Introduction
Pulmonary immaturity is a major cause of morbidity
and mortality in premature infants. For >30 years
glucocorticoids have been clinically administered to mothers at
risk of premature delivery in order to induce maturation of preterm
fetal lungs and prevent the development of respiratory distress
syndrome (1,2). However, glucocorticoids have been
reported to affect the stimulation of surfactant synthesis
(3,4), structural lung growth (5–9), and the
maturation of pulmonary epithelial cells and differentiation of
type 2 cells (10,11) during lung development.
Drake et al (12) have demonstrated the intergenerational
consequences of fetal programming in utero by exposure to
glucocorticoids in rats. Furthermore, increasing evidence in humans
suggests that prenatal overexposure to glucocorticoids may result
in adverse adult cardiovascular, metabolic, neuroendocrine and
behavioral phenotypes, and these effects appear to be transmitted
across generations (13). This
transmission across generations without further exposure to
glucocorticoids suggests an epigenetic mechanism (13). The authors of the present study have
previously demonstrated that high dose prenatal dexamethasone (DEX)
treatment increases the expression levels of TNF-α and decreased
the levels of histone deacetylase 2 protein in rat lungs in the
acute stage (14).
In addition to methylation and histone acetylation,
epigenetic alterations include regulation of micro (mi)RNA. It is
hypothesized that miRNAs are involved in the regulation of almost
every cellular and physiological process; however, the specific
biological and physiological functions of many miRNAs remain
unknown (15). Certain miRNAs have
been reported to modulate the development of the lungs, surfactant
secretion and PPAR-γ expression during rat lung development
(15–17). Furthermore, Williams et al
(18) have demonstrated that the
overall miRNA expression profile is similar for mouse and human
lung tissue, which suggests that miRNA expression is evolutionarily
conserved during lung development. Various miRNAs, including
miR-150, miR-375 and miR-26a, have been reported to be involved in
the regulation of pulmonary surfactant secretion (15,19,20).
Since miRNA expression profiling is important for the investigation
of potential differences between physiological and
pathophysiological status, the aim of the present study was to
investigate alterations in miRNA profiles following prenatal
glucocorticoid treatment for fetal lung development.
Materials and methods
Animals
This study was conducted in strict accordance with
the recommendations outlined in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The
protocol was approved by the Institutional Animal Care and Use
Committee of the Kaohsiung Chang Gung Memorial Hospital (Kaohsiung,
Taiwan). Virgin Sprague-Dawley (SD) rats (12–16 weeks old) were
obtained (BioLASCO Taiwan Co., Ltd., Taipei, Taiwan), and housed
and maintained in a facility accredited by the Association for
Assessment and Accreditation of Laboratory Animal Care
International. Virgin SD female rats were allowed to mate with male
rats for 24 h, and were separated from the male rats and housed
individually in a standard plastic home cage. Following
confirmation of pregnancy on day 14 following mating, pregnant
females were randomly assigned into prenatal steroid treatment or
untreated until delivery groups. Pregnant rats were checked for
litters daily at 10 a.m. The day of birth was designated postnatal
day 0 (D0) and rat pups were weaned on postnatal day 21 (D21), with
ad libitum access to standard chow and water. Only male rat
pups were used in the present study. In the short-term prenatal
steroid group, pregnant rats were administered i.p. DEX (0.1
mg/kg/day; 026804; Taiwan Biotech Co., Ltd., Taoyuan, Taiwan) at
gestational day 19–20 (DEX2 group); whereas pregnant rats in the
long-term antenatal steroid group were administered i.p. DEX (0.1
mg/kg/day) at gestational day 14–20 (DEX7 group). The early effects
of prenatal programming using glucocorticoids were assessed on
postnatal day 7 (D7), whereas the late programming effects were
assessed on postnatal day 120 (D120). The vehicle group comprised
of pregnant SD rats at gestational day 14–20 that received i.p.
normal saline.
Histological examination
Rats were anesthetized by an intramuscular injection
of a 1:1 mixture (100 mg/kg) of ketamin (#003542; United
Biomedical, Inc., Hsinchu, Taiwan) and rompun (#06713; Bayer Taiwan
Co., Ltd., Taipei, Taiwan), followed by cardiac puncture and
perfusion. Immediately after the rats were sacrificed as described
previously (14), the lungs were
harvested and stored in saline on ice and were subsequently
dissected from the surrounding tissues. Lung tissue was then fixed
in 10% formalin neutral buffer solution, pH 7.4 (Wako Pure Chemical
Industries, Ltd., Osaka, Japan). Subsequently, the tissues were cut
into 4-µm sections and stained with hematoxylin (Merck, Darmstadt,
Germany) and eosin Y (HT110180; Sigma-Aldrich, St. Louis, MO, USA)
for morphometric analysis. Images were captured using a digital
camera (CTR 5000; Leica) and a Nikon Eclipse E600 microscope
(magnification, ×10).
Western blot analysis
A 50-mg lung tissue sample was homogenized using 500
µl PRO-PREP Protein Extraction Solution (#17081; iNtRON
Biotechnology, Inc., Seongnam, Korea). Cell lysis was induced by
incubation for 30 min on ice and the samples was centrifuged at
14,000 × g for 20 min at 4°C. Protein concentrations were
determined using a Bio-Rad Protein Assay kit II (#5000002; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Protein samples (50 µg)
were boiled with gel-loading buffer (10 mM Tris-HCl, 1% sodium
dodecyl sulfate (SDS), 25% glycerol, 0.1 mM β-mercaptoethanol and
0.03% bromophenol blue; pH 6.8) for 5 min and separated by 10%
SDS-polyacrylamide gel electrophoresis. Following transfer to a
polyvinylidene fluoride membrane (Roche Diagnostics, Basel,
Switzerland) and blocking with phosphate-buffered saline-Tween
containing 5% dry milk, the membranes were incubated for 2 h with
anti-rat lamin A/C antibody (#4777; Cell Signaling Technology,
Inc., Danvers, MA, USA) diluted 1:200 in Tris-buffered saline (TBS)
containing 1.21 g Tris (#640310, Merck) and 8.76 g NaCl (#106404;
Merck) in 1 L ddH2O (pH 7.6), supplemented with 1%
skimmed milk. Following five washes with 0.1% Tween-TBS (T-TBS),
the membranes were incubated for 1 h with horseradish
peroxidase-labeled secondary antibody (#7076; Cell Signaling
Technology, Inc.) diluted 1:1,000 in T-TBS. Bands of interest were
visualized using Western Lightning Plus enhanced chemiluminescence
reagents (NEL105001EA; PerkinElmer, Inc., Waltham, MA, USA) and
quantified using densitometry (Quantity One Analysis software,
version 4.6.7; Bio-Rad Laboratories, Inc.) as integrated optical
density after subtraction of background. The integrated optical
density was factored for Ponceau S staining (PonS; P7170;
Sigma-Aldrich) to correct for any variations in total protein
loading. The protein abundance was represented as integrated
optical density/PonS.
RNA isolation
Total RNA was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. Purified RNA was
quantified at an optical density of 260 nm using a ND-1000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA) and measured using a Bioanalyzer 2100
with an RNA 6000 LabChip kit (both Agilent Technologies, Inc.,
Santa Clara, CA, USA).
Library preparation and
sequencing
Small RNA library construction and deep sequencing
were performed by Welgene Biotech, Co., Ltd. (Taipei, Taiwan).
Samples were prepared using an Illumina Sample Preparation kit
according to the TruSeq® Small RNA Sample Preparation Guide
(Illumina, Inc., San Diego, CA, USA). Subsequently, 5′ and 3′
adaptors (Illumina, Inc.) were ligated to total RNA, followed by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) amplification (described below). Enriched cDNA constructs
were size-fractionated and purified by electrophoresis in 6%
polyacrylamide gel with 30% acrylamide/Bis solution (#1610156) at a
ratio of 29:1, and using a Mini-PROTEAN Tetra Vertical
Electrophoresis System (Bio-Rad Laboratories, Inc.). Bands
containing 18–40 nucleotide RNA fragments, 140–155 nucleotides in
length with both adapters, were harvested. Libraries were sequenced
using an Genome Analyzer IIx (50 cycle single read; Illumina,
Inc.).
