washing three times with phosphate-buffered saline (PBS), the
tissue sections were incubated for 10 min with 3% hydrogen peroxide
at room temperature. Subsequently, the lung tissue sections were
incubated for 1 h with rabbit anti-rat JAK2 (1:50; sc-294) and
STAT3 (1:50; sc-482) antibodies (Santa Cruz Biotechnology, Inc.) at
room temperature. After washing with PBS, the tissue sections were
incubated for 30 min at room temperature with
poly-peroxidase-conjugated anti-rabbit immunoglobulin G.
Diaminobenzidine (Baier Di Biotechnology Co., Ltd.) was used as a
substrate for the immunoperoxidase reaction. After rinsing with
water, the tissue sections were stained with hematoxylin and
incubated with goat anti-rat ICAM-1 and NF-κB antibodies for 1 h.
Imunofluorescence was detected following incubation of the tissue
sections with 4′,6-diamidino-2-phenylindole for 10 min. The tissue
samples were observed using an inverted fluorescence microscope
(Leica Microsystems, Mannheim, Germany). Imaging was conducted
using the Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.).
Statistical analysis
The target protein and β-actin band intensities were
analyzed using ImageJ software, version 1.35d (National Institutes
of Health, Bethesda, MD, USA). The target protein/β-actin groups
were then compared with the control/β-actin group, statistically
analyzed with SPSS software, version 16.0 (SPSS, Inc., Chicago, IL,
USA). Data are presented as the mean ± standard deviation. Groups
were compared using one-way analysis of variance and the Student
Newman-Keuls method. P<0.05 was considered to indicate a
statistically significant difference.
Results
ICAM-1 and NF-κB expression levels are
increased following SAP induction and decreased following DEX
treatment
In order to assess the role of ICAM-1 in SAP-ALI,
the effects of SAP induction followed by DEX treatment on the mRNA
and protein expression levels of ICAM-1 and NF-κB were investigated
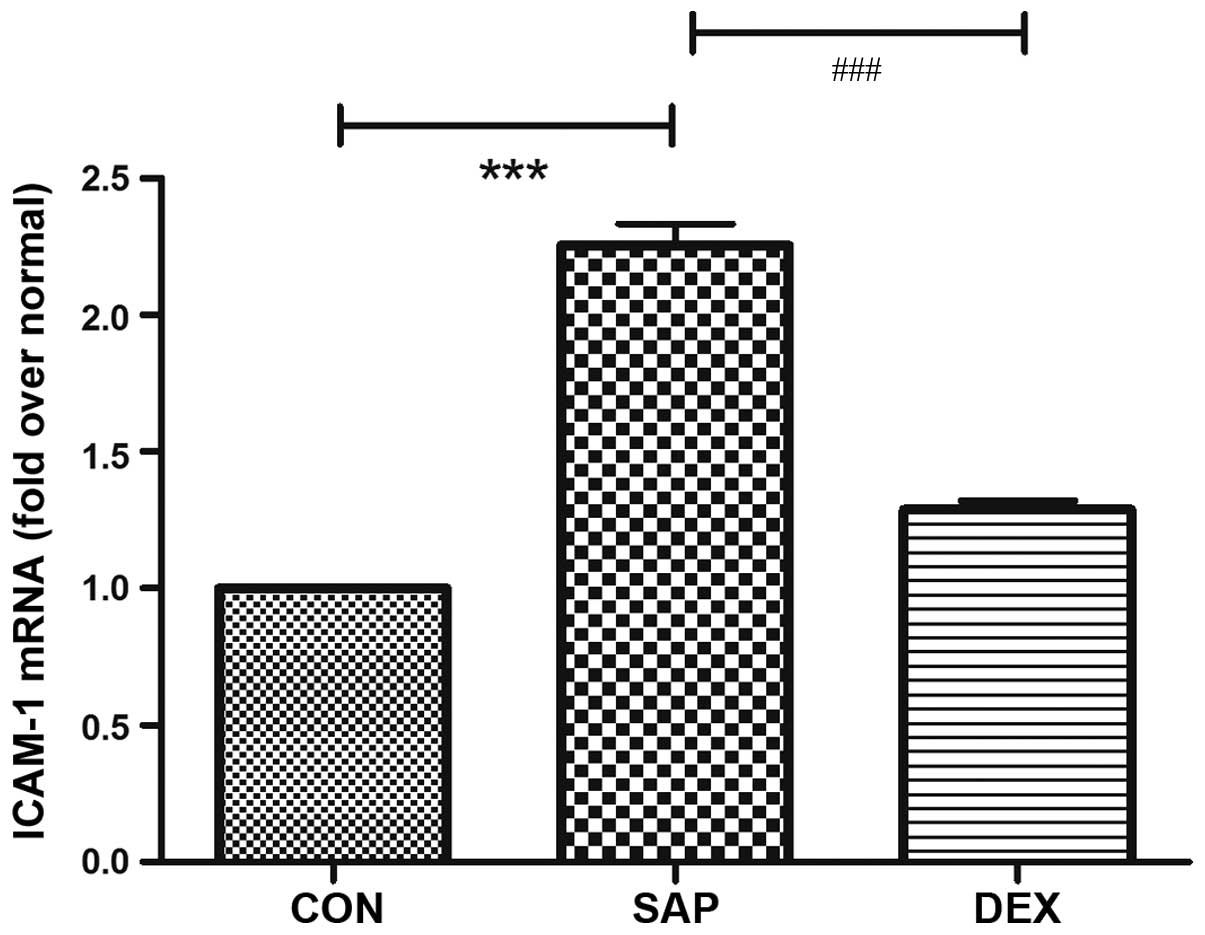
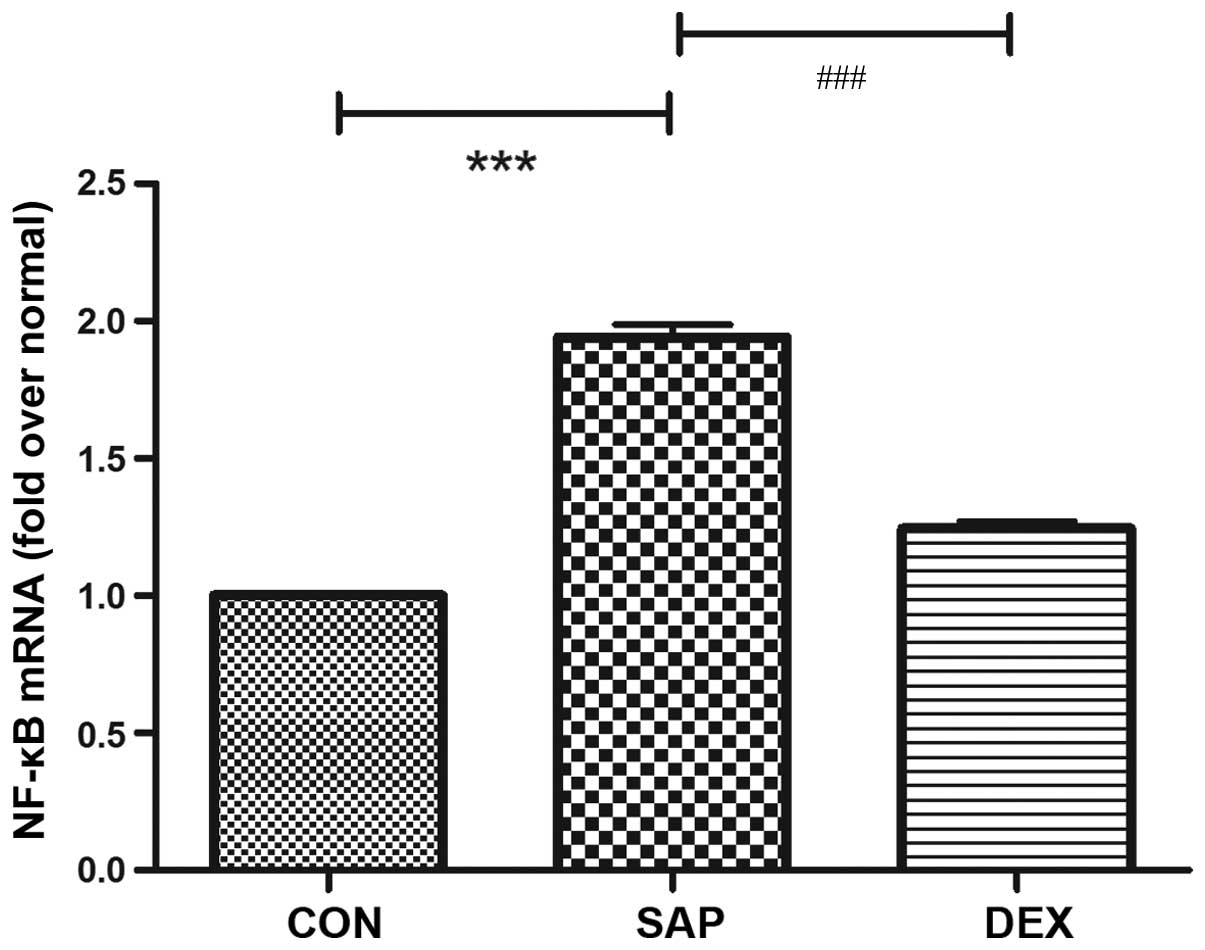
in a rat model of SAP-ALI. ICAM-1 and NF-κB mRNA expression levels
