Introduction
Septic encephalopathy (SE), defined as altered
mental status and presenting with behavioral or cognitive
abnormalities, is one of the most common complications in septic
patients and is likely to be under-diagnosed (1). SE is associated with a higher mortality
rate and is also a reliable indicator of a poor clinical outcome
(2,3). Numerous animal and human studies have
been performed to elucidate the etiology of SE (4–6) but, at
present, the pathogenesis of SE is unknown. However, several
potential mechanisms have been investigated, such as alterations to
the blood-brain barrier (BBB), reduction in cerebral blood flow,
the inflammatory response and activation of microglia and
astrocytes and amino acid imbalance (7–10). The
impact of an increased inflammatory response on the central nervous
system (CNS) has been a key focus of investigation during the last
two decades. Excessive inflammation, often termed a ‘cytokine
storm’, characterizes early sepsis (11–14). As
sepsis progresses, patients frequently develop multiple organ
dysfunction and nosocomial infections by opportunistic pathogens
(15–17) and the nervous system is particularly
vulnerable to damage in response to systemic inflammation. Previous
studies (11–13) suggest that patients with sepsis
present with an immune factor imbalance, and there is a clear
connection between immune imbalance and the occurrence of SE.
Extensive previous evidence indicates that anti-inflammatory action
in the brain and the resolution of neuroinflammation requires
balance between the various branches of the immune system (18–20). An
imbalance within the immune system and the systemic inflammation
that results may promote CNS damage. The present retrospective
study aimed to investigate the role of immune imbalance in SE, in
addition to its effect on prognosis, using clinical data from
patients with severe sepsis.
Subjects and methods
Ethics statement
The present study was approved by the Medical Ethics
Committee of Zhongshan Hospital at Xiamen University (Xiamen,
China). The present study did not increase the patient's medical
expenses or pain and all research materials and results were used
for research purposes. The requirement for informed consent was
waived by the Medical Ethics Committee as the present study was an
observational, respective study using a database from which the
patients' identifying information had been removed.
Subjects
Severe sepsis was defined as sepsis combined with
sepsis-induced organ dysfunction or tissue hypoperfusion, in
accordance with criteria set out during the 2001
SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definition Conference
(21). Symptoms of SE include
somnolence, stupor, coma, confusion, disorientation, agitation,
irritability and a decreased score on the Glasgow Coma Scale. SE
was defined as an altered mental status with behavioral or
cognitive abnormalities, but there is no current unified standard
for SE diagnoses (22,23). Patients suffering from the following
underlying conditions that may affect brain and CNS function and
symptomatic diagnosis were excluded: i) Intracranial organic
diseases; ii) severe nutritional deficiency; iii) hypoglycemia; iv)
hypernatremia; v) hepatic encephalopathy; and vi) a history of
exposure to drugs, toxic substances, alcohol, industrial agents,
heavy metals or any substance established to cause altered
consciousness.
In the present study, 127 patients demonstrating
severe sepsis were admitted to the Intensive Care Unit of Zhongshan
Hospital at Xiamen University between January 2014 and January
2015. Of these patients, 41 patients were excluded due to the
aforementioned exclusion criteria, predominantly linked to exposure
to sedative drugs or intracranial organic disease, thus 86 patients
were analyzed in the current study. In total, 57 men and 29 women
were included, with an average age of 58.7 years. During their
hospital stay, 34 patients developed SE although 52 patients did
not, and patients were subsequently assigned to the SE and non-SE
groups. Eighteen patients across the two groups succumbed to the
disease during the 28-day study, representing a frequency of 20.93%
(18/86 patients).
Treatment
The patients were treated with a standardized
therapy based on the Severe Sepsis Campaign sepsis treatment
guidelines (24). This therapy
involved fluid resuscitation, antibiotic therapy, identification
and control of infected tissue, mechanical ventilation, renal
replacement, glucose control and supportive treatments, such as
vasoactive drugs and steroid therapies.
Data collection
The medical record for each patient was reviewed.
Patient demographics, mean arterial pressure, heart rate, duration
of ventilator treatment, Acute Physiology and Chronic Health
Evaluation (APACHE) II score, Sequential Organ Failure Assessment
(SOFA) score and outcome were recorded. APACHE II score and SOFA
score at the time of admission to the Intensive Care Unit were also
calculated.
