Introduction
Gastric cancer (GC) is the third leading cause of
cancer-associated mortality across the two genders, and the fifth
most common malignancy worldwide. Half of the total GC cases
worldwide occur in Eastern Asia, mainly in China (1), and the management of the disease
continues to evolve. In Asia, an extended resection followed by
adjuvant chemotherapy represents the standard care regimen
(2). Oxaliplatin (L-OHP) forms the
basis of treatment of multiple types of cancer, and the current GC
adjuvant therapy regimen in Eastern Asia is S-1 or capecitabine
combined with L-OHP (2,3). Although L-OHP responsiveness is
initially high, patients ultimately develop L-OHP resistance
(4). Nevertheless, the exact
mechanisms of L-OHP resistance in GC cells have not been
elucidated. Strategies to decrease the resistance of GC to
platinum-based chemotherapy must, therefore, be developed.
DNA repair capability is a key contributor to the
resistance to L-OHP; specifically, the excision repair
cross-complementation group 1 (ERCC1) protein has an essential role
in nucleotide excision repair (5).
ERCC1 may be an effective prognostic factor for gastric cancer
patients following radical resection; it has previously been
suggested that ERCC1-negative patients may benefit more from
adjuvant chemotherapy (6).
Furthermore, high ERCC1 expression may be a critical indicator of a
poor clinical outcome, and may be predictive of resistance to
pharmaceutical treatment in advanced GC patients (7). Although L-OHP resistance may be
partially explained by ERCC1 expression, the effects of other L-OHP
resistance-associated genes, including taxol resistance gene 1
(Txr1), should also be evaluated.
Txr1 is a drug resistance gene receiving increasing
attention; this gene decreases thrombospondin-1 (TSP1) secretion
and causes taxol resistance (8).
Previous studies using lung (9) and
breast (10) cancer cells have
revealed that Txr1 upregulation may induce drug resistance in
cancer cells. Our previous study revealed that Txr1 expression is
also associated with the 5-year overall survival (OS) rate of GC
patients and may represent a target to reverse L-OHP resistance in
GC (11).
The aim of the present study was to identify the
molecular mechanism of Txr1-associated L-OHP resistance. A variety
of proteins, including ERCC1 and TSP1, were detected; these
proteins may have clinical potential as predictive biomarkers.
Materials and methods
Cell culture and treatments
The human GC cell lines, AGS, SGC-7901, MNK-45 and
BGC-823, were purchased from China Infrastructure of Cell Line
Resources (Beijing, China) and subsequently cultured in Gibco
Dulbecco's modified Eagle's medium supplemented with 10%
heat-inactivated fetal calf serum, 100 U/ml penicillin and 100
µg/ml streptomycin (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The cells were cultured in a 5% CO2, humidified
incubator at 37°C. Goat polyclonal antibodies against Txr1 (1:100;
cat. no., sc-244548), and mouse monoclonal antibodies against
β-actin (1:1,000; cat. no., sc-8432) and TSP1 (1:100; cat. no.,
sc-59887) were acquired from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The mouse anti-ERCC1 monoclonal antibody (1:500;
cat. no., MA5–13830) was purchased from Thermo Fisher Scientific,
Inc.
Quantitative polymerase chain reaction
(qPCR)
Total RNA from cell line samples was prepared using
TRIzol reagent (Thermo Fisher Scientific, Inc.) in accordance with
the manufacturer's protocols. RNA was diluted to 100 ng/µl using
DNase/RNase-free water and the quality and quantity of RNA was
assessed using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA), and the RNA samples were
subsequently stored at −80°C. Complementary DNA synthesis was
performed with reverse transcriptase using the Reverse
Transcription system (cat. no., A3500; Promega Corporation,
Madison, WI, USA) in accordance with the manufacturer's protocols.
Total RNA (10 ng/µl) in 5 µl nuclease-free water was added to 3 µl
5X RT primer, 1.5 µl 10X reverse transcriptase buffer, 0.15 µl 100
mM dNTPs, 0.19 µl RNase inhibitor, 4.16 µl nuclease-free water and
50 units reverse transcriptase in a total volume of 20 µl. The
reaction was performed for 60 min at 42°C, then for 5 min at 95°C,
in triplicate.
mRNA levels were quantified using a SYBR Green
Real-Time PCR Master mix (Thermo Fisher Scientific, Inc.) and a
7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Primers to amplify the cDNA were obtained from
Sangon Biotech Co., Ltd. (Shanghai, China). Thermal cycling was
performed as follows: 95°C for 5 min, followed by 38 cycles of 95°C
for 15 sec and 60°C for 60 sec. Relative expression was calculated
using the comparative cycle quantification (Cq) method
(ΔΔCq), and β-actin was used for normalization. All PCR
amplifications were performed in triplicate and the experiment was
repeated three times. The primers used are listed in Table I.
 | Table I.Primers used to detect Txr1, TSP1 and
ERCC1 expression by quantitative PCR. |
Table I.
Primers used to detect Txr1, TSP1 and
ERCC1 expression by quantitative PCR.
| Primer | Sequence (5′–3′) |
|---|
| Txr1 |
|
|
Forward |
AAGGTTGCTGGGAAGTAGAGTC |
|
Reverse |
ATTGGGCTAAGGAGGAGAGGTA |
| TSP1 |
|
|
Forward |
CGTGGTCATCTTGTTCTGTGA |
|
Reverse |
AGGGTTTCCCGTTCATCTG |
| ERCC1 |
|
|
Forward |
CCTCAGACCTACGCCGAATA |
|
Reverse |
GGCTCACAATGATGCTGTTG |
Lentivirus-mediated RNA interference
and overexpression
Human Txr1 cDNA was subcloned into the plasmid
pLenti6/V5. Txr1 and an empty control recombinant lentivirus
(Lv.Txr1 and Lv.Empty) were generated by Shanghai GenePharma Co.,
Ltd. (Shanghai, China). A lentivirus expressing Txr1 small
interfering (si)RNA (siTxr1) and a negative control lentivirus
(siCON) were also generated by Shanghai GenePharma Co., Ltd.