in the lung tissue sections from the CON group at 24 h following
SAP injury were used as a baseline. Similar alterations in mRNA
expression levels were detected for ICAM-1 and NF-κB in the SAP
group (Figs. 1 and 2), and were characterized by a significant
increase following SAP induction (P<0.01; Figs. 1 and 2). DEX treatment significantly decreased
the mRNA expression levels of ICAM-1 and NF-κB in the lung tissue
post-injury, as compared with those in the SAP group (P<0.01;
Figs. 1 and 2).
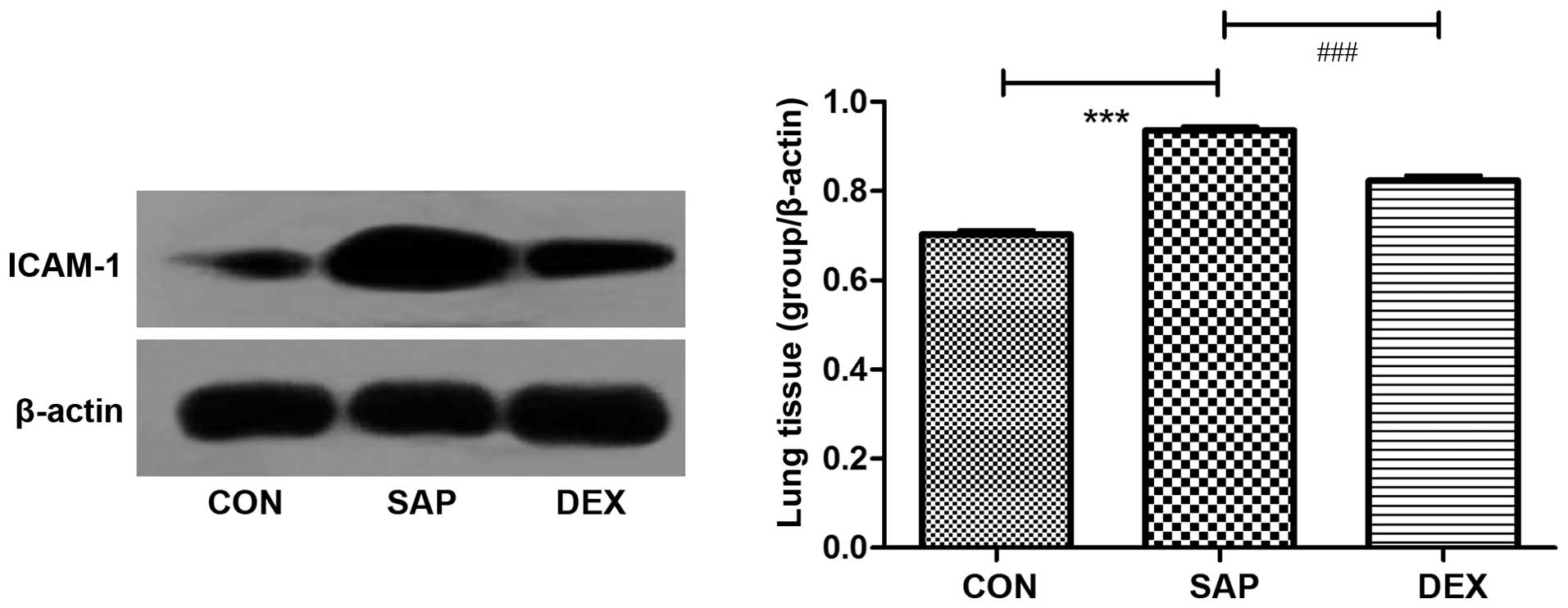
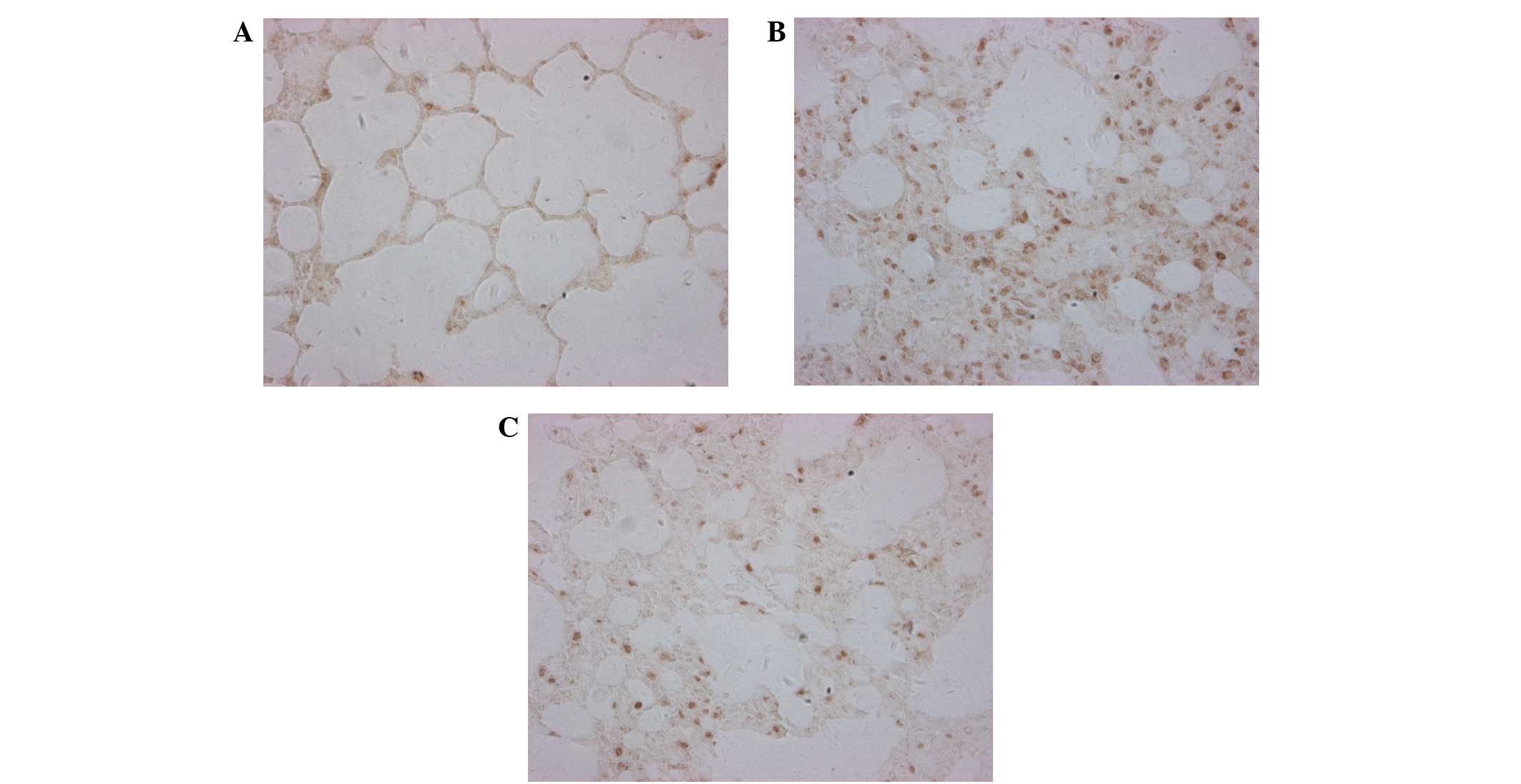
The protein expression levels of ICAM-1 in the lung
tissue of the rats were analyzed by western blotting and
immunofluorescence. Western blotting demonstrated that ICAM-1
protein expression levels were significantly increased in the SAP
group, as compared with the CON group (P<0.01; Fig. 3). Conversely, ICAM-1 protein
expression levels were significantly decreased in the DEX-treated
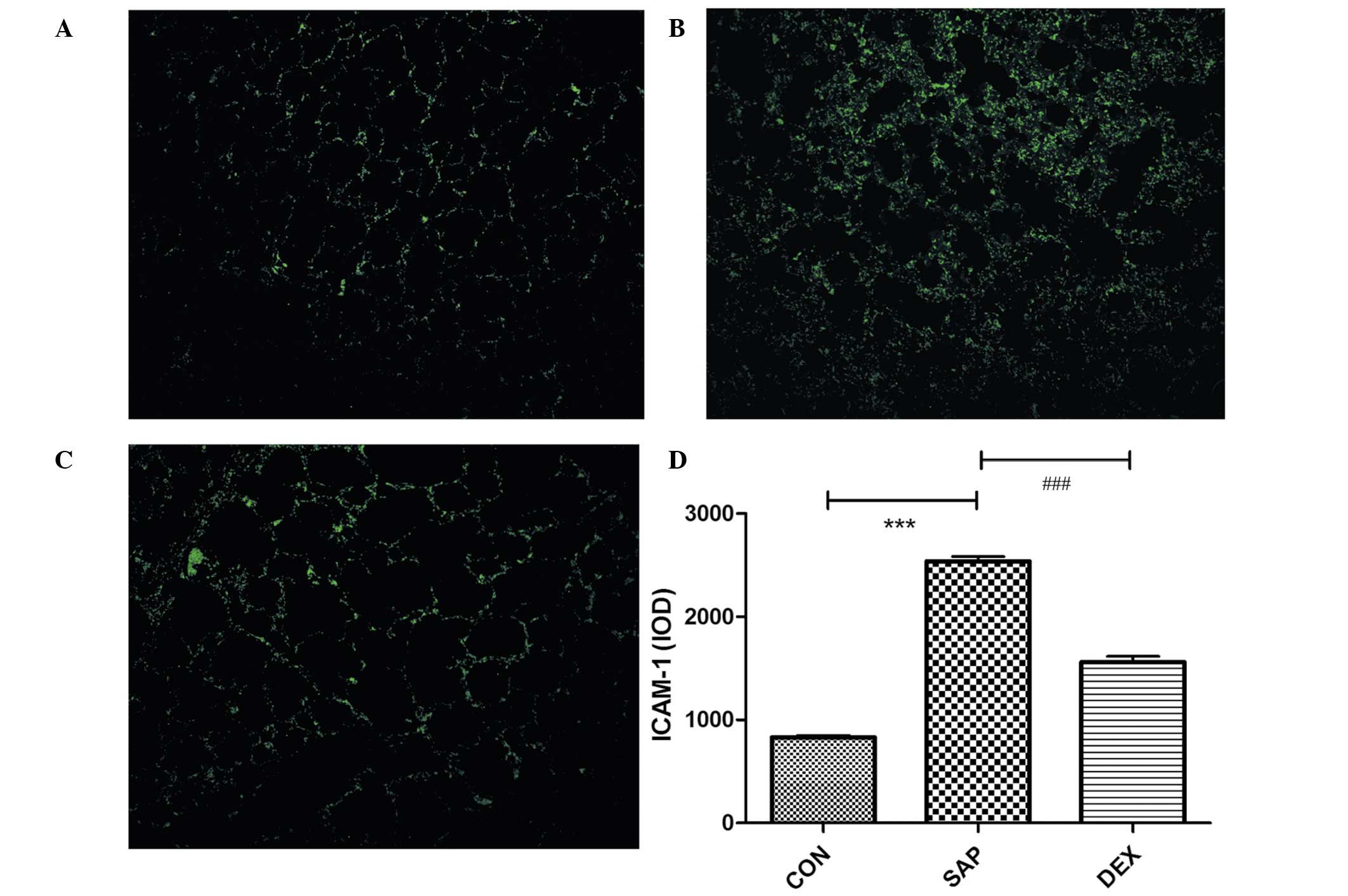