Blood samples were obtained for routine examination
of metrics, including liver and kidney function, such as alanine
aminotransferase (ALT), bilirubin, activated partial thromboplastin
time and serum creatinine levels; blood glucose; 6-h lactate
clearance; B-type natriuretic peptide; blood gas analysis,
including reporting of pH, arterial partial pressure of oxygen,
arterial partial pressure of carbon dioxide and bicarbonate; brain
injury markers, including levels of neuron specific enolase (NSE)
and S-100β protein; and immune parameters, including white blood
cell count, C-reactive protein, procalcitonin and the percentages
of cluster of differentiation 4+ (CD4+) and
cluster of differentiation 8+ (CD8+)
T-lymphocytes and natural killer (NK) cells present. Flow
cytometery (Elite XL4; Beckman Coulter, Inc., Brea, CA, USA) was
used to detect the proportion of CD4+ and
CD8+ T-lymphocytes, to calculate the
CD4+/CD8+ ratio. Samples were collected in a
test tube at 4°C containing tripotassium hydrogen
ethylenediaminetetraacetate and analyzed within 30 min of
collection.
The specimens were collected from the sputum in the
lower respiratory tract using fiberbronchoscopy (Olympus BF P-30;
Olympus Corporation, Tokyo, Japan), from wound excretions, from
urine and from blood for cultivation and diagnosis of pathogenic
bacteria in a 37°C incubator.
Statistical analysis
Statistical analysis was conducted using SPSS v.
19.0 software (IBM SPSS, Armonk, NY, USA). Measurement data were
expressed as mean ± SD and compared using independent t-tests.
Enumeration data were compared using a χ2 test or with
Fishers exact test, as appropriate. For detection of correlation,
Pearson's correlation analysis was performed. P<0.05 was
considered to indicate a statistically significant difference.
Statistically significant variables were subsequently analyzed
using a binary logistic regression to identify the risk factors
associated with SE. Only variables markedly associated with SE
(P<0.05) were included in the final model. Receiver operating
characteristic (ROC) curves and the area under the curves (AUCs)
were examined in significant variables associated with SE, to
determine a cut-off level and to predict mortality.
Results
Baseline data of the patients
During the period of the present study, 127 patients
with severe sepsis were initially admitted, 86 of whom were
included in the study. Patient characteristics of the two groups
are provided in Table I. No
significant differences were identified between the groups in age,
gender, underlying diseases, mean arterial pressure or heart rate
(P>0.05). However, duration of ventilator treatment and 28-day
mortality were significantly higher in the SE group compared to the
non-SE group (13.12±3.89 vs. 8.28±3.32 days, P<0.01; and 38 vs.
10%, P=0.001, respectively). With regard to the disease severity
index, the SE group had higher APACHE II and SOFA scores than the
non-SE group (21.74±2.96 vs. 16.25±2.62, P<0.01; and 8.21±1.45
vs. 5.38±1.84, P<0.01, respectively), indicating a greater
degree of organ dysfunction.
 | Table I.Baseline characteristics of the SE
and non-SE groups. |
Table I.
Baseline characteristics of the SE
and non-SE groups.
|
Characteristics | SE group
(n=34) | Non-SE group
(n=52) | P-value |
|---|
| Age in years, mean
± SD | 59.15±8.80 | 58.39±8.14 |
0.69 |
| Male/female, n | 24/10 | 33/19 |
0.49 |
| Underlying
diseases, n (%) |
|
|
|
| Chronic
lung disease | 9
(26) | 11 (21) |
0.57 |
|
Hypertension | 8
(24) | 16 (31) |
0.46 |
|
Hyperlipidemia | 10 (29) | 17 (68) |
0.75 |
|
Coronary artery disease | 4
(12) | 8
(15) |
0.76 |
| Chronic
liver disease | 3 (9) | 9
(17) |
0.35 |
| Chronic
renal disease | 2 (6) | 2 (4) |
0.65 |
|
Diabetes mellitus | 11 (32) | 19 (37) |
0.69 |
| Clinical
presentation, mean ± SD |
|
|
|
| Mean
arterial pressure, mmHg | 78.52±7.15 | 79.23±5.93 |
0.62 |
| Heart
rate, beats/min | 104.71±15.79 | 109.21±15.03 |
0.19 |
|
Ventilator treatment duration,
days | 13.12±3.89 |
8.28±3.32 |
<0.01a |
| 28-day
mortality, n (% of cases) | 13 (38) | 5 (10) |
0.001a |
| Disease severity
index, mean ± SD |
|
|
|
| APACHE
II score | 21.74±2.96 | 16.25±2.62 |
<0.01a |
| SOFA
score |
8.21±1.45 |
5.38±1.84 |
<0.01a |
Table II reports the
sources of infection and the causative microorganisms in the two
groups of patients. No significant difference was identified in the
infection source between the groups: the SE group did not report
significantly different numbers of patients with gram-positive
cocci, gram-negative bacilli or epiphyte infection compared with
the non-SE group, nor any different frequency of each microorganism
(P>0.05).