Cell proliferation assay
Cell proliferation was analyzed in triplicate using
the CellTiter 96 AQueous One Solution Cell Proliferation assay (MTS
assay; Promega Corporation) in accordance with the manufacturer's
protocols. Briefly, 5,000 cells/well were grown in RPMI-1640 medium
(100 µl/well; Gibco; Thermo Fisher Scientific, Inc.) in 96-well
plates and exposed to various concentrations of the experimental
drug (0.001, 0.01, 0.1, 1, 10, 100 and 1,000 µg/ml). After 48 h of
drug exposure, 20 µl MTS was added to the medium and the cells were
incubated in a 5% CO2 incubator at 37°C for 2–4 h. The
absorbance, which is directly proportional to the number of viable
cells in culture, was measured at 490 nm using a plate reader
(Model 680; Bio-Rad Laboratories, Hercules, CA, USA). Relative
growth was, therefore, calculated using the following formula:
Growth (%) = [(Atreated -
Azero)/(Acontrol - Azero)] × 100,
where ‘Atreated’ was the number of living cells in the
treated wells, ‘Acontrol’ was the number of living cells
in the untreated wells and ‘Azero’ was the background
absorbance, which was subtracted to correct for error.
Western blot analysis
Whole-cell extracts were prepared using a keyGEN
Whole Cell Lysis assay (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). The samples were denatured by boiling for 5 min in loading
buffer, separated using a 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and electroblotted onto a polyvinylidene
difluoride membrane (Thermo Fisher Scientific, Inc.). The membranes
were incubated with primary antibodies for 2 h, washed with
Tris-buffered saline plus Tween 20 (TBST; Bio-Rad Laboratories,
Inc.) buffer for 30 min and incubated with horseradish
peroxidase-conjugated bovine anti-mouse IgG (1:10,000; cat. no.,
sc-2371) and chicken anti-goat immunoglobulin (Ig)G (1:10,000; cat.
no., sc-2953) secondary antibodies at room temperature for 1 h. The
membranes were subsequently washed again with TBST for 30 min, and
the immunolabeled bands were detected using an enhanced
chemiluminescence kit (SuperSignal West Pico Chemiluminescent
Substrate kit; Thermo Fisher Scientific, Inc.). The blots were
scanned using an Epson Perfection Photo Scanner (Tokyo, Japan) and
analysis of these was performed with ImageJ (National Institutes of
Health, Bethesda, MA, USA) by measuring the densities of the
immunoreactive bands.
Flow cytometry (FCM) analysis
An Annexin V/propidium iodide (PI) double staining
assay was performed using an Annexin V-fluorescein isothiocyanate
apoptosis detection kit (Sigma-Aldrich, St. Louis, MO, USA) in
accordance with the manufacturer's protocols. Briefly, after a 48-h
transfection with the aforementioned treatments, 1×106
cells/well were harvested and washed twice with phosphate-buffered
saline in 6-well plates. The cells were resuspended in 1X binding
buffer and incubated with Annexin V-FITC and PI for 15 min, in the
dark, at room temperature. Apoptosis was detected by FCM using BD
CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
All experiments were performed independently in
triplicate. The results are reported as the mean ± standard
deviation, and statistical analyses were performed using the SPSS
17.0 software (SPSS Inc., Chicago, IL, USA). Significant
differences were assessed using a standard one-way analysis of
variance and two-tailed unpaired Student's t-test. P<0.05 was
considered to represent a statistically significant difference.
Results
L-OHP sensitivity of GC cells is
associated with endogenous Txr1 expression
L-OHP sensitivity was detected in the GC cell lines
(AGS, MNK-45, BGC-823 and SGC-7901) using an MTS assay; in these
cells, viability was inhibited by L-OHP in a dose-dependent manner.
The half-maximal inhibitory concentration values in 48 h of L-OHP
treatment in the AGS, MNK-45, BGC-823 and SGC-7901 cells were 3.78,
4.79, 5.74 and 8.45 mg/l, respectively. Although the trends were
similar, the L-OHP sensitivities of the 4 GC cell lines differed
(Fig. 1A), suggesting that other
factors may mediate L-OHP sensitivity. Endogenous Txr1 expression
in the AGS, MNK-45, BGC-823 and SGC-7901 cells was evaluated using
qPCR. As reported in Fig. 1B, Txr1
mRNA levels differed amongst the cell types. Notably, Txr1 levels
were significantly higher in the SGC-7901 cells, as compared with
the other GC cell lines (P<0.05 vs. AGS; Fig. 1B), and Txr1 expression varied between
the cell lines in a pattern that was converse to the variation
observed in L-OHP sensitivity. Furthermore, higher Txr1 expression
was correlated with lower L-OHP sensitivity in the SGC-7901 cells,
suggesting a negative association between L-OHP sensitivity and
endogenous Txr1 expression (Fig.
1C). These data indicated that L-OHP sensitivity is associated
with endogenous Txr1 expression in GC cell lines.