Next-generation sequencing (NGS) data
analysis
According to the manufacturer's protocol, the
generated NGS data were analyzed using the miRSeq software package
(21), which enables the acquisition
of miRNA expression profiles and the evaluation of the overall
quality of the NGS libraries. Using the readPro tool in miRSeq, a
15–27 length parameter was specified. Each sample used 1 ng RNA in
the NGS experiment.
Prediction of target genes of
differentially-expressed miRNAs
Targets of miRNAs were predicted using an online
target prediction tool; TargetScan 6.2 (http:www.targetscan.org/). A prediction score of
>0.5 was selected as the criterion for target genes with each
miRNA.
RT-qPCR analysis
In order to validate the miRNA profiling results,
RT-qPCR was conducted on all samples using a SYBR® Green
miRNA-based assay including the Universal cDNA Synthesis Kit
(#203301) and an ExiLENT SYBR® Green Master Mix Kit (#203420;
Exiqon, Inc., Woburn, MA, USA). Total RNA was extracted from the
lung tissue using TRIzol® reagent. A Universal cDNA Synthesis kit
(#203301; Exiqon, Inc.) was used to reverse transcribe 20 ng RNA
from the tissues using the 2X ExiLENT SYBR® Green Master Mix,
according to the manufacturer's protocol. A total of 50 ng RNA was
reverse transcribed to cDNA in qPCR experiment. The mixture was
incubated at 42°C for 60 min, followed by 95°C for 5 min. RT-qPCR
reactions were subsequently performed using a total reaction volume
of 10 µl containing 4 µl cDNA, 1 µl PCR primer mix and 5 µl ExiLENT
SYBR® Green master mix (203420; Exiqon, Inc.). The PCR cycling was
conducted as follows: Pre-incubation at 95°C for 10 min; then
amplification for 45 cycles of 95°C for 10 sec and 60°C for 10 min;
then melting cycle of 95°C for 5 sec, 65°C for 1 min and 97°C with
continuous per 5°C acquisition of fluorescence; and then cooling at
40°C for 10 sec. All RT-qPCR reactions, including the controls,
were performed in triplicate using a LightCycler® 480 system (Roche
Diagnostics) (14). Relative miRNA
expression levels were analyzed using the Cq method and normalized
against U6 snRNA (203907; Exiqon, Inc.) expression levels. Target
miRNA primer sequences were as follows: Rno-let-7b-5p,
5′-UGAGGUAGUAGGUUGUGUGGUU-3′; rno-miR-26a-5p,
5′-UUCAAGUAAUCCAGGAUAGGCU-3′; rno-miR-27a-3p,
5′-UUCACAGUGGCUAAGUUCCGC-3′; rno-miR-101a-3p,
5′-UACAGUACUGUGAUAACUGAA-3′; rno-miR-375–3p,
5′-UUUGUUCGUUCGGCUCGCGUGA-3′. Data were analyzed as previously
described (22).
Statistical analysis
Data are expressed as mean ± standard error of the
mean. Mann-Whitney U test was used when two groups were analyzed.
All statistical tests were performed using SPSS software, version
19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistical significant difference.
Results
Prenatal DEX treatment induces
alveolar tissue dysplasia and lamin A/C reduction
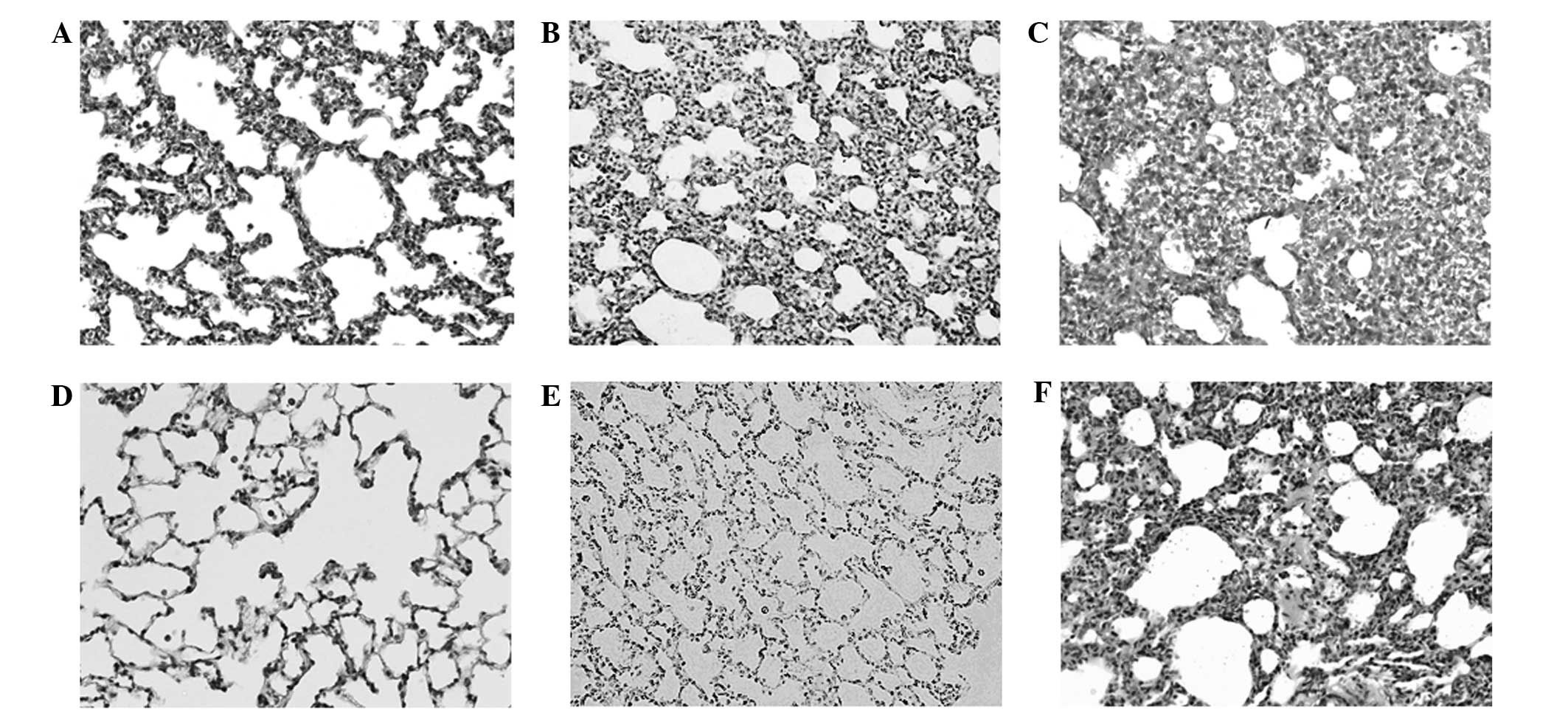
Histological examination of the prenatal DEX
treatment group using hematoxylin and eosin staining demonstrated
obvious dysplasia of the lung alveolar tissue at D7 and D120, as
compared with the vehicle group (Fig.