 | Table II.Sources of infection in the SE and
non-SE groups, expressed as n (%). |
Table II.
Sources of infection in the SE and
non-SE groups, expressed as n (%).
| Sources of
infection | SE group
(n=34) | Non-SE group
(n=52) | P-value |
|---|
| Organ system
infected |
|
|
|
|
Respiratory system | 10 (29) | 17 (33) | 0.75 |
|
Digestive system | 12 (35) | 20 (39) | 0.77 |
| Urinary
system | 6
(18) | 8
(15) | 0.78 |
| Skin
and soft tissue | 4
(12) | 5
(10) | 0.74 |
|
Other | 2 (6) | 2 (4) | 0.65 |
| Concurrent
bacteremia episodes | 6
(18) | 9
(17) | 0.97 |
| Causative
pathogens |
|
|
|
|
Gram-positive | 15 (44) | 25 (48) | 0.72 |
|
Staphylococcus
aureus | 8
(23) | 11 (21) | 0.80 |
|
Streptococcus
pneumonia | 1 (3) | 4 (8) | 0.64 |
|
Enterococcus
faecium | 5
(15) | 6
(12) | 0.75 |
|
Enterococcus
faecalis | 1 (3) | 4 (8) | 0.64 |
|
Gram-negative | 24 (71) | 39 (75) | 0.65 |
|
Acinetobacterbaum
anni | 7
(21) | 13 (25) | 0.64 |
|
Pseudomonas
aeruginosa | 5
(15) | 10 (19) | 0.59 |
|
Escherichia
coli | 4
(12) | 8
(15) | 0.76 |
|
Klebsiella
pneumoniae |
4 (11.8) | 5
(9.6) | 0.74 |
|
Proteus
mirabilis | 2
(5.9) | 2
(3.8) | 0.65 |
|
Entembacter
cloacae | 2
(5.9) | 1
(1.9) | 0.56 |
|
Epiphyte |
4 (11.8) | 5
(9.6) | 0.74 |
Laboratory data are shown in Table III. A significant increase in the
serum levels of ALT and S-100β protein in the SE group compared to
the non-SE group was revealed using independent t-tests
(156.79±33.57 vs. 98.69±38.12 U/l, P<0.01; and 1.21±0.15 vs.
0.98±0.20 µg/l, P<0.01, respectively), using the
Karman-Worblewski method (25). In
regard to inflammatory markers and immune parameters, the
percentage of CD4+ T lymphocytes and the
CD4+/CD8+ ratio were significantly lower in
the SE group (51.67±7.12 vs. 60.72±3.70% in the non-SE group,
P<0.01; and 1.59±0.32 vs. 1.85±0.26 in the non-SE group,
P<0.01, respectively), while the percentage of NK cells was
significantly higher in the SE group compared to the non-SE group
(11.80±1.44 vs. 9.19±2.36%, P<0.01). No significant differences
were identified in the other laboratory variables examined
(P>0.05).
 | Table III.Laboratory data of the SE and non-SE
groups, presented as mean ± SD. |
Table III.