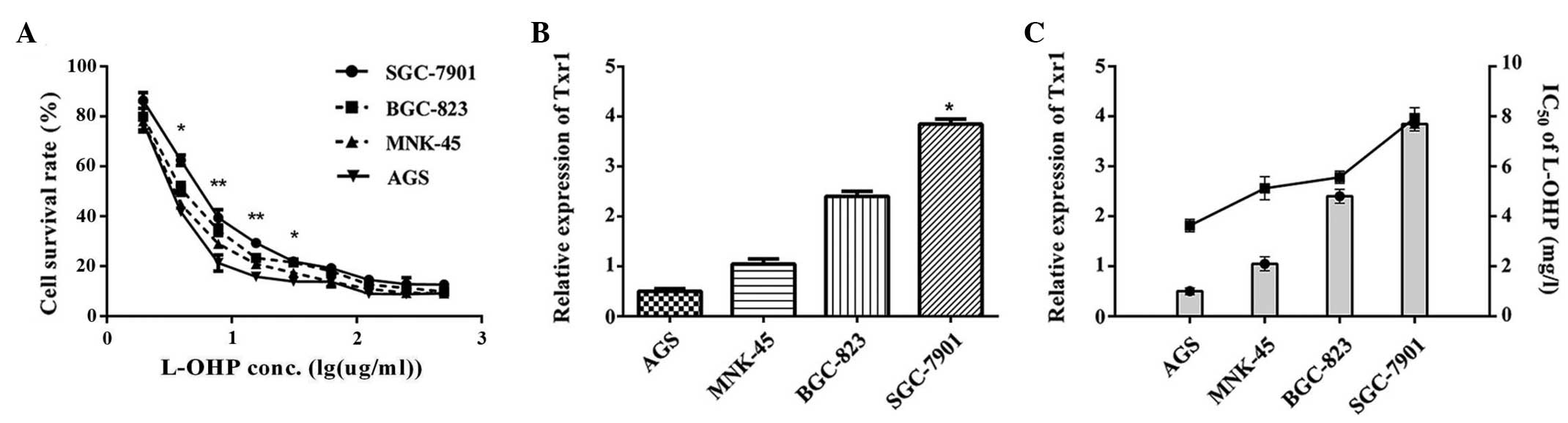 | Figure 1.L-OHP sensitivity of GC cells is
correlated with endogenous Txr1 expression. (A) Cell viability,
assessed using an MTS assay. Cells were all treated with the same
dose of L-OHP (7.8 mg/l) for 48 h. *P<0.05 and **P<0.01,
SGC-7901 vs. AGS. (B) Txr1 expression in GC cell lines, measured by
qPCR, indicating that Txr1 expression is significantly higher in
SGC-7901 cells. *P<0.05 vs. the AGS group. (C) Sensitivity of
AGS, MNK-45, BGC-823 and SGC-7901 cells to L-OHP was based on their
half-maximal inhibitory concentration values (3.78±0.32, 4.79±0.21,
5.74±0.17 and 8.45±0.10 mg/l, respectively). Results are
representative of 3 independent experiments. Error bars represent
the standard error of the mean. L-OHP, oxaliplatin; GC, gastric
cancer; Txr-1, taxol resistance gene 1. |
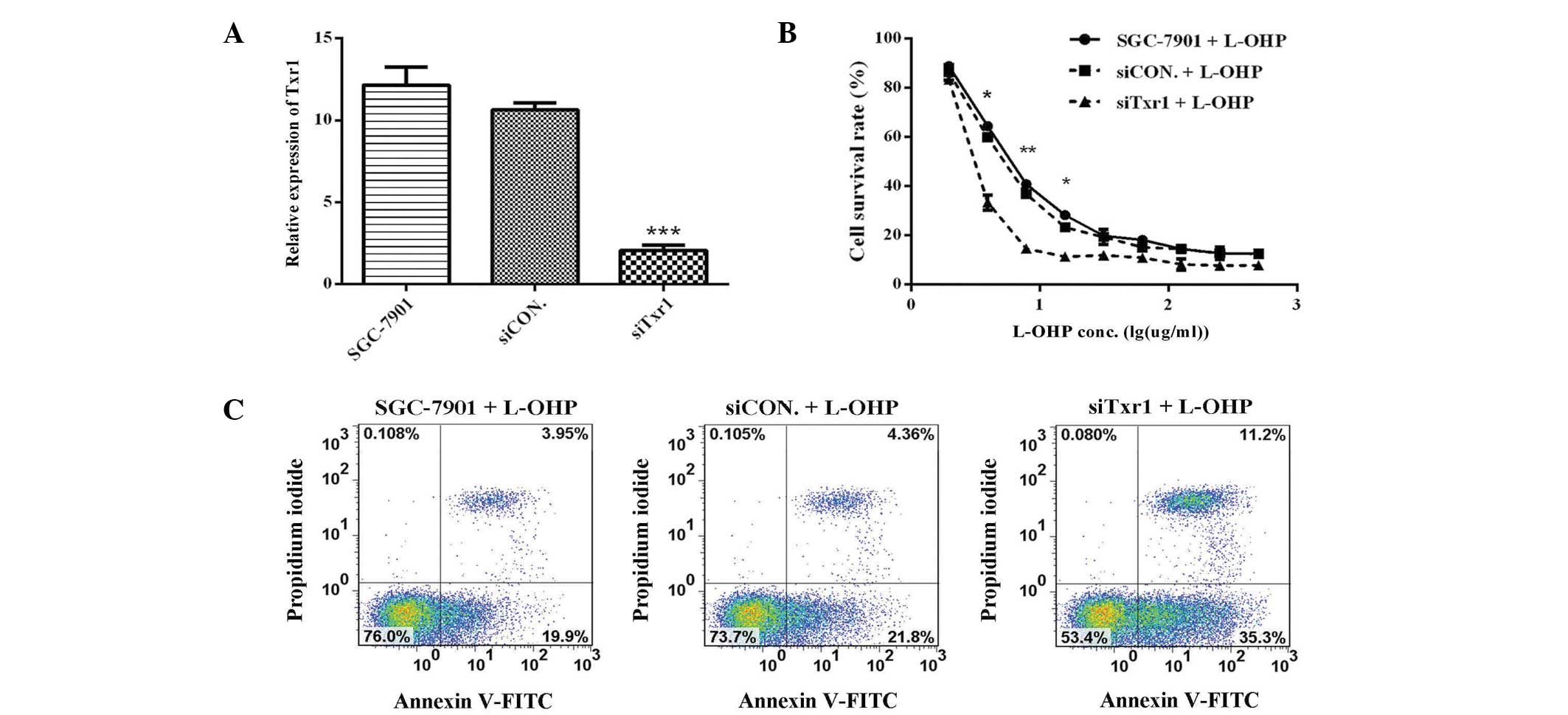
Txr1-knockdown sensitizes SGC-7901
cells to L-OHP
Txr1 downregulation was hypothesized to increase
L-OHP sensitivity. SGC-7901 cells were selected as an in
vitro model, and Txr1 was knocked down in these cells by siTxr1
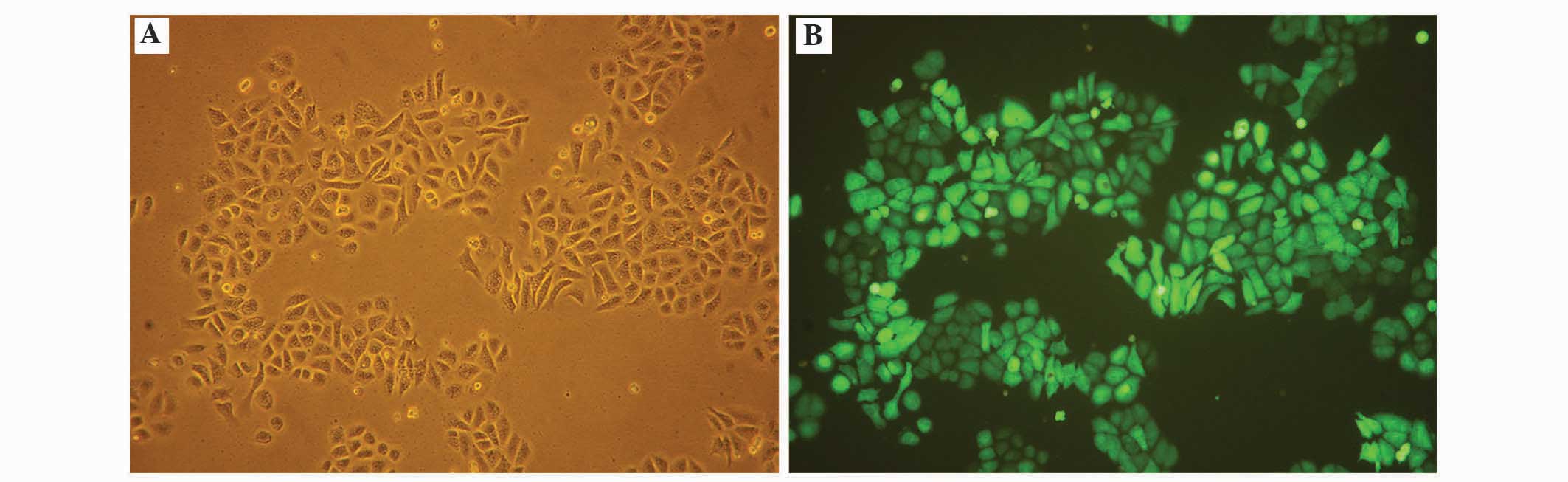
lentiviral transfection. The lentiviral transfection efficiencies
in the SGC-7901 cells were evaluated by microscopy 48 h after
transfection, revealing >90% lentiviral transfection efficiency
(Fig. 2). Txr1 expression was also