1). The greatest dysplastic change was detected in the rat
lungs of the DEX7 group, which were administered prenatal DEX for 7
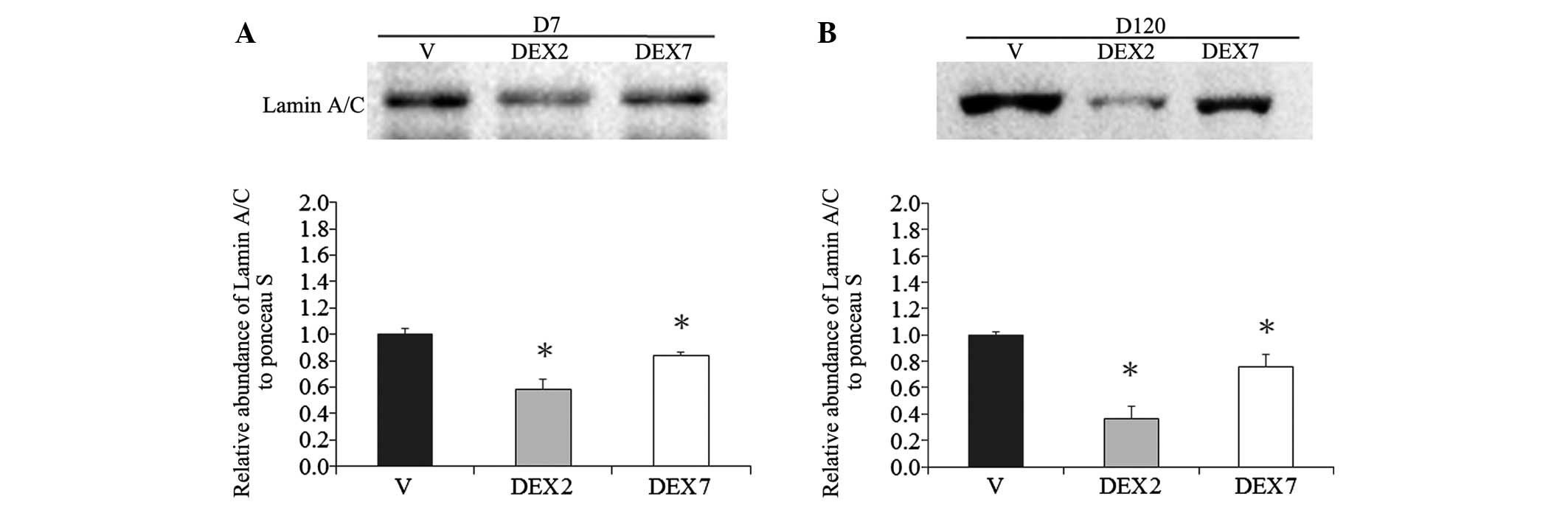
days. Since lamin A/C level has been demonstrated to be an
important determinant for lineage-specific differentiation during
embryonic development (23), the
abundance of lamin A/C was determined using western blotting. A
reduction in lamin A/C was detected in the lung alveolar tissue of
the prenatal DEX group at D7 and D120, as compared with the vehicle
group (Fig. 2). The lowest lamin A/C
expression levels were detected in the rat lungs of the DEX2 group,
which were administered prenatal DEX for 2 days.
Summaries of NGS libraries
In the present study, six libraries were used from
rat lung tissues sequenced using the NGS platform and the generated
NGS data were analyzed using the miRSeq software package. The
results demonstrated that the six libraries had similar
performances regarding self-ligation reads, which are the reads
generated from 5′ and 3′ adaptor self-ligation at the sample
preparation step, and clean reads, which are the reads with the 3′
adaptor identified and trimmed. However, further investigation
demonstrated that the clean reads of the D7 libraries tended to be
less enriched in 22-nt length (Fig.
3A). The length distribution patterns of the D120 libraries
were more homologous to the rat miRNAs in miRBase 20; therefore,
these had a higher likelihood of being miRNAs (Fig. 2B). The D120 libraries accounted for a
significantly higher proportion of the miRNA reads (P=0.015
pair-wise t-test) (Fig. 3B).
Furthermore, among the three D120 libraries, a peak at 24 nt was
detected at D120 in the DEX7 group, resulting in a higher
proportion of non-coding RNA reads. Therefore, the sample
preparation steps performed better in the D120 libraries, among
which the D120 V and D120 DEX2 groups were particularly
successful.
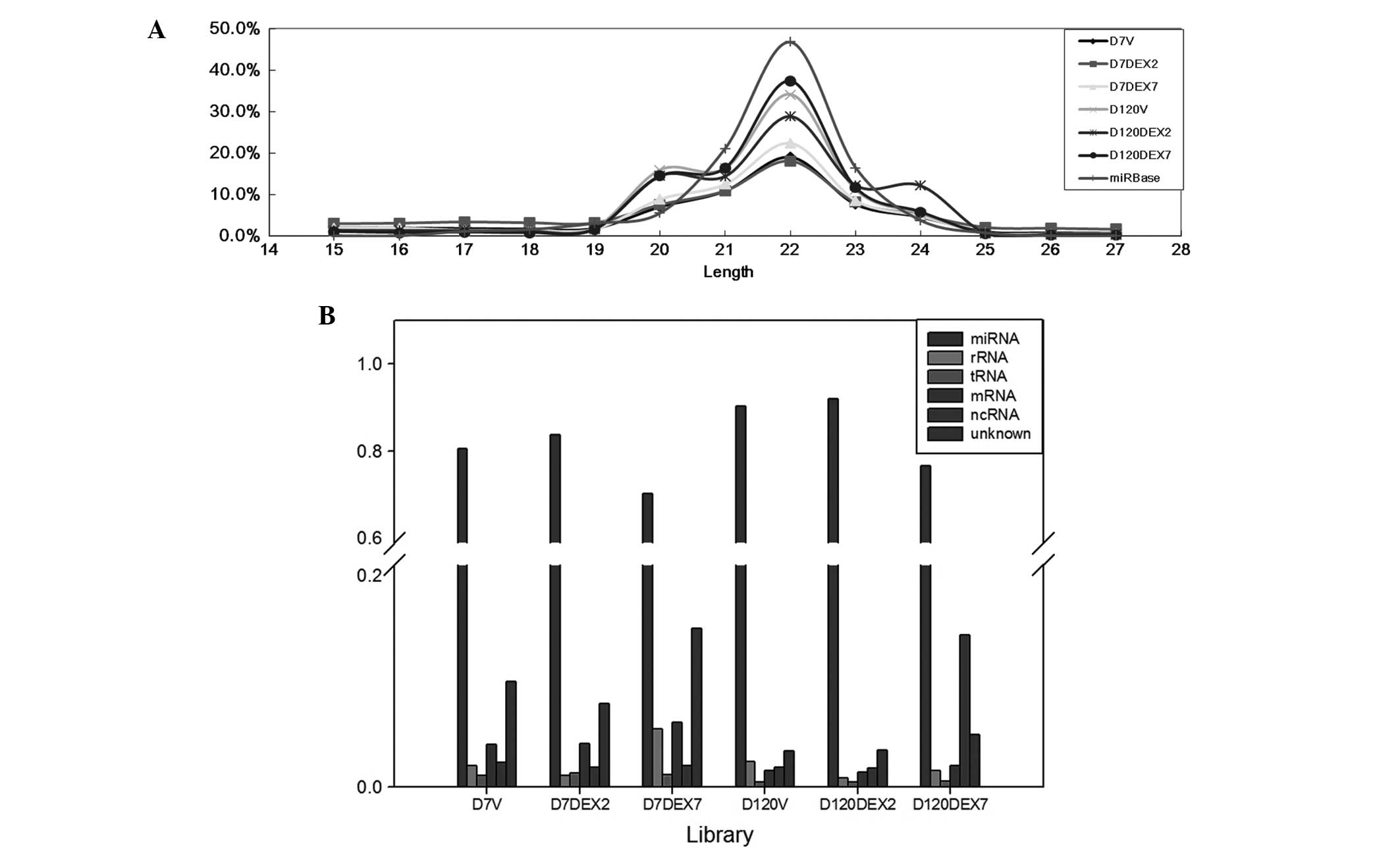 | Figure 3.Next generation sequencing data. (A)
Length distributions of the clean reads in the libraries, which
were defined as reads with the 3′ adaptor identified and trimmed.
(B) The read category of the next generation sequencing reads.