group, as compared with the SAP group (P<0.01; Fig. 3). Low levels of ICAM-1 were detected
on the vascular endothelial and bronchiolar and alveolar epithelial
surfaces of the CON group using immunofluorescence (Fig. 4A), and these were significantly
increased in the SAP group at 24 h following SAP induction
(P<0.01; Fig. 4B). Conversely, in
the rats treated with DEX, ICAM-1 levels were significantly
decreased, as compared with those in the SAP group (P<0.01;
Fig. 4C). These results suggest that
DEX is able to decrease the SAP-induced expression of ICAM-1
(Fig. 4D).
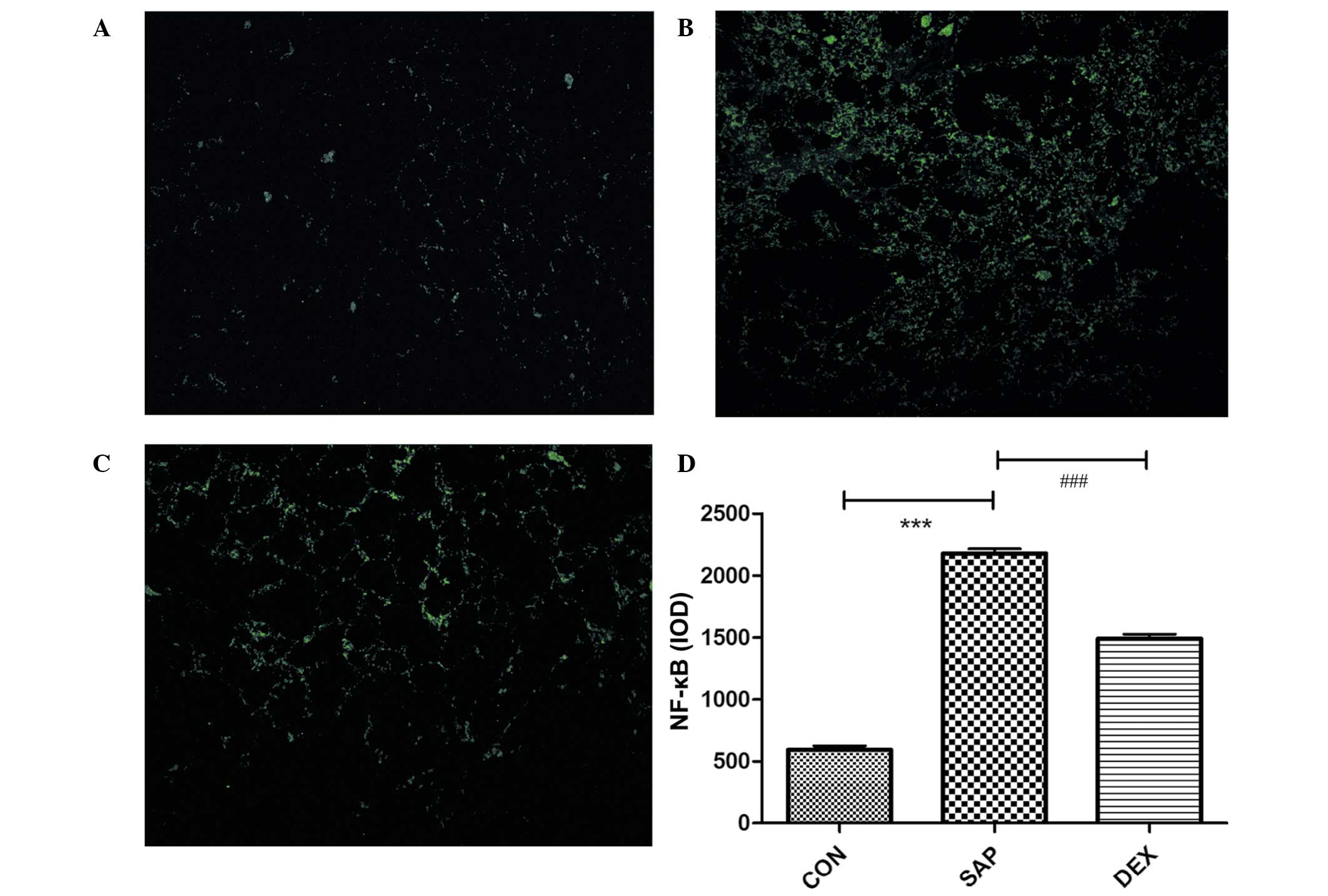
The protein expression levels of NF-κB were analyzed
using immunofluorescence, which demonstrated that the levels of
NF-κB were significantly increased in the SAP group, as compared
with the CON group (P<0.01; Fig.
5). Conversely, NF-κB protein expression levels were
significantly decreased in the DEX-treated group, as compared with
the SAP group (P<0.01; Fig. 5).
These results suggest that DEX is able to decrease the SAP-induced
upregulation of NF-κB.