examined in the SGC-7901 cells following lentiviral transfection
using qPCR. Txr1 expression was significantly decreased by 80.5%
(P<0.001) in siTxr1-transfected cells, as compared with
siCON-transfected cells (Fig. 3A),
demonstrating that the lentiviral-mediated siRNA effectively and
specifically reduced Txr1 expression in the SGC-7901 cells. To
assess whether decreased Txr1 expression enhanced L-OHP
sensitivity, the cells transfected with the siCON or siTxr1
lentivirus were treated with L-OHP and viability was measured using
an MTS assay. siTxr1-lentiviral transfection enhanced the
L-OHP-mediated suppression of SGC-7901 cell viability, particularly
at lower L-OHP doses (7.8 mg/l) (Fig.
3B). Txr1-knockdown therefore allowed the L-OHP dose used for
treatment to be reduced.
Apoptosis is the predominant mechanism of
L-OHP-induced toxicity. An Annexin V/PI assay was therefore used to
measure the apoptotic frequency in Txr1-knockdown SGC7901 cells
treated with L-OHP (7.8 mg/l). Compared with L-OHP treatment alone,
L-OHP treatment with Txr1-knockdown increased the induction of
apoptosis in SGC-7901 cells (35.3 vs. 19.9%, 21.8% of cells in
early apoptosis; and 11.2 vs. 3.95%, 4.36% of cells in late
apoptosis) (Fig. 3C). Txr1-knockdown
therefore sensitized the cells to L-OHP-induced apoptosis, thereby
enhancing the cytotoxic effect of L-OHP.