Length distribution patterns suggested that miRNA reads accounted
for a higher proportion of the reads in the D120 libraries, as
compared with the D7 libraries, which was confirmed by the read
category. D7, postnatal day 7; D120, postnatal day 120; DEX2,
prenatal dexamethasone treatment for 2 days; DEX7, prenatal
dexamethasone treatment for 7 days; miRNA, micro RNA; rRNA,
ribosomal RNA; tRNA, transfer RNA;mRNA, messenger RNA; ncRNA,
non-coding RNA. |
miRNA expression profiles varied
between D7 and D120
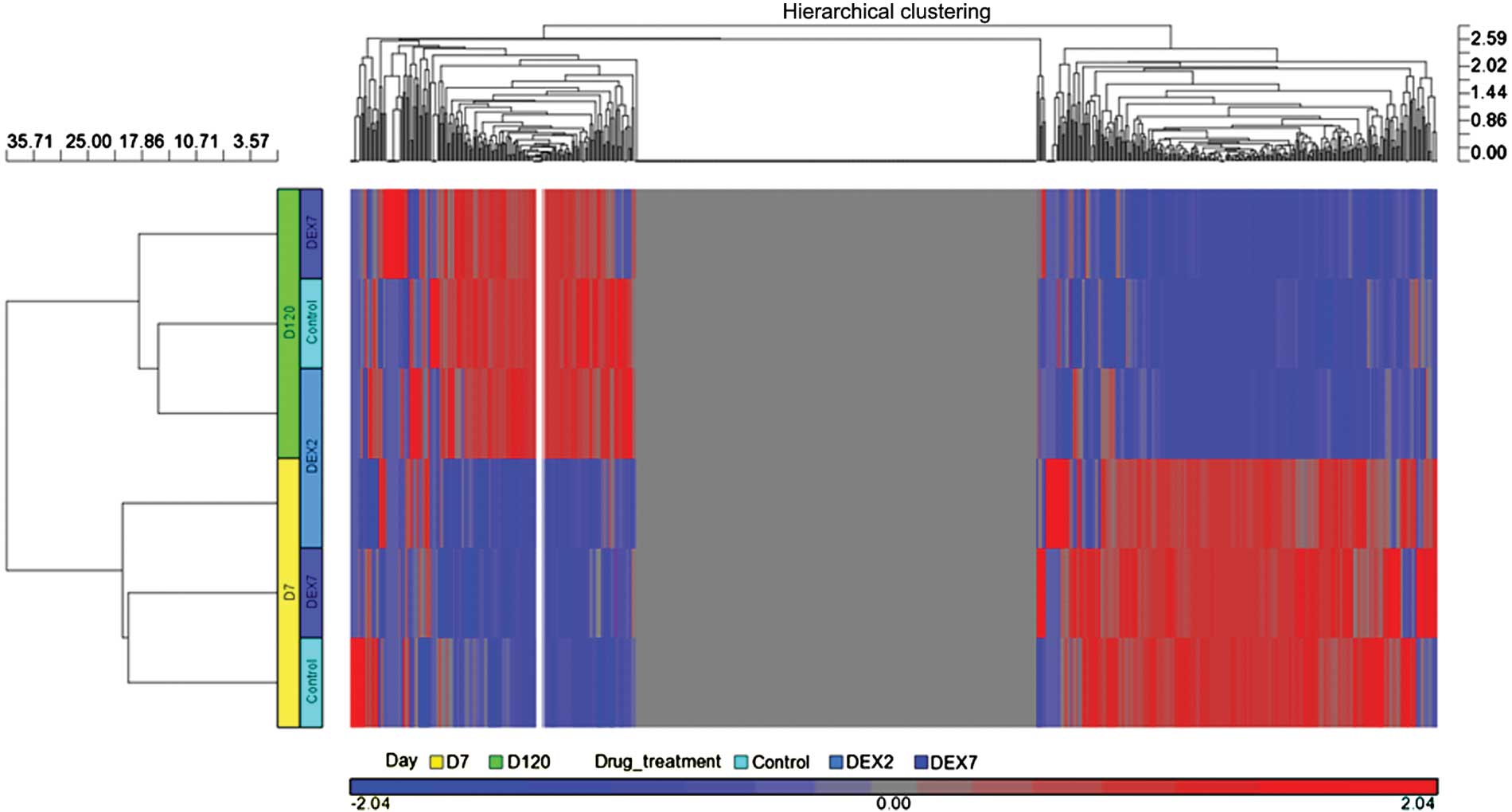
Following miRSeq analysis, miRNA expression profiles
in six libraries were acquired. In order to compare the overall
miRNA expression profiles among the six libraries, respective heat
maps were plotted using the Partek® analysis toolkit (Partek, Inc.,
St. Louis, MO, USA). The six libraries were first classified into
two clusters belonging to D7 and D120, respectively (Fig. 4). Therefore, age was the predominant
factor for the observed differences in the miRNA expression
profiles. In the D7 libraries, the vehicle and DEX7 groups
demonstrated more homologous patterns; whereas, in the D120
libraries, the vehicle group was most homologous to the DEX2 group.
Therefore, the results of the present study suggest that DEX
treatment produced inconsistent effects on the miRNA expression
profiles between the D7 and D120 libraries.
Since age was associated with notable differences,
alterations in the miRNA expression profiles of rat lung tissues
were compared between D7 and D120. miRNA profiles were grouped
according to D7 or D120, regardless of prenatal DEX treatment. In
this NGS profile, 167 differentially expressed miRNAs were detected
between the D7 and D120 groups, and 12 of these miRNAs demonstrated
a >2-fold change, including six upregulated miRNAs: miR-101a-3p;
let-7b-5p; let-7c-5p, miR-23b-3p; miR-27a-3p; and miR30b-5p, and
six downregulated miRNAs: miR-99b-5p; miR-146b-5p; miR-181b-5p;
miR-182; miR-199a-3p; and mir-351-5p. (Table I). When the fold-change cutoff was
set to 1.5–2.0, seven upregulated miRNAs and nine downregulated
miRNAs were detected at D120 (Table
II).
 | Table I.Differentially expressed miRNAs with
>2-fold change between D7 and D120 (transcripts per
million). |
Table I.
Differentially expressed miRNAs with
>2-fold change between D7 and D120 (transcripts per
million).
|
| D7 | D120 |
|
|---|
|
|
|
|
|
|---|
| miR_ID | V | DEX2 | DEX7 | Mean ± SE | V | DEX2 | DEX7 | Mean ± SE | D120/D7 ratio |
|---|
|
rno-miR-101a-3p |
1,944.1 |
2,008.1 |
1,735.1 | 1,896±82 |
8,774.1 |
9,926.1 | 10,070.1 |
9,590±410 | 5.06 |
| rno-miR-30b-5p |
1,872.1 |
1,583.1 |
1,487.1 |
1,647±116 |
3,959.1 |
4,839.1 |
4,319.1 |
4,372±255 | 2.65 |
| rno-miR-27a-3p |
2,278.1 |
1,866.1 |
2,149.1 |
2,098±122 |
4,409.1 |
5,952.1 |
3,981.1 |
4,781±599 | 2.28 |
| rno-miR-23b-3p |
1,665.1 |
1,677.1 |
1,755.1 | 1,699±28 |
3,747.1 |
3,861.1 |
3,966.1 | 3,858±63 | 2.27 |
| rno-let-7c-5p |
6,885.2 |
8,289.2 |
7,993.2 |
7,723±427 | 15,630.2 | 16,148.2 | 16,302.2 | 16,027±203 | 2.08 |
| rno-let-7b-5p |
1,907.1 |
2,133.1 |
2,355.1 |
2,132±129 |
4,463.1 |
4,397.1 |
4,405.1 | 4,422±21 | 2.07 |
|
rno-miR-146b-5p |
5,756.1 |
6,051.1 |
6,828.1 |
6,212±320 |
3,845.1 |
2,190.1 |
3,173.1 |
3,069±481 | 0.49 |
|
rno-miR-181b-5p |
3,553.2 |
3,839.2 |
4,497.2 |
3,963±279 |
1,895.2 |
1,890.2 |
1,968.2 | 1,918±25 | 0.48 |
| rno-miR-182 | 11,330.1 |
9,066.1 |
9,610.1 | 10,002±682 |
4,050.1 |
5,689.1 |
4,013.1 |
4,584±553 | 0.46 |
|
rno-miR-199a-3p | 11,301.1 | 13,021.1 | 11,249.1 | 11,857±582 |
4,014.1 |
4,244.1 |
4,002.1 | 4,087±79 | 0.34 |
| rno-miR-351–5p |
9,872.1 |
8,745.1 | 10,055.1 |
9,557±410 |
1,829.1 |
2,052.1 |
1,649.1 |
1,843±117 | 0.19 |
| rno-miR-99b-5p | 38,160.1 | 34,937.1 | 37,580.1 | 36,892±992 |
5,815.1 |
6,246.1 |
6,643.1 |
6,235±239 | 0.17 |
 | Table II.Differentially expressed miRNAs with
a fold change of 1.5–2-fold between D7 and D120 (transcripts per
million). |
Table II.