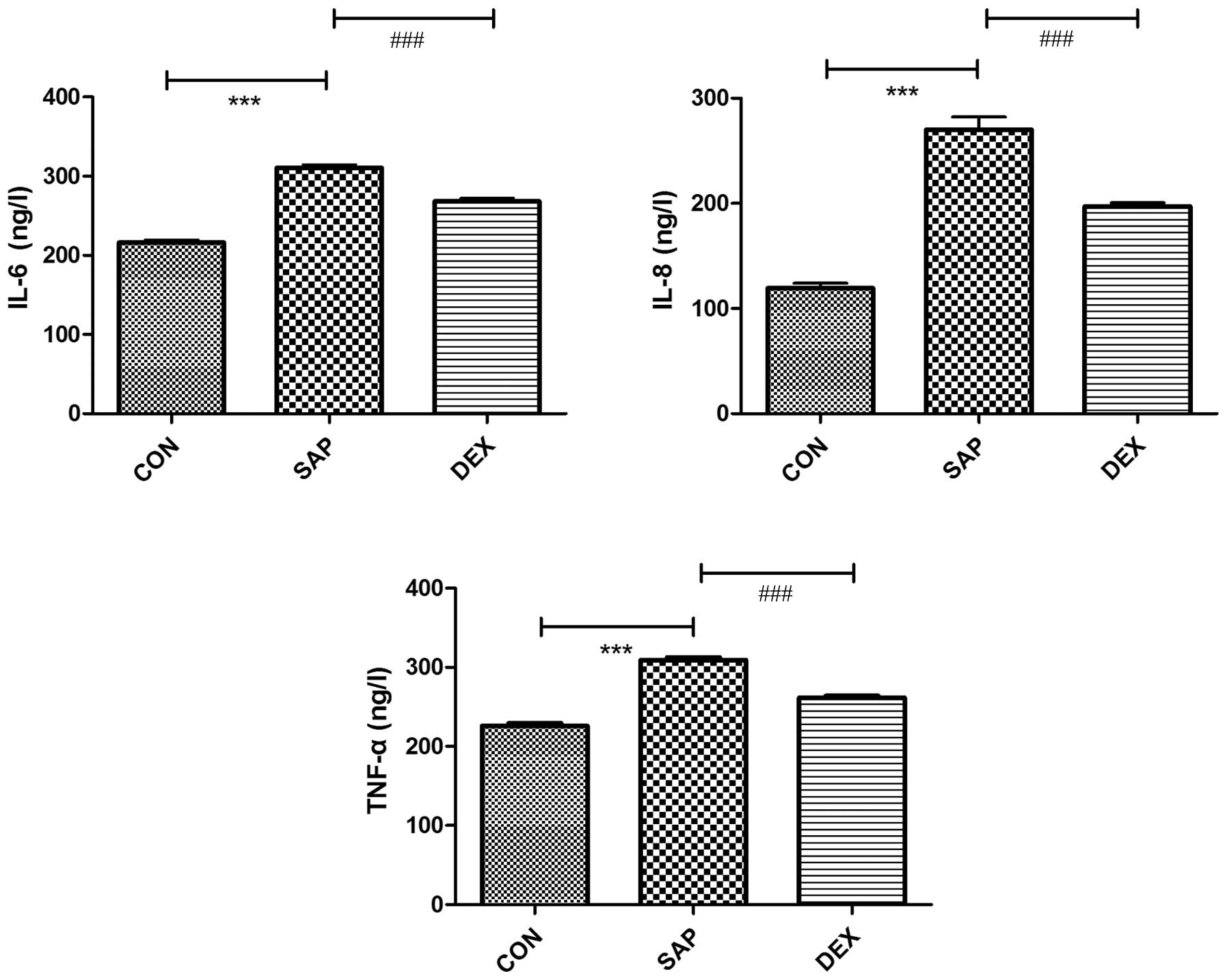
IL-6, IL-8 and TNF-α concentrations
increase following SAP induction
The concentrations of IL-6, IL-8 and TNF-α in plasma
were detected using an ELISA. In the CON group, the concentrations
of IL-6, IL-8 and TNF-α were 216.06±11.04, 119.28±15.2 and
225.97±10.56 ng/l, respectively. The concentrations of the
cytokines were significantly increased following SAP induction to
310.47±12.28, 270.06±38.36 and 309.16±10.62 ng/l, respectively
(P<0.01; Fig. 6). Treatment with
DEX significantly inhibited the SAP-induced production of IL-6,
IL-8 and TNF-α (P<0.01; Fig.
6).
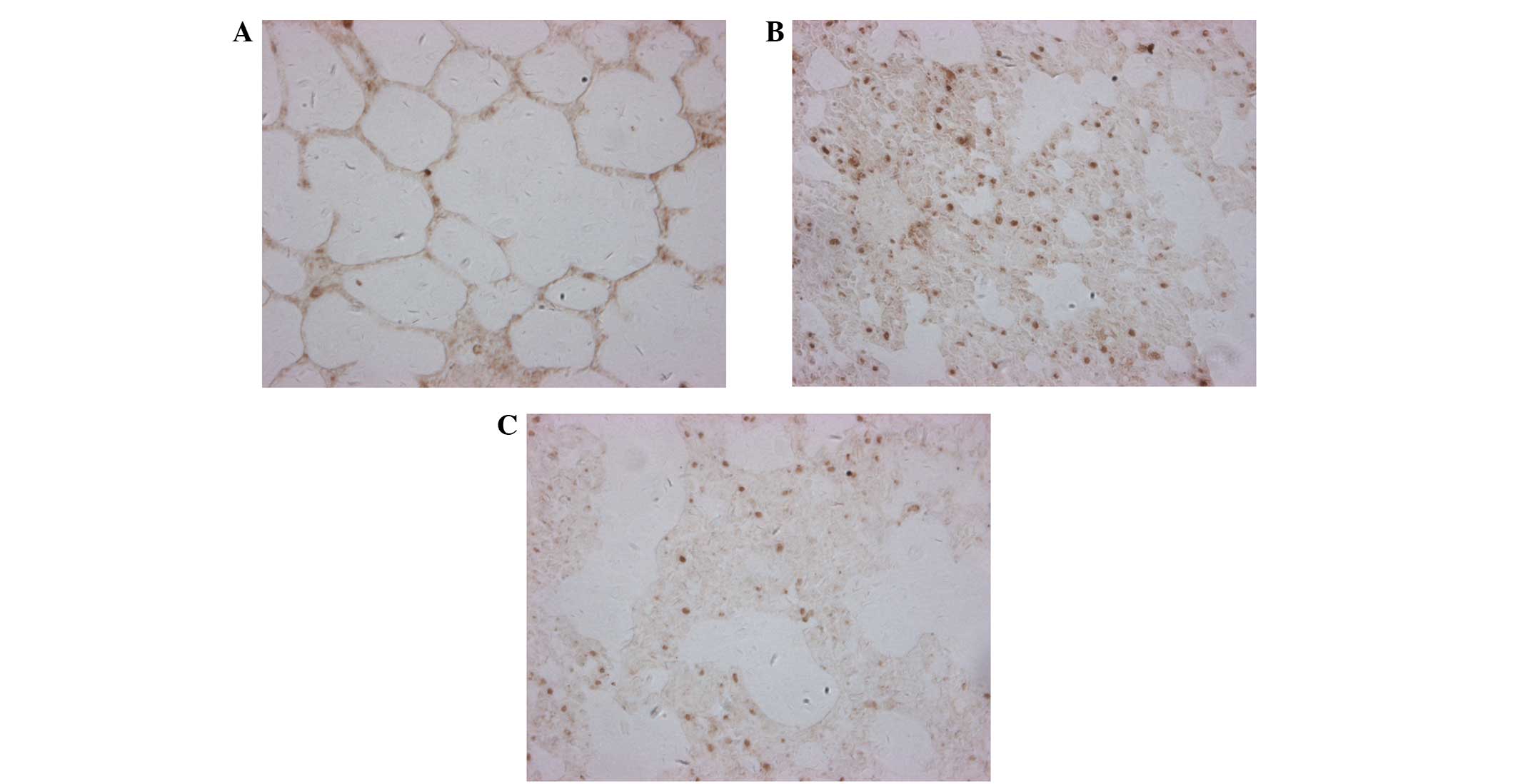
Analysis of the JAK2/STAT3 signaling
pathway in lung tissue
A previous study demonstrated that IL-6-induced
MCP-1 production by HUVECs was mediated by the JAK2/STAT3 pathway
(15). In order to evaluate the role
of this signaling cascade in SAP-ALI-induced ICAM-1 expression, the
protein expression levels of JAK2 and STAT3 were analyzed by
immunohistochemistry. The protein expression levels of JAK2 and
STAT3 in the lung tissue sections were significantly upregulated in
the SAP group, as compared with the CON group (P<0.01; Figs. 7 and 8). Conversely, JAK2 and STAT3 protein
expression levels were significantly decreased in the DEX group, as
compared with the SAP group (P<0.01; Figs. 7 and 8). These results suggest that the
JAK2/STAT3 signaling pathway may upregulate ICAM-1 expression in
response to SAP injury, and that DEX treatment is able to attenuate
JAK2/STAT3 signaling.
Analysis of lung tissue
morphology
ICAM-1 staining was increased in the lung tissue of
the SAP group, as compared with the CON group (Fig. 4). The rats in the SAP group exhibited
expanded and congested minute pulmonary vessels, and alveolar
septum capillaries. In addition, the alveolar walls had burst, the
alveolar space was narrowed, partial alveoli were damaged and the
alveolar septum was broadened. Furthermore, there was marked
infiltration of PMN cells into the alveoli and pulmonary
interstitial tissue of the SAP rats, and this was associated with
hemorrhage and pulmonary interstitial edema. Therefore, increased
ICAM-1 staining was an indication of a severe injury. DEX treatment
markedly reduced the severity of the SAP-induced pulmonary
histopathological injury, as demonstrated by decreased protein
expression levels of JAK2 and STAT3 in the DEX-treated rats, as
compared with the SAP group. However, the role of the JAK2/STAT3
signaling pathway in SAP-induced upregulation of ICAM-1, IL6 and
TNF-α, should be investigated in future studies.
Discussion
Infiltration of monocytes and macrophages into local
areas of inflammation in pulmonary tissues is a key event in
SAP-ALI, and ICAM-1 has previously been shown to have an important
role in mediating the inflammatory response (18). A previous study reported protective
effects for angiopoietin-like protein-4 (Angptl4) in PMVECs in a