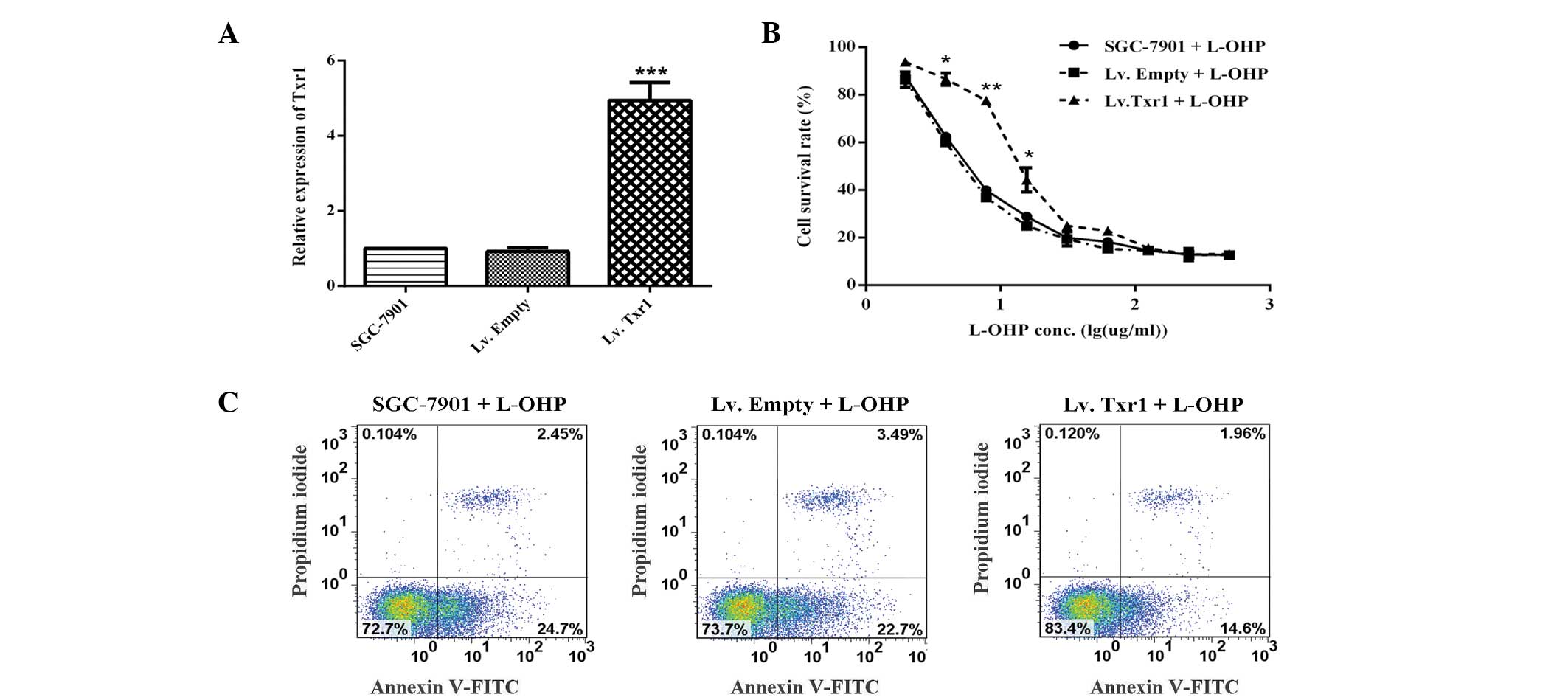
Txr1 overexpression promotes L-OHP
resistance in SGC-7901 cells
In order to investigate the role of Txr1 in the
L-OHP sensitivity of the SGC-7901 cells, Txr1-overexpressing cells
were used. Following transfection with Lv.Empty or Lv.Txr1, the
SGC-7901 cells were treated with L-OHP at various concentrations
(Fig. 4A and B). As expected, Txr1
overexpression significantly decreased apoptosis (14.6 vs. 22.7%,
24.7% of cells in early apoptosis; and 1.96 vs. 3.49%, 2.54% of
cells in late apoptosis) (Fig. 4C).
These gain- and loss-of-function experiments therefore indicated an
important function of Txr1 in the L-OHP sensitivity of SGC-7901
cells.
Effects of Txr1 on the expression of
resistance-associated factors in SGC-7901 GC cells
Due to the inverse correlation observed between Txr1
levels and L-OHP sensitivity, additional mechanistic experiments
were warranted. TSP1 and ERCC1 expression was examined by qPCR and
western blotting; TSP1 protein levels were significantly increased
and ERCC1 levels were decreased in the siTxr1-transfected group
compared with the CON groups (P<0.001; Fig. 5A and B). Whereas, in the
lentivirus-mediated overexpression Txr1 group, TSP1 protein levels
were decreased and ERCC1 levels were increased, as compared with
the CON groups (P<0.001; Fig. 5C and
D). These data suggest that Txr1-knockdown may mediate GC
sensitization to L-OHP via TSP1 and ERCC1.