Differentially expressed miRNAs with
a fold change of 1.5–2-fold between D7 and D120 (transcripts per
million).
|
| D7 | D120 |
|
|---|
|
|
|
|
|
|---|
| miR_ID | V | DEX2 | DEX7 | Mean ± SE | V | DEX2 | DEX7 | Mean ± SE | D120/D7 ratio |
|---|
| rno-miR-24–3p |
1,478.2 |
1,652.2 |
1,588.2 | 1,573±51 |
3,586.2 |
2,718.2 |
3,068.2 |
3,124±252 | 1.99 |
| rno-miR-22–3p | 34,445.1 | 31,739.1 | 32,412.1 | 32,865±813 |
62,815.1 |
72,094.1 |
59,302.1 |
64,737±3,816 | 1.97 |
| rno-miR-30a-5p | 70,582.1 | 62,221.1 | 57,131.1 |
63,311±3,921 | 112,501.1 | 112,489.1 | 124,269.1 |
116,420±3,925 | 1.84 |
| rno-miR-26b-5p |
4,819.1 |
3,493.1 |
4,496.1 |
4,269±399 |
8,013.1 |
8,604.1 |
6,330.1 |
7,649±681 | 1.79 |
|
rno-miR-126a-3p | 11,682.1 | 10,692.1 | 12,468.1 | 11,614±514 |
19,567.1 |
21,152.1 |
18,153.1 | 19,624±866 | 1.69 |
| rno-miR-34c-5p |
2,917.1 |
3,165.1 |
3,207.1 | 3,096±90 |
4,733.1 |
5,210.1 |
5,355.1 |
5,099±188 | 1.65 |
| rno-miR-375–3p |
2,200.1 |
1,742.1 |
2,183.1 |
2,042±150 |
3,145.1 |
3,007.1 |
3,116.1 | 3,089±42 | 1.51 |
| rno-miR-16–5p | 19,969.1 | 17,390.1 | 21,315.1 |
19,558±1,152 |
13,711.1 |
12,966.1 |
12,399.1 | 13,025±380 | 0.67 |
| rno-miR-151–5p |
5,036.1 |
5,846.1 |
4,952.1 |
5,278±285 |
3,568.1 |
3,745.1 |
3,210.1 |
3,508±157 | 0.66 |
|
rno-miR-450a-5p |
3,921.1 |
3,304.1 |
3,100.1 |
3,442±247 |
2,560.1 |
2,426.1 |
1,716.1 |
2,234±262 | 0.65 |
| rno-miR-92a-3p | 13,189.2 | 11,645.2 | 14,568.2 | 13,134±844 |
7,602.2 |
8,403.2 |
9,040.2 |
8,349±416 | 0.64 |
| rno-miR-25–3p |
3,437.1 |
3,411.1 |
3,578.1 | 3,475±52 |
2,060.1 |
2,220.1 |
2,338.1 | 2,206±81 | 0.63 |
| rno-miR-151–3p |
7,984.1 |
7,373.1 |
8,401.1 |
7,919±299 |
4,718.1 |
4,124.1 |
5,128.1 |
4,657±291 | 0.59 |
|
rno-miR-125a-5p |
7,931.1 |
7,749.1 |
7,260.1 |
7,647±200 |
4,633.1 |
4,911.1 |
3,730.1 |
4,425±356 | 0.58 |
| rno-miR-92b-3p |
2,728.1 |
2,191.1 |
3,271.1 |
2,730±312 |
1,450.1 |
1,435.1 |
1,571.1 | 1,485±43 | 0.54 |
|
rno-miR-181c-5p |
6,190.1 |
6,712.1 |
6,829.1 |
6,577±196 |
3,264.1 |
3,235.1 |
3,586.1 |
3,362±112 | 0.51 |
miRNA expression profile of rat lungs
in the vehicle and prenatal DEX treatment groups
miRNA expression profiles of the rat lungs were
compared between the vehicle and prenatal DEX treatment groups,
including day 7 in the vehicle group (D7 V), prenatal DEX treatment
for 2 days (D7 DEX2) and prenatal DEX treatment for 7 days (D7
DEX7). Since prenatal DEX treatment did not cause a > 2-fold
increase in the miRNA expression levels, as compared with the
vehicle, the cutoff value was set as a 1.2-fold change between the
D7 DEX2 and D7 V groups or the D7 DEX7 and D7 V groups. Following
the implementation of the 1.2-fold change cutoff, 12 upregulated
and 20 downregulated miRNAs were detected following prenatal DEX
treatment at D7 (Table III).
 | Table III.Differences in miRNA expression
profiles at D7 (transcripts per million). |
Table III.
Differences in miRNA expression
profiles at D7 (transcripts per million).