rat model of acute inflammatory stroke (19). The present study demonstrated that
the mRNA and protein expression levels of ICAM-1 were upregulated
in the lung tissue of a rat model of SAP-ALI, and that this could
be attenuated by treatment with DEX.
ICAM-1 (CD54) is an inducible surface glycoprotein
with a molecular weight of 80–114 kDa, and is a member of the Ig
superfamily, which consists of five Ig-like domains, a hydrophobic
transmembrane domain and a short cytoplasmic C-terminal domain
(20). Under stable conditions,
ICAM-1 is expressed at low levels in endothelial and epithelial
cells, or constitutively on the surface of alveolar cells, where it
is involved in cell recognition, activation, proliferation,
differentiation and motility, thereby helping to stabilize the
internal environment of the body (10). During inflammation, ICAM-1 binds to
two integrins, CD11a/CD18 (LFA-1) and CD11b/CD18 (Mac-1), which are
members of the β2 subfamily. LFA-1 and Mac-1 are expressed by
leukocytes and promote the adhesion and transendothelial migration
of these cells (20,21). In addition, ICAM-1 has a key role in
pathological events associated with inflammatory reactions,
including acute renal failure and acute pancreatitis (22).
Upregulation of ICAM-1 expression in the lungs
during ALI, which is an often fatal complication of SAP, has
previously been associated with leukocyte adhesion and activation,
as well as induction of the ‘cascade effect’ of inflammatory
mediators, pulmonary microcirculation dysfunction, acute
respiratory distress syndrome (ARDS), multiple organ failure, and
mortality (7,23). TNF-α has previously been shown to
activate ICAM-1 expression via a NF-κB cis-element located in the
5′-flanking region of the ICAM-1 gene (24,25).
NF-κB is a proinflammatory transcription factor that triggers
inflammatory cascades during inflammatory responses, and NF-κB
activation has been shown to regulate the expression of numerous
genes encoding proinflammatory cytokines, chemokines, adhesion
molecules and inducible enzymes (26,27). The
results of the present study suggested that high levels of TNF-α
and NF-κB may contribute to the development of SAP-ALI via
induction of ICAM-1 expression.
ECs under inflammatory stimulation produce
cytokines, chemokines and cell adhesion molecules, which
participate in various biological processes, including vascular
remodeling and atherosclerosis (28); and therefore suppressing the release
of these mediators is important for controlling inflammation. The
present study demonstrated that the SAP-induced upregulation of the
inflammation-associated proteins ICAM-1 and NF-κB in the lung
tissue could be suppressed by DEX treatment, which is a widely used
steroid that has previously been shown to exert anti-inflammatory
and immunosuppressant effects (29).
Serum ICAM-1 is an early diagnostic and predictive marker of SAP
(30). Therefore, ICAM-1 may be
considered a key target for potential attenuation of inflammation
in patients with SAP-ALI.