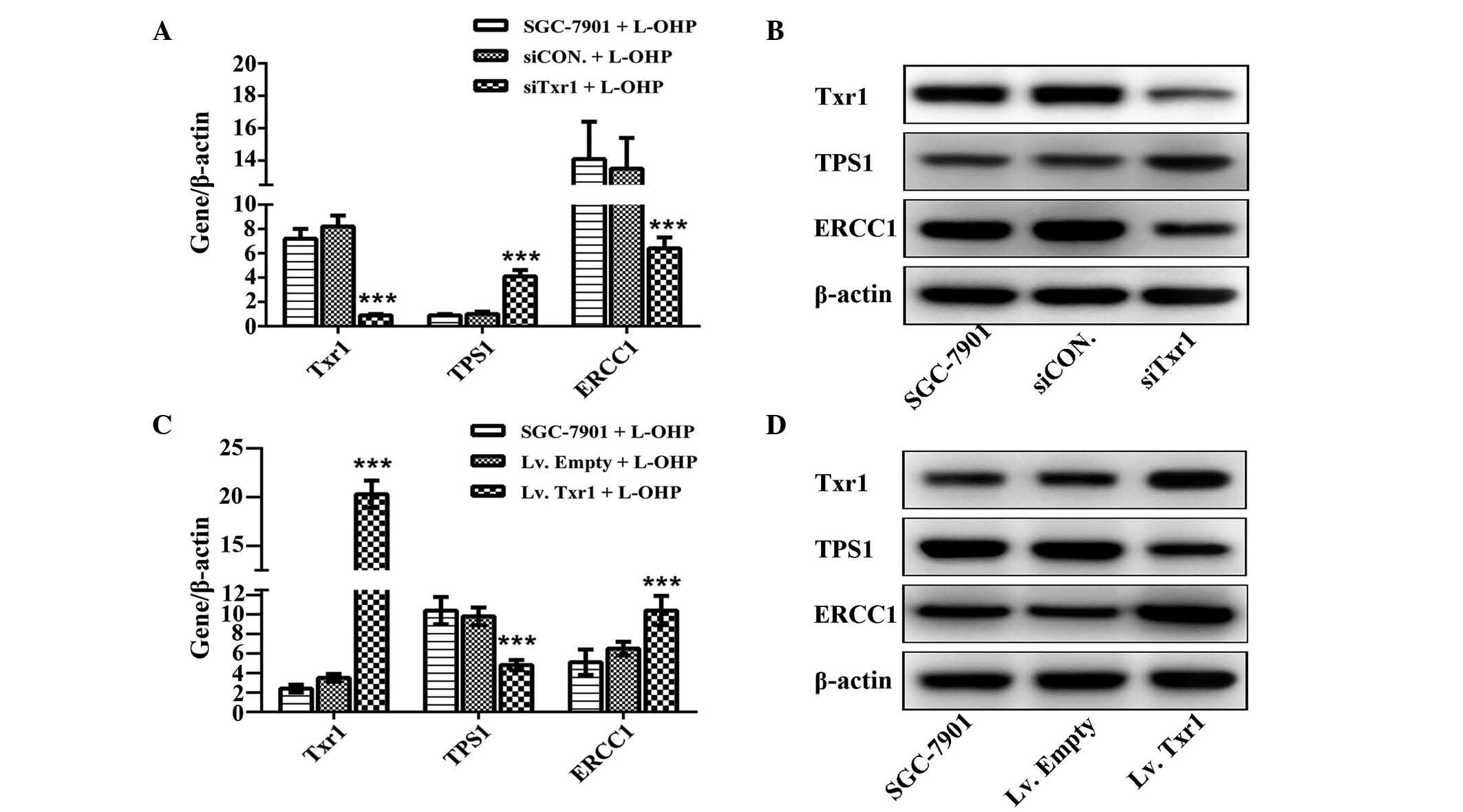 | Figure 5.TSP1 and ERCC1 are involved in
Txr1-mediated L-OHP sensitivity. (A) Txr1, TSP1 and ERCC1 mRNA
levels in SGC7901 cells following siCON or siTxr1 lentiviral
transfection and L-OHP treatment, detected using qPCR
(***P<0.001 vs. CON). (B) Txr1, TSP1 and ERCC1 protein levels,
examined by western blotting and using β-actin as a loading
control. (C) Txr1, TSP1 and ERCC1 mRNA levels in SGC-7901 cells
following control or Txr1 lentiviral transfection and L-OHP
treatment, detected by qPCR (***P<0.001 vs. CON). (D) Txr1, TSP1
and ERCC1 protein levels, examined by western blotting and using
β-actin as a loading control. Txr-1, taxol resistance gene 1; CON,
negative control; Lv, recombinant lentivirus; TSP1,
thrombospondin-1; ERCC, excision repair cross-complementing 1
protein; L-OHP, oxaliplatin; si, small interfering; qPCR,
quantitative polymerase chain reaction. |
Discussion
Surgical resection for GC is the only potentially
curative treatment method, however, the majority of patients
relapse following resection; therefore, combinatorial treatment
approaches are standard for disease that is advanced beyond stage
1B (12). A previous, large
meta-analysis of adjuvant chemotherapy in GC conducted at the
individual patient level confirmed a 6% improvement in outcomes
following 5-fluorouracil (5-FU)-based chemotherapy compared with
surgery alone (hazard ratio, 0.82; 95% confidence interval,
0.76–0.90; P<0.001) in all subgroups tested (13). Combination regimens based on a
platinum-fluoropyrimidine doublet are typically used as first-line
chemotherapy options (14). L-OHP
has been a crucial component in combination therapies since its
clinical introduction, resulting in modest survival improvements
across multiple malignancies (15).
The Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer
trial (16), conducted in South
Korea, China and Taiwan, evaluated an adjuvant chemotherapy
combining capecitabine with L-OHP following curative D2 gastrectomy
and compared results with surgery alone, reporting significantly
improved OS and disease-free survival.
L-OHP forms the basis of multiple treatment
regimens. Despite its modest activity as a single agent, L-OHP
exerts significant activity when used in combination with other
drugs (17). As with other
anticancer drugs, tumor cells can acquire resistance to the
cytotoxic effects of L-OHP. The mechanism of L-OHP resistance has
previously been demonstrated to be mediated by Txr1, which
downregulates TSP1 in tumors (11,18). In
our previous study, we reported that the 5-year OS rate of GC
patients with high Txr1 expression was lower than that of patients
with low Txr1 expression; Txr1 expression may therefore be used as
an independent prognostic indicator (11).
To assess the role of Txr1 in the L-OHP resistance
of GC cells, the present study first examined Txr1 expression in 4
GC cell lines and revealed a negative association between L-OHP
sensitivity and endogenous Txr1 expression. Following transfection
with siTxr1, the SGC-7901 cells demonstrated increased L-OHP
sensitivity, indicating that endogenous Txr1 may protect cells from
L-OHP cytotoxicity. To demonstrate that Txr1 conferred L-OHP
resistance, cells overexpressing exogenous Txr1 using Lv.Txr1 were
used; these cells exhibited increased L-OHP resistance. A likely
association between Txr1 expression and L-OHP resistance was
therefore identified.