| miR_ID | V | DEX2 | DEX7 | DEX2/V ratio | DEX7/V ratio |
|---|
| rno-let-7b-5p |
1,907.1 |
2,133.1 |
2,355.1 | 1.12 | 1.23 |
| rno-let-7c-5p |
6,885.2 |
8,289.2 |
7,993.2 | 1.20 | 1.16 |
| rno-miR-10a-5p |
54,083.1 |
64,522.1 |
55,017.1 | 1.19 | 1.02 |
| rno-miR-21–5p |
10,869.1 |
13,347.1 |
11,374.1 | 1.23 | 1.05 |
| rno-miR-26a-5p |
70,422.1 |
56,575.1 |
58,489.1 | 0.80 | 0.83 |
| rno-miR-26b-5p |
4,819.1 |
3,493.1 |
4,496.1 | 0.72 | 0.93 |
| rno-miR-27a-3p |
2,278.1 |
1,866.1 |
2,149.1 | 0.82 | 0.94 |
| rno-miR-28–3p |
3,702.1 |
2,921.1 |
3,449.1 | 0.79 | 0.93 |
| rno-miR-30a-5p |
70,582.1 |
62,221.1 |
57,131.1 | 0.88 | 0.81 |
| rno-miR-30b-5p |
1,872.1 |
1,583.1 |
1,487.1 | 0.85 | 0.79 |
| rno-miR-30c-5p |
7,164.2 |
6,454.2 |
5,816.2 | 0.90 | 0.81 |
| rno-miR-30d-5p |
23,793.1 |
19,892.1 |
22,603.1 | 0.84 | 0.95 |
| rno-miR-92b-3p |
2,728.1 |
2,191.1 |
3,271.1 | 0.80 | 1.20 |
| rno-miR-100-5p |
1,970.1 |
2,304.1 |
2,399.1 | 1.17 | 1.22 |
|
rno-miR-126a-5p |
39,461.1 |
33,312.1 |
36,780.1 | 0.84 | 0.93 |
| rno-miR-127–3p |
19,146.1 |
14,062.1 |
17,011.1 | 0.73 | 0.89 |
| rno-miR-143–3p | 205,095.1 | 245,257.1 | 215,878.1 | 1.20 | 1.05 |
|
rno-miR-146a-5p |
1,401.1 |
1,523.1 |
2,035.1 | 1.09 | 1.45 |
|
rno-miR-146b-5p |
5,756.1 |
6,051.1 |
6,828.1 | 1.05 | 1.19 |
|
rno-miR-148b-3p |
1,184.1 |
1,659.1 |
1,333.1 | 1.40 | 1.13 |
|
rno-miR-181a-5p |
61,870.2 |
71,522.2 |
79,498.2 | 1.16 | 1.28 |
|
rno-miR-181b-5p |
3,553.2 |
3,839.2 |
4,497.2 | 1.08 | 1.27 |
| rno-miR-182 |
11,330.1 |
9,066.1 |
9,610.1 | 0.80 | 0.85 |
| rno-miR-183–5p |
1,652.1 |
1,318.1 |
1,536.1 | 0.80 | 0.93 |
| rno-miR-186–5p |
2,128.1 |
1,372.1 |
1,653.1 | 0.64 | 0.78 |
|
rno-miR-200b-3p |
1,791.1 |
1,428.1 |
1,658.1 | 0.80 | 0.93 |
| rno-miR-322–5p |
2,786.1 |
2,097.1 |
2,181.1 | 0.75 | 0.78 |
| rno-miR-375–3p |
2,200.1 |
1,742.1 |
2,183.1 | 0.79 | 0.99 |
| rno-miR-411–5p |
3,302.1 |
2,481.1 |
2,920.1 | 0.75 | 0.88 |
| rno-miR-429 |
1,646.1 |
1,258.1 |
1,313.1 | 0.76 | 0.80 |
| rno-miR-434–3p |
1,903.1 |
1,044.1 |
1,393.1 | 0.55 | 0.73 |
|
rno-miR-449a-5p |
2,708.1 |
2,806.1 |
3,497.1 | 1.04 | 1.29 |
|
rno-miR-450a-5p |
3,921.1 |
3,304.1 |
3,100.1 | 0.84 | 0.79 |
Differences in the miRNA expression profiles of the
rat lungs at D120 were compared among the vehicle (D120 V),
prenatal DEX treatment for 2 days (D120 DEX2) and D120 DEX7)
groups. Following the implementation of the 1.2-fold change cutoff,
four upregulated and five downregulated miRNAs were detected
following prenatal DEX treatment at D120 (Table IV). These results suggest that the
efficacy of prenatal treatment with DEX on the miRNA profiles in
rat lung tissues is markedly decreased in the chronic stage.
 | Table IV.Differences in miRNA expression
profiles at D120 (transcripts per million). |
Table IV.
Differences in miRNA expression
profiles at D120 (transcripts per million).
| miR_ID | V | DEX2 | DEX7 | DEX2/V ratio | DEX7/V ratio |
|---|
| rno-miR-24–3p |
3,586.2 |
2,718.2 |
3,068.2 | 0.76 | 0.86 |
| rno-miR-26a-5p | 83,002.1 | 99,966.1 | 71,257.1 | 1.20 | 0.86 |
| rno-miR-26b-5p |
8,013.1 |
8,604.1 |
6,330.1 | 1.07 | 0.79 |
| rno-miR-27a-3p |
4,409.1 |
5,952.1 |
3,981.1 | 1.35 | 0.90 |
| rno-miR-28–3p |
1,883.1 |
2,265.1 |
1,961.1 | 1.20 | 1.04 |
| rno-miR-30b-5p |
3,959.1 |
4,839.1 |
4,319.1 | 1.22 | 1.09 |
| rno-miR-92a-3p |
7,602.2 |
8,403.2 |
9,040.2 | 1.11 | 1.19 |
|
rno-miR-125a-5p |
4,633.1 |
4,911.1 |
3,730.1 | 1.06 | 0.81 |
|
rno-miR-126a-5p | 40,074.1 | 30,726.1 | 38,860.1 | 0.77 | 0.97 |
| rno-miR-145–5p |
1,204.1 |
1,897.1 |
1,417.1 | 1.58 | 1.18 |
|
rno-miR-146b-5p |
3,845.1 |
2,190.1 |
3,173.1 | 0.57 | 0.83 |
| rno-miR-150–5p |
1,623.1 |
1,810.1 |
1,341.1 | 1.12 | 0.83 |
|
rno-miR-181a-5p | 66,412.2 | 54,932.2 | 63,020.2 | 0.83 | 0.95 |
| rno-miR-182 |
4,050.1 |
5,689.1 |
4,013.1 | 1.40 | 0.99 |
| rno-miR-192–5p |
1,683.1 |
1,760.1 |
2,144.1 | 1.05 | 1.27 |
|
rno-miR-450a-5p |
2,560.1 |
2,426.1 |
1,716.1 | 0.95 | 0.67 |
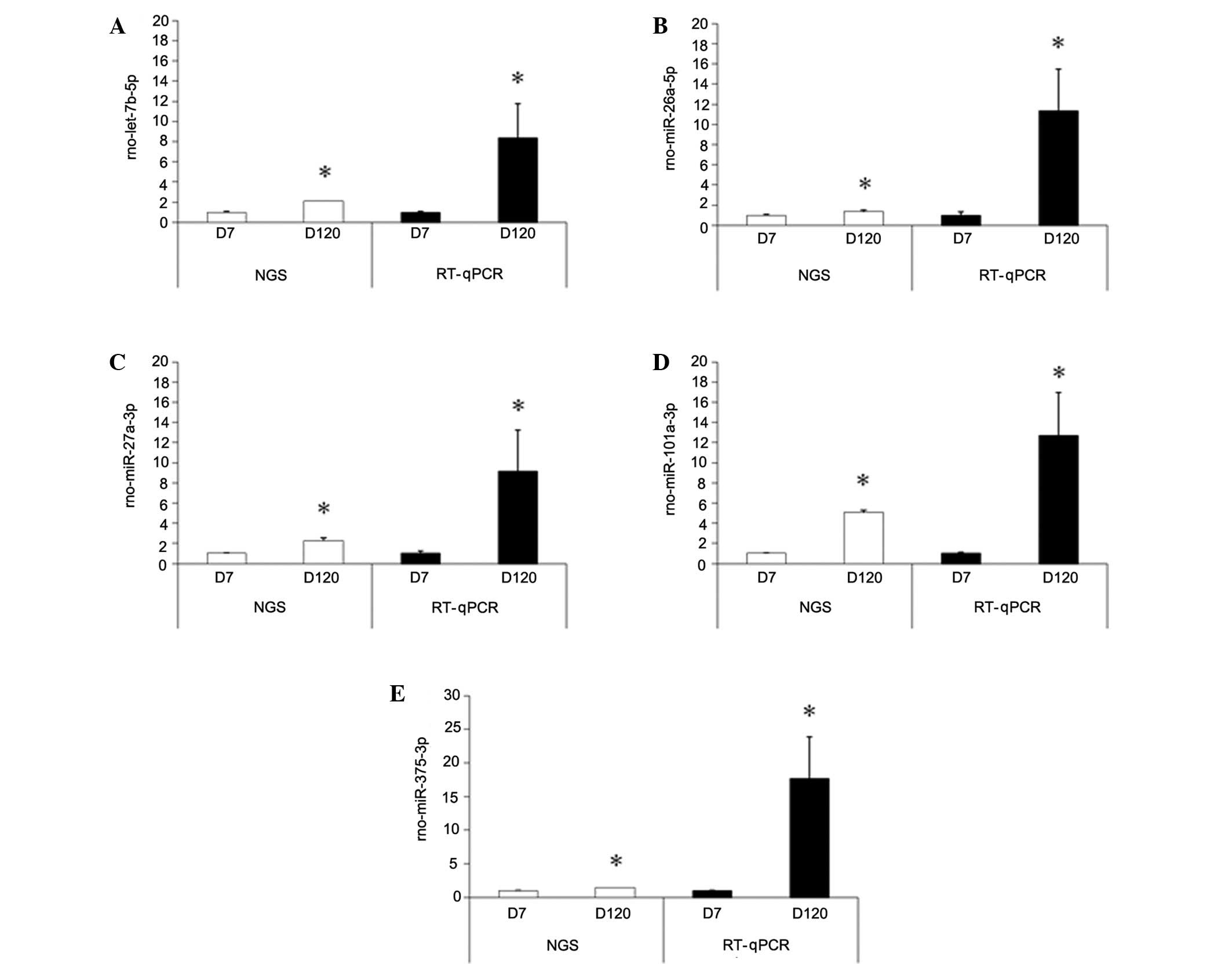
RT-qPCR validation
The following differentially expressed miRNAs were
selected to perform RT-qPCR, based on the NGS data: let-7b-5p,
miR-26a-5p, miR-27a-3p, miR-101a-3p, and miR-375. The relative
expression of these five miRNAs are shown in Fig. 5. These five miRNAs were selected for
further study as, among the consistently upregulated miRNAs,
let-7b-5p, miR-27a-3p, and miR-101a-3p demonstrated the greatest
fold changes between the D7 and D120 groups. Furthermore,
let-7b-5p, miR-27a-3p, and miR-101a-3p are reportedly associated
with lung tumor development (24),
and miR-26a and miR-375 have been associated with the regulation of
pulmonary surfactant secretion. The alterations in miRNAs following
prenatal DEX treatment were not validated by RT-qPCR as the effects
of prenatal DEX treatment appear to be limited.