The mechanism underlying SAP-induced ALI is
currently unknown. Previous studies investigating MAPK signaling
pathways have greatly enriched the current understanding of the
molecular mechanisms underlying various biological stimuli,
including inflammatory mediators (31–33).
Wang et al (19) demonstrated
that, in rat PMVECs treated with lipopolysaccharides,
overexpression of Angptl4 or rosiglitazone treatment inhibited the
Raf/[MAPK/extracellular signal-regulated kinase (ERK)]/MAPK cascade
and was able to protect against increased permeability induced by
F-actin depolymerization. In addition, phosphorylated-AKT/AKT
downregulation was shown to inactivate phosphorylation of NF-κB,
inhibiting the downstream production of inflammatory cytokines,
including TNF-α (19,34). Conversely, the present study
demonstrated that JAK2/STAT3 levels were upregulated in a SAP-ALI
rat model, and were decreased following treatment with DEX.
Previous studies have demonstrated a role for DEX in reducing STAT3
expression levels (35). In the
present study, JAK2 and STAT3 upregulation was associated with
inflammation in a rat model of SAP-ALI, and may have also been
involved in cellular responses to various cytokines, growth factors
and hormones. Further experiments are required in order to explore
the network of MAPK/ERK/JAK signaling that may contribute to the
SAP-ALI-associated inflammatory response.
ALI/ARDS is an inflammatory injury of the lungs that
is predominantly characterized by PMN cell infiltration (36). It has previously been reported that
the flavonols, luteolin, apigenin and quercetin, were able to
inhibit ICAM-1 mRNA and protein expression by inactivating NF-κB in
TNF-α-stimulated HUVECs (37).
Furthermore, it has been demonstrated that 2′-hydroxychalcone, an
achalcone derivative, was able to attenuate TNF-α-induced ICAM-1
expression via NF-κB inactivation, leading to reduced adhesion of
neutrophils to HUVECs (38). In the
present study, SAP-induced TNF-α and NF-κB upregulation was shown
to be attenuated by DEX treatment; however, a previous study
suggested that DEX treatment was unable to entirely attenuate
leukocyte recruitment during sodium taurocholate-induced acute
pancreatitis (39). DEX treatment
may attenuate cytokine production via inhibition of ICAM-1; a
possible explanation for its anti-inflammatory effect. However,
this does not fully explain the ability of DEX to inhibit the
production of cytokines and inflammatory factors; thus suggesting
that further research is required.
In conclusion, the present study demonstrated that
in vivo activation of JAK2/STAT3 signaling may be involved
in the pathogenesis of SAP-ALI. These results suggested that ICAM-1
and JAK/STAT signaling intermediates, as well as components of
other acute inflammatory signaling pathways, including the MAPK
pathway, may be important targets for intervention in the treatment
of patients with SAP-ALI. The present study demonstrated that
downregulation of the ICAM-1-mediated JAK2/STAT3 signaling cascade
was able to attenuate inflammatory responses that promote leukocyte
trafficking during inflammation. Further elucidation of the
molecular mechanisms underlying SAP-ALI will be crucial for the
development of novel treatments in the future.
Acknowledgements
The present study was supported by The National
Natural Science Foundation of China (grant no. 81173452). The
present study was conducted at the Laboratory of the First
Affiliated Hospital of Dalian Medical University and Dalian Center
Hospital (Dalian, China). The authors would like to thank Dr Hao
Zhang for assistance with editing.
References
|
1
|
Chen C, Xu S, Wang WX, Ding YM, Yu KH,
Wang B and Chen XY: Rosiglitazone attenuates the severity of sodium
taurocholate-induced acute pancreatitis and pancreatitis-associated
lung injury. Arch Med Res. 40:79–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lund H, Tønnesen H, Tønnesen MH and Olsen
O: Long-term recurrence and death rates after acute pancreatitis.
Scand J Gastroenterol. 41:234–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Renzulli P, Jakob SM, Täuber M, Candinas D
and Gloor B: Severe acute pancreatitis: Case-oriented discussion of
interdisciplinary management. Pancreatology. 5:145–156. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Williams M and Simms HH: Prognostic
usefulness of scoring systems in critically ill patients with
severe acute pancreatitis. Crit Care Med. 27:901–907. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Storme L, Aubry E, Rakza T, Houeijeh A,
Debarge V, Tourneux P, Deruelle P and Pennaforte T: French
Congenital Diaphragmatic Hernia Study Group: Pathophysiology of
persistent pulmonary hypertension of the newborn: Impact of the
perinatal environment. Arch Cardiovasc Dis. 106:169–177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shields CJ, Winter DC and Redmond HP: Lung
injury in acute pancreatitis: Mechanisms, prevention and therapy.
Curr Opin Crit Care. 8:158–163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan Q, Jiang YW, Ma TT, Fang QH and Pan
L: Attenuating effect of Ginsenoside Rb1 on LPS-induced lung injury
in rats. J Inflamm (Lond). 11:402014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rao RM, Yang L, Garcia-Cardena G and
Luscinskas FW: Endothelial-dependent mechanisms of leukocyte
recruitment to the vascular wall. Circ Res. 101:234–247. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams MR, Azcutia V, Newton G, Alcaide
P and Luscinskas FW: Emerging mechanisms of neutrophil recruitment
across endothelium. Trends Immunol. 32:461–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meager A: Cytokine regulation of cellular
adhesion molecule expression in inflammation. Cytokine Growth
Factor Rev. 10:27–39. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roebuck KA and Finnegan A: Regulation of
intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc
Biol. 66:876–888. 1999.PubMed/NCBI
|
|
12
|
Minhajuddin M, Bijli KM, Fazal F, Sassano
A, Nakayama KI, Hay N, Platanias LC and Rahman A: Protein kinase
C-delta and phosphatidylinositol 3-kinase/Akt activate mammalian
target of rapamycin to modulate NF-kappaB activation and
intercellular adhesion molecule-1 (ICAM-1) expression in
endothelial cells. J Biol Chem. 284:4052–4061. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amin MA, Haas CS, Zhu K, Mansfield PJ, Kim
MJ, Lackowski NP and Koch AE: Migration inhibitory factor
up-regulates vascular cell adhesion molecule-1 and intercellular
adhesion molecule-1 via Src, PI3 kinase, and NFkappaB. Blood.