TSP1 gene expression was also analyzed in the
present study, revealing that TSP1 levels were positively
correlated with L-OHP sensitivity. TSP1 was previously reported to
have anti-angiogenic and pro-apoptotic activities in oncogenesis
(19). TSP1 is an important mediator
of taxane-induced apoptosis via the prevention of angiogenesis and
the induction of apoptosis in malignant cells (20).
Papadaki et al (9) reported that, amongst lung
adenocarcinoma patients administered a combinatorial treatment of
docetaxel and gemcitabine, patients demonstrating low Txr1 and high
TSP1 expression levels experienced higher survival rates compared
with patients demonstrating the converse. Similar results were
obtained in non-small cell lung cancer patients treated with
docetaxel in association with cisplatin or gemcitabine (21). Txr1 is therefore likely to have
diverse effects on different cell types in a TSP1-dependent manner.
An inverse correlation has previously been identified between TSP1
mRNA levels and the gastric cardia adenocarcinoma
tumor-node-metastasis stage (22).
In our previous study, exogenous Txr1 expression was associated
with decreased TSP1 mRNA in BGC-823 cells, possibly contributing to
taxol resistance (11). In the
present study, Txr1-knockdown in SGC-7901 cells led an to increased
TSP1 expression level, apoptosis rate and L-OHP sensitivity. Txr1
overexpression decreased TSP1 expression, resulting in apoptosis
inhibition, which may be the underlying etiology of L-OHP
resistance in GC.
DNA repair is a principal mechanism underlying the
resistance to platinum-based therapy (23). The repair of platinum-associated DNA
damage requires resolution of the associated DNA break, which
involves a variety of repair proteins, including ERCC1, which may
have clinical potential as a predictive biomarker (24). In the present study, ERCC1 expression
was increased when Txr1 was exogenously expressed, meaning that
Txr1 and ERCC1 mRNA levels are negatively correlated with L-OHP
sensitivity. Several pre-clinical studies have demonstrated that
ERCC1 has an important role in determining platinum drug
sensitivity; increased ERCC1 expression is associated with platinum
drug resistance (25–27). Clinically, high ERCC1 levels are
correlated with poor responses to platinum-based chemotherapy in GC
(28). ERCC1 acts on larger lesions
covering 20–25 nucleotides, therefore, an efficient DNA repair
capacity of ERCC1 appears to be critical for resistance to platinum
drugs (29,30). Previous data from in vitro
systems have revealed that suppression of ERCC1 expression enhances
or restores the sensitivity to platinum. This has significant
clinical implications, as the inclusion of factors targeting ERCC1
may increase platinum activity and/or oppose resistance, although
this remains unreported in patients (31).
Drug resistance in GC patients appears to be
complex. The presence of one protein can markedly affect a
multitude of proteins and pathways, and the associations between
apparently unrelated proteins and pathways are only now being
revealed (32). The screening and
validating of molecular biomarkers capable of predicting the
response to different chemotherapeutic agents represent significant
steps toward personalized treatment for cancer patients. The
present study focused on Txr1, as this gene has previously been
identified as a drug resistance gene in numerous solid tumors,
including GC. The current study demonstrated that Txr1-knockdown in
SGC7901 cells enhanced their chemosensitivity to L-OHP. The
identification of Txr1 as a potential resistance gene in L-OHP
therapy provides a fundamental basis for novel approaches in
developing L-OHP-based therapeutic strategies. Furthermore, the
introduction of novel anti-microtubule platinum agents requires
elucidation of the biological mechanisms mediating the resistance
effects of these drugs.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172317) and
Beijing Municipal Administration of Hospitals Clinical Medicine
Development of Special Funding Support (grant no. ZYLC201504).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Foo M and Leong T: Adjuvant therapy for
gastric cancer: Current and future directions. World J
Gastroenterol. 20:13718–13727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park SC and Chun HJ: Chemotherapy for
advanced gastric cancer: Review and update of current practices.
Gut Liver. 7:385–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao Q, Zhang H, Li Y, Liu J, Hu X and Fan
L: Anti-tumor effects of CIK combined with oxaliplatin in human
oxaliplatin-resistant gastric cancer cells in vivo and in vitro. J
Exp Clin Cancer Res. 29:1182010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Zhou XQ, Li JY, Cheng JF, Zeng XN,
Li X and Liu P: Prognostic significance of ERCC1 expression in
postoperative patients with gastric cancer. Chin J Cancer Res.
26:323–330. 2014.PubMed/NCBI
|
|
7
|
Yao A, Wang Y, Peng X, Ye R, Wang Q, Qi Y
and Zhou F: Predictive value of excision repair
cross-complementation group 1 expression for platinum-based
chemotherapy and survival in gastric cancer: A meta-analysis. J
Cancer Res Clin Oncol. 140:2107–2117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lih CJ, Wei W and Cohen SN: Txr1: A
transcriptional regulator of thrombospondin-1 that modulates
cellular sensitivity to taxanes. Genes Dev. 20:2082–2095. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Papadaki C, Mavroudis D, Trypaki M,
Koutsopoulos A, Stathopoulos E, Hatzidaki D, Tsakalaki1 E,
Georgoulias V and Souglakos J: Tumoral expression of TXR1 and TSP1
predicts overall survival of patients with lung adenocarcinoma
treated with first-line docetaxel-gemcitabine regimen. Clin Cancer
Res. 15:3827–3833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bai Z, Zhang Z, Qu X, Han W and Ma X:
Sensitization of breast cancer cells to taxol by inhibition of
taxol resistance gene 1. Oncol Lett. 3:135–140. 2012.PubMed/NCBI
|
|
11
|
Bai ZG, Qu X, Han W, Ma XM, Zhao XM and
Zhang ZT: Expression of taxol resistance gene 1 correlates with
gastric cancer patient clinical outcome and induces taxol
resistance. Mol Med Rep. 3:1071–1078. 2010.PubMed/NCBI
|
|
12
|
Waddell T, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D: European Society for Medical Oncology
(ESMO); European Society of Surgical Oncology (ESSO); European
Society of Radiotherapy and Oncology (ESTRO): ESMO; ESSO; ESTRO:
Gastric cancer: cancer: Gastric cancer: ESMO-ESSO-ESTRO clinical
practice guidelines for diagnosis, treatment and follow-up. J Eur J
Surg Oncol. 40:584–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
GASTRIC (Global Advanced/Adjuvant Stomach
Tumor Research International Collaboration) Group. Paoletti X, Oba
K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P,
Sakamoto J, Sargent D, Sasako M, et al: Benefit of adjuvant
chemotherapy for resectable gastric cancer: A meta-analysis. JAMA.