Discussion
In the present study, miRSeq was used to analyze six
libraries from rat lung tissues. The D7 libraries were less
enriched as they contained unknown reads with nucleotides of 31, 32
and 33 bp in length (data not shown).
miRNAs serve a crucial function in cellular
proliferation, differentiation and organ development; however, few
previous miRNA studies have investigated their connection with
diseases of lung development (25).
In the present study, differences in miRNA expression profiles were
compared between the D7 and D120 groups, and the miRNAs with a
>2-fold change were selected. Six upregulated and six
downregulated miRNAs were detected at D120, as compared with D7.
Among these differentially expressed miRNAs, miR-101-3p and
miR-99b-5p were responsible for the lowest and highest expressions
of miRNA at D7, respectively.
It has previously been demonstrated that miR-101-3p
is downregulated following infection with hepatitis B virus
(26); however, the role of
miR-101-3p in lung development remains unclear. Li et al
(27) demonstrated that the altered
serum levels of miR-21, miR-155 and miR-101-3p were associated with
the degree of forced vital capacity and radiographic features in
idiopathic pulmonary fibrosis. miR-101 and miR-144 have also been
demonstrated to regulate the expression levels of the cystic
fibrosis transmembrane conductance regulator in the lungs (28). Furthermore, previous studies have
demonstrated that mir-101 exerts a tumor suppressive function in
certain lung cancer conditions (29,30), and
the overexpression of miR-101-3p has been reported to inhibit
cellular proliferation, migration and reduce apoptosis (31). Low expression levels of miR-101-3p at
D7 in the present study suggested its immaturity.
The mature form of let-7 and its family members are
highly conserved. The key functions of let-7 genes are to promote
terminal differentiation in development and tumor suppression
(32,33). The let-7 family has previously been
identified as a tumor marker and a potential therapeutic regimen
for the treatment of certain tumors (32,34–36).
Furthermore, previous studies have demonstrated that mir-27a may be
associated with malignant changes in bronchial epithelial cells and
signal interactions in lung cancer (37,38).
However, to the best of our knowledge, the associations between
mir-23b-3p and mir-30b-5p, and lung development is yet to be
investigated.
It has previously been suggested that miR-99b is
capable of potentiating endothelial cell differentiation from stem
cells (39). Enhanced miR-99b levels
following lentiviral-mediated transfer were demonstrated to
potentiate the mRNA and protein expression of endothelial cell
specific markers, increase nitric oxide production and improve
neovascularization in vivo (39). The high expression levels of miR-99b
detected at D7 in the present study are consistent with growth and
vascularization. Furthermore, among the downregulated miRNAs
following lung maturation (Table I),
miR-99b-5p was responsible for the least miRNA in D120 rat lungs.
In order to elucidate the underlying mechanisms of its regulatory
role, the predicted target genes of miR-99b-5p were collected from
TargetScan 6.2 and the pathways in which the target genes were
enriched were derived. Among the enriched pathways, the Hippo
signaling pathway (Kyoto Encyclopedia of Genes and Genomes,
map04390) was significantly associated with organ growth (P=0.0003)
(40). In animals, the Hippo
signaling pathway controls organ size through the regulation of
cell proliferation and apoptosis (40); therefore, it is reasonable that the
Hippo signaling pathway is activated during lung tissue maturation
in order to restrict organ size overgrowth by suppressing
miR-99b-5p.
In the present study, the differences in the miRNA
expression profiles of rat lung tissues were compared between
vehicle and prenatal DEX treatment groups. Prenatal DEX treatment
had a limited impact on the miRNA profiles of lung tissue at D7 and
D120. Therefore, the fold-change cutoff value was set at 1.2, which
facilitated the detection of 12 upregulated and 20 downregulated
miRNAs at D7 following prenatal DEX treatment (Table III). Among these 20 downregulated
miRNAs, miR-434–3p was demonstrated to be the most suppressive.
Various miRNAs, including miR-150, miR-375, and miR-26a have been
associated with the regulation of pulmonary surfactant secretion
(15,19,20). The
results of the present study agree in part with these previous
studies, and downregulated miR-375 and miR-26a were compatible with
prenatal glucocorticosteroid treatment. However, further miRNAs
that are downregulated following prenatal DEX treatment have been
detected in our study. The authors of the present study hypothesize
that these newly identified miRNAs may be crucially involved in the
development of fetal lungs, and the results of the present study
may aid further clarification of the mechanisms of normal lung
development. However, the results of the present study cannot fully
represent the effects of prenatal DEX treatment on the lung
development of premature babies since significant prenatal
DEX-induced changes in the miRNA profiles may appear earlier;
therefore more intensive time course studies are required.
Acknowledgements
The present study was supported in part by grants
from the National Science Council, Taiwan (grant nos. CMRPG8B0141,
CMRPG8B0142, CMRPG8C0171 and NSC 102-2314-B-182A-042-MY3). The
authors thank the Genomics and Proteomics Core Laboratory,
Department of Medical Research at Kaohsiung Chang Gung Memorial
Hospital for their technical support.
References
|
1
|
Crowley P, Chalmers I and Keirse MJ: The
effects of corticosteroid administration before preterm delivery:
An overview of the evidence from controlled trials. Br J Obstet
Gynaecol. 97:11–25. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts D and Dalziel S: Antenatal
corticosteroids for accelerating fetal lung maturation for women at
risk of preterm birth. Cochrane Database Syst Rev.
19:CD0044542006.
|
|
3
|
Liggins GC: The role of cortisol in
preparing the fetus for birth. Reprod Fertil Dev. 6:141–150. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gross I: Regulation of fetal lung
maturation. Am J Physiol. 259:L337–L344. 1990.PubMed/NCBI
|
|
5
|
Schittny JC, Djonov V, Fine A and Burri
PH: Programmed cell death contributes to postnatal lung
development. Am J Respir Cell Mol Biol. 18:786–793. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Whitsett JA and Stahlman MT: Impact of
advances in physiology, biochemistry and molecular biology on
pulmonary disease in neonates. Am J Respir Crit Care Med.