107:2252–2261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boengler K, Hilfiker-Kleiner D, Drexler H,
Heusch G and Schulz R: The myocardial JAK/STAT pathway: From
protection to failure. Pharmacol Ther. 120:172–185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SC, Chang YL, Wang DL and Cheng JJ:
Herbal remedy magnolol suppresses IL-6-induced STAT3 activation and
gene expression in endothelial cells. Br J Pharmacol. 148:226–232.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chatterjee PK, Al-Abed Y, Sherry B and
Metz CN: Cholinergic agonists regulate JAK2/STAT3 signaling to
suppress endothelial cell activation. Am J Physiol Cell Physiol.
297:C1294–C1306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong LH, Liu ZM, Wang SJ, Zhao SJ, Zhang
D, Chen Y and Wang YS: Corticosteroid therapy for severe acute
pancreatitis: A meta-analysis of randomized, controlled trials. Int
J Clin Exp Pathol. 8:7654–7660. 2015.PubMed/NCBI
|
|
18
|
Kojima R, Kawachi M and Ito M: Butein
suppresses ICAM-1 expression through the inhibition of IκBα and
c-Jun phosphorylation in TNF-α- and PMA-treated HUVECs. Int
Immunopharmacol. 24:267–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Chen H, Li H, Zhang J and Gao Y:
Effect of angiopoietin-like protein 4 on rat pulmonary
microvascular endothelial cells exposed to LPS. Int J Mol Med.
32:568–576. 2013.PubMed/NCBI
|
|
20
|
Staunton DE, Marlin SD, Stratowa C, Dustin
ML and Springer TA: Primary structure of ICAM-1 demonstrates
interaction between members of the immunoglobulin and integrin
supergene families. Cell. 52:925–933. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Diamond MS, Staunton DE, Marlin SD and
Springer TA: Binding of the integrin Mac-1 (CD11b/CD18) to the
third immunoglobulin-like domain of ICAM-1 (CD54) and its
regulation by glycosylation. Cell. 65:961–971. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang N, Luo M, Li R, Huang Y, Zhang R, Wu
Q, Wang F, Li Y and Yu X: Blockage of JAK/STAT signalling
attenuates renal ischaemia-reperfusion injury in rat. Nephrol Dial
Transplant. 23:91–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou S, Ding H, Lv Q, Yin X, Song J, Landén
NX and Fan H: Therapeutic effect of intravenous infusion of
perfluorocarbon emulsion on LPS-induced acute lung injury in rats.
PLoS One. 9:e878262014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Collins T, Read MA, Neish AS, Whitley MZ,
Thanos D and Maniatis T: Transcriptional regulation of endothelial
cell adhesion molecules: NF-kappa B and cytokine-inducible
enhancers. FASEB J. 9:899–909. 1995.PubMed/NCBI
|
|
25
|
Min JK, Kim YM, Kim SW, Kwon MC, Kong YY,
Hwang IK, Won MH, Rho J and Kwon YG: TNF-related activation-induced
cytokine enhances leukocyte adhesiveness: Induction of ICAM-1 and
VCAM-1 via TNF receptor-associated factor and protein kinase
C-dependent NF-kappaB activation in endothelial cells. J Immunol.
175:531–40. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li HL, Chen HL, Li H, Zhang KL, Chen XY,
Wang XW, Kong QY and Liu J: Regulatory effects of emodin on
NF-kappaB activation and inflammatory cytokine expression in RAW
264.7 macrophages. Int J Mol Med. 16:41–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu R, Kim CS, Kawada T, Kwon TW, Lim TH,
Kim YW and Kwon BS: Involvement of leukotactin-1, a novel CC
chemokine, in human atherosclerosis. Atherosclerosis. 174:35–42.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung J, Ko SH, Yoo Doy, Lee JY, Kim YJ,
Choi SM, Kang KK, Yoon HJ, Kim H, Youn J and Kim JM:
5,7-Dihydroxy-3,4,6-trimethoxyflavone inhibits intercellular
adhesion molecule 1 and vascular cell adhesion molecule 1 via the
Akt and nuclear factor-κB-dependent pathway, leading to suppression
of adhesion of monocytes and eosinophils to bronchial epithelial
cells. Immunology. 137:98–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu HH and Jiang LL: Serum inter-cellular
adhesion molecule 1 is an early marker of diagnosis and prediction
of severe acute pancreatitis. World J Gastroenterol. 18:2554–2560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brown MD and Sacks DB: Protein scaffolds
in MAP kinase signalling. Cell Signal. 21:462–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu L, Cai B, Zheng S, Liu X, Cai H and Li
H: Effect of emdoin on endoplasmic reticulum stress in rats with
severe acute pancreatitis. Inflammation. 36:1020–1029. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rani N, Bharti S, Bhatia J, Tomar A, Nag
TC, Ray R and Arya DS: Inhibition of TGF-β by a novel PPAR-Y
agonist, chrysin, salvages β-receptor stimulated myocardial injury
in rats through MAPKs-dependent mechanism. Nutr Metab (Lond).
12:112015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu F, Wang H, Li J, Liang J and Ma S:
Homoplantaginin modulates insulin sensitivity in endothelial cells
by inhibiting inflammation. Biol Pharm Bull. 35:1171–1177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu T, Fei Z, Gangavarapu KJ, Agbenowu S,
Bhushan A, Lai JC, Daniels CK and Cao S: Interleukin-6 and
JAK2/STAT3 signaling mediate the reversion of dexamethasone
resistance after dexamethasone withdrawal in 7TD1 multiple myeloma
cells. Leuk Res. 37:1322–1328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Castillo RL, Loza Carrasco R and
Romero-Dapueto C: Pathophysiological approaches of acute
respiratory distress syndrome: Novel bases for study of lung
injury. Open Respir Med J. 9:83–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi JS, Choi YJ, Park SH, Kang JS and
Kang YH: Flavones mitigate tumor necrosis factor-alpha-induced
adhesion molecule upregulation in cultured human endothelial cells:
Role of nuclear factor-kappa B. J Nutr. 134:1013–1019.
2004.PubMed/NCBI
|
|
38
|
Madan B, Batra S and Ghosh B:
2′-hydroxychalcone inhibits nuclear factor-kappaB and blocks tumor
necrosis factor-alpha- and lipopolysaccharide-induced adhesion of
neutrophils to human umbilical vein endothelial cells. Mol
Pharmacol. 58:526–534. 2000.PubMed/NCBI
|
|
39
|
Ramudo L, Yubero S, Manso MA,
Sanchez-Recio J, Weruaga E and De Dios I: Effects of dexamethasone
on intercellular adhesion molecule 1 expression and inflammatory
response in necrotizing acute pancreatitis in rats. Pancreas.
39:1057–1063. 2010. View Article : Google Scholar : PubMed/NCBI
|