303:1729–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Waddell T, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D: European Society for Medical Oncology
(ESMO); European Society of Surgical Oncology (ESSO); and European
Society of Radiotherapy and Oncology (ESTRO): Gastric cancer:
ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 24(Suppl 6): vi57–vi63. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alian OM, Azmi AS and Mohammad RM: Network
insights on oxaliplatin anti-cancer mechanisms. Clin Transl Med.
1:262012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noh SH, Park SR, Yang HK, Chung HC, Chung
IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, et al: CLASSIC trial
investigators: Adjuvant capecitabine plus oxaliplatin for gastric
cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an
open-label, randomised phase 3 trial. Lancet Oncol. 15:1389–1396.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Francia R, Siesto RS, Valente D, Del
Buono A, Pugliese S, Cecere S, Cavaliere C, Nasti G, Facchini G and
Berretta M: Current strategies to minimize toxicity of oxaliplatin:
Selection of pharmacogenomic panel tests. Anticancer Drugs.
24:1069–1078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bi J, Bai Z, Ma X, Song J, Guo Y, Zhao J,
Yi X, Han S and Zhang Z: Txr1: An important factor in oxaliplatin
resistance in gastric cancer. Med Oncol. 31:8072014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sid B, Sartelet H, Bellon G, El Btaouri H,
Rath G, Delorme N, Haye B and Martiny L: Thrombospondin. 1 A
multifunctional protein implicated in the regulation of tumor
growth. Crit Rev Oncol Hematol. 49:245–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Amerongen R and Berns A: TXR1-mediated
thrombospondin repression: A novel mechanism of resistance to
taxanes? Genes Dev. 20:1975–1981. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Papadaki C, Tsaroucha E, Kaklamanis L,
Lagoudaki E, Trypaki M, Tryfonidis K, Mavroudis D, Stathopoulos E,
Georgoulias V and Souglakos J: Correlation of BRCA1, TXR1 and TSP1
mRNA expression with treatment outcome to docetaxel-based
first-line chemotherapy in patients with advanced/metastatic
non-small-cell lung cancer. Br J Cancer. 104:316–323. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo W, Dong Z, He M, Guo Y, Guo J, Chen Z,
Yang Z and Kuang G: Aberrant methylation of thrombospondin-1 and
its association with reduced expression in gastric cardia
adenocarcinoma. J Biomed Biotechnol. 2010:7214852010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reardon JT, Vaisman A, Chaney SG and
Sancar A: Efficient nucleotide excision repair of cisplatin,
oxaliplatin, and Bis-aceto-ammine-dichloro-cyclohexylamine-platinum
(IV) (JM216) platinum intrastrand DNA diadducts. Cancer Res.
59:3968–3971. 1999.PubMed/NCBI
|
|
24
|
Deng Q, Yang H, Lin Y, Qiu Y, Gu X, He P,
Zhao M, Wang H, Xu Y, Lin Y, et al: Prognostic value of ERCC1 mRNA
expression in non-small cell lung cancer, breast cancer, and
gastric cancer in patients from Southern China. Int J Clin Exp
Pathol. 7:8312–8321. 2014.PubMed/NCBI
|
|
25
|
Lee KB, Parker RJ, Bohr V, Cornelison T
and Reed E: Cisplatin sensitivity/resistance in UV repair-deficient
Chinese hamster ovary cells of complementation groups 1 and 3.
Carcinogenesis. 14:2177–2180. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Melton DW, Ketchen AM, Núñez F,
Bonatti-Abbondandolo S, Abbondandolo A, Squires S and Johnson RT:
Cells from ERCC1-deficient mice show increased genome instability
and a reduced frequency of S-phase-dependent illegitimate
chromosome exchange but a normal frequency of homologous
recombination. J Cell Sci. 111:395–404. 1998.PubMed/NCBI
|
|
27
|
Youn CK, Kim MH, Cho HJ, Kim HB, Chang IY,
Chung MH and You HJ: Oncogenic H-Ras up-regulates expression of
ERCC1 to protect cells from platinum-based anticancer agents.
Cancer Res. 64:4849–4857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Metzger R, Leichman CG, Danenberg KD,
Danenberg PV, Lenz HJ, Hayashi K, Groshen S, Salonga D, Cohen H,
Laine L, et al: ERCC1 mRNA levels complement thymidylate synthase
mRNA levels in predicting response and survival for gastric cancer
patients receiving combination cisplatin and fluorouracil
chemotherapy. J Clin Oncol. 16:309–316. 1998.PubMed/NCBI
|
|
29
|
Reed E: Platinum-DNA adduct, nucleotide
excision repair and platinum based anti-cancer chemotherapy. Cancer
Treat Rev. 24:331–344. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wood RD, Mitchell M, Sgouros J and Lindahl
T: Human DNA repair genes. Science. 291:1284–1289. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reed E, Yu JJ, Davies A, Gannon J and
Armentrout SL: Clear cell tumors have higher mRNA levels of ERCC1
and XPB than other histological types of epithelial ovarian cancer.
Clin Cancer Res. 9:5299–5305. 2003.PubMed/NCBI
|
|
32
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|