157:S67–S71. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walther FJ, Ikegami M, Warburton D and
Polk DH: Corticosteroids, thyrotropin-releasing hormone and
antioxidant enzymes in preterm lamb lungs. Pediatr Res. 30:518–521.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pinkerton KE, Willet KE, Peake JL, Sly PD,
Jobe AH and Ikegami M: Prenatal glucocorticoid and T4 effects on
lung morphology in preterm lambs. Am J Respir Crit Care Med.
156:624–630. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beck JC, Mitzner W, Johnson JW, Hutchins
GM, Foidart JM, London WT, Palmer AE and Scott R: Betamethasone and
the rhesus fetus: Effect on lung morphometry and connective tissue.
Pediatr Res. 15:235–240. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vyas J and Kotecha S: Effects of antenatal
and postnatal corticosteroids on the preterm lung. Arch Dis Child
Fetal Neonatal Ed. 77:F147–F150. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Kuliszewski M, Yee W, Sedlackova
L, Xu J, Tseu I and Post M: Cloning and expression of
glucocorticoid-induced genes in fetal rat lung fibroblasts.
Transforming growth factor-beta 3. J Biol Chem. 270:2722–2728.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drake AJ, Walker BR and Seckl JR:
Intergenerational consequences of fetal programming by in utero
exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp
Physiol. 288:R34–R38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Drake AJ, Tang JI and Nyirenda MJ:
Mechanisms underlying the role of glucocorticoids in the early life
programming of adult disease. Clin Sci (Lond). 113:219–232. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu HR, Kuo HC, Chen CC, Sheen JM, Tiao MM,
Chen YC, Chang KA, Tain YL and Huang LT: Prenatal dexamethasone
exposure in rats results in long-term epigenetic histone
modifications and tumor necrosis factor-α production decrease.
Immunology. 143:651–660. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Mishra A, Chintagari NR, Gou D
and Liu L: Micro-RNA-375 inhibits lung surfactant secretion by
altering cytoskeleton reorganization. IUBMB Life. 62:78–83.
2010.PubMed/NCBI
|
|
16
|
Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim
MM, Srikantan S, Martindale JL, Hutchison ER, Kim HH, Marasa BS, et
al: MiR-130 suppresses adipogenesis by inhibiting peroxisome
proliferator-activated receptor gamma expression. Mol Cell Biol.
31:626–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhaskaran M, Wang Y, Zhang H, Weng T,
Baviskar P, Guo Y, Gou D and Liu L: MicroRNA-127 modulates fetal
lung development. Physiol Genomics. 37:268–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams AE, Moschos SA, Perry MM, Barnes
PJ and Lindsay MA: Maternally imprinted microRNAs are
differentially expressed during mouse and human lung development.
Dev Dyn. 236:572–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weng T, Mishra A, Guo Y, Wang Y, Su L,
Huang C, Zhao C, Xiao X and Liu L: Regulation of lung surfactant
secretion by microRNA-150. Biochem Biophys Res Commun. 422:586–589.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang XQ, Zhang P, Yang Y, Qiu J, Kan Q,
Liang HL, Zhou XY and Zhou XG: Regulation of pulmonary surfactant
synthesis in fetal rat type II alveolar epithelial cells by
microRNA-26a. Pediatr Pulmonol. 49:863–872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan CT, Tsai KW, Hung TM, Lin WC, Pan CY,
Yu HR and Li SC: MiRSeq: A user-friendly standalone toolkit for
sequencing quality evaluation and miRNA profiling. Biomed Res Int.
2014:4621352014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu HR, Chang JC, Chen RF, Chuang H, Hong
KC, Wang L and Yang KD: Different antigens trigger different
Th1/Th2 reactions in neonatal mononuclear cells (MNCs) relating to
T-bet/GATA-3 expression. J Leukoc Biol. 74:952–958. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sehgal P, Chaturvedi P, Kumaran RI, Kumar
S and Parnaik VK: Lamin A/C haploinsufficiency modulates the
differentiation potential of mouse embryonic stem cells. PLoS One.
8:e578912013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang QZ, Xu W, Habib N and Xu R: Potential
uses of microRNA in lung cancer diagnosis, prognosis, and therapy.
Curr Cancer Drug Targets. 9:572–594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sessa R and Hata A: Role of microRNAs in
lung development and pulmonary diseases. Pulm Circ. 3:315–328.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sheng Y, Li J, Zou C, Wang S, Cao Y, Zhang
J, Huang A and Tang H: Downregulation of miR-101-3p by hepatitis B
virus promotes proliferation and migration of hepatocellular
carcinoma cells by targeting Rab5a. Arch Virol. 159:2397–2410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li P, Li J, Chen T, Wang H, Chu H, Chang
J, Zang W, Wang Y, Ma Y, Du Y, et al: Expression analysis of serum
microRNAs in idiopathic pulmonary fibrosis. Int J Mol Med.
33:1554–1562. 2014.PubMed/NCBI
|
|
28
|
Hassan F, Nuovo GJ, Crawford M, Boyaka PN,
Kirkby S, Nana-Sinkam SP and Cormet-Boyaka E: MiR-101 and miR-144
regulate the expression of the CFTR chloride channel in the lung.
PLoS One. 7:e508372012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho HM, Jeon HS, Lee SY, Jeong KJ, Park
SY, Lee HY, Lee JU, Kim JH, Kwon SJ, Choi E, et al: MicroRNA-101
inhibits lung cancer invasion through the regulation of enhancer of
zeste homolog 2. Exp Ther Med. 2:963–967. 2011.PubMed/NCBI
|
|
30
|
Zhang JG, Guo JF, Liu DL, Liu Q and Wang
JJ: MicroRNA-101 exerts tumor-suppressive functions in non-small
cell lung cancer through directly targeting enhancer of zeste
homolog 2. J Thorac Oncol. 6:671–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sheng Y, Ding S, Chen K, Chen J, Wang S,
Zou C, Zhang J, Cao Y, Huang A and Tang H: Functional analysis of
miR-101-3p and Rap1b involved in hepatitis B virus-related
hepatocellular carcinoma pathogenesis. Biochem Cell Biol.
92:152–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barh D, Malhotra R, Ravi B and Sindhurani
P: MicroRNA let-7: An emerging next-generation cancer therapeutic.
Curr Oncol. 17:70–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnson SM, Grosshans H, Shingara J, Byrom
M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack
FJ: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Childs G, Fazzari M, Kung G, Kawachi N,
Brandwein-Gensler M, McLemore M, Chen Q, Burk RD, Smith RV,
Prystowsky MB, et al: Low-level expression of microRNAs let-7d and
miR-205 are prognostic markers of head and neck squamous cell
carcinoma. Am J Pathol. 174:736–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schubert M, Spahn M, Kneitz S, Scholz CJ,
Joniau S, Stroebel P, Riedmiller H and Kneitz B: Distinct microRNA
expression profile in prostate cancer patients with early clinical
failure and the impact of let-7 as prognostic marker in high-risk
prostate cancer. PLoS One. 8:e650642013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Acunzo M, Romano G, Palmieri D, Laganá A,
Garofalo M, Balatti V, Drusco A, Chiariello M, Nana-Sinkam P and
Croce CM: Cross-talk between MET and EGFR in non-small cell lung
cancer involves miR-27a and Sprouty2. Proc Natl Acad Sci USA.
110:8573–8578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Q, Li DC, Li ZF, Liu CX, Xiao YM,
Zhang B, Li XD, Zhao J, Chen LP, Xing XM, et al: Upregulation of
miR-27a contributes to the malignant transformation of human
bronchial epithelial cells induced by SV40 small T antigen.
Oncogene. 30:3875–3886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kane NM, Howard L, Descamps B, Meloni M,
McClure J, Lu R, McCahill A, Breen C, Mackenzie RM, Delles C, et
al: Role of microRNAs 99b, 181a and 181b in the differentiation of
human embryonic stem cells to vascular endothelial cells. Stem
cells. 30:643–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pan D: Hippo signaling in organ size
control. Genes Dev. 21:886–897. 2007. View Article : Google Scholar : PubMed/NCBI
|