Laboratory data of the SE and non-SE
groups, presented as mean ± SD.
| Laboratory
variable | SE group
(n=34) | Non-SE group
(n=53) | P-value |
|---|
| Biochemistry |
|
|
|
|
Glucose, mmol/l |
9.63±3.21 |
9.20±2.94 |
0.52 |
| ALT,
U/l |
156.79±33.57 |
98.69±38.12 |
<0.01a |
|
Creatinine, mmol/l |
86.49±24.62 |
79.28±22.04 |
0.16 |
| 6 h
lactate clearance, % |
16.71±7.73 |
18.15±8.08 |
0.41 |
| Total
bilirubin, mmol/l |
13.28±4.94 |
12.80±4.52 |
0.64 |
| BNP,
pg/ml |
1224.20±586.99 |
1042.89±507.23 |
0.13 |
|
PaO2, mmHg |
77.20±17.92 |
81.19±20.91 |
0.36 |
| APTT,
sec |
38.56±6.87 |
40.08±7.59 |
0.36 |
| NSE,
µg/l |
10.02±1.48 |
9.86±0.91 |
0.58 |
| S-100β,
µg/l |
1.21±0.15 |
0.98±0.20 |
<0.01a |
| Inflammatory
markers |
|
|
|
| WBC, n
× 109/l |
15.89±6.51 |
16.39±7.24 |
0.75 |
| CRP,
mg/l |
230.99±67.59 |
205.99±102.81 |
0.18 |
| PCT,
ng/ml |
10.69±5.41 |
9.97±4.94 |
0.53 |
|
CD4+, % of total
cells |
51.67±7.12 |
60.72±3.70 |
<0.01a |
|
CD8+, % of total
cells |
32.92±2.48 |
33.26±2.71 |
0.56 |
|
CD4+/CD8+ |
1.59±0.32 |
1.85±0.26 |
<0.01a |
| NK, %
of total cells |
11.80±1.44 |
9.19±2.36 |
<0.01a |
Correlation between immune parameters
and disease severity
The percentage of CD4+ T lymphocytes, the
CD4+/CD8+ ratio and the percentage of NK
cells were significantly different between the SE and non-SE groups
(Table III). Thus, additional
correlation analysis between these immune parameters and disease
severity was conducted. Based on the results of Pearson's
correlation analysis (Tables IV and
V), the percentage of
CD4+ T-lymphocytes, the CD4+/CD8+
ratio and the percentage of NK cells demonstrated a marked
correlation with APACHE II scores (r=−0.854, −0.824 and 0.816,
respectively; P<0.01), SOFA scores (r=−0.878, −0.853 and 0.871,
respectively; P<0.01), NSE levels (r=−0.738, −0.872 and 0.683,
respectively; P<0.01) and S-100β protein levels (r=−0.696,
−0.719 and 0.795, respectively; P<0.01). These analyses revealed
that the percentage of CD4+ T lymphocytes and NK cells
and the CD4+/CD8+ ratio were correlated with
sepsis and brain injury severity. The results of the present
analyses also indicated that the most marked correlation in sepsis
severity was between the percentage of CD4+ T
lymphocytes and SOFA score (R2=0.771) and, in brain
injury severity, was between the CD4+/CD8+
ratio and NSE levels (R2=0.760).
 | Table IV.Correlation between immune parameters
and sepsis severity. |
Table IV.
Correlation between immune parameters
and sepsis severity.
|
| APACHE II | SOFA |
|---|
|
|
|
|
|---|
| Immune
parameter | r | P-value | R2 | 95% CI | r | P-value | R2 | 95% CI |
|---|
|
CD4+ | −0.854 | <0.01 | 0.729 | −0.905 to
−0.787 | −0.878 | <0.01 | 0.771 | −0.914
to −0.827 |
|
CD4+/CD8+ | −0.824 | <0.01 | 0.679 | −0.883 to
−0.741 | −0.853 | <0.01 | 0.728 | −0.909
to −0.783 |
| NK |
0.816 | <0.01 | 0.666 | 0.756 to 0.869 |
0.871 | <0.01 | 0.759 | 0.803
to 0.920 |
 | Table V.Correlation between immune parameters
and brain injury severity. |
Table V.
Correlation between immune parameters
and brain injury severity.
|
| NSE | S-100β |
|---|
|
|
|
|
|---|
| Immune
parameter | r | P-value | R2 | 95% CI | r | P-value | R2 | 95% CI |
|---|
|
CD4+ | −0.738 | <0.01 | 0.545 | −0.828 to
−0.613 | −0.696 | <0.01 | 0.484 | −0.863 to
−0.457 |
|
CD4+/CD8+ | −0.872 | <0.01 | 0.760 | −0.919 to
−0.811 | −0.719 | <0.01 | 0.517 | −0.886 to
−0.484 |
| NK |
0.683 | <0.01 | 0.466 | 0.555 to 0.778 |
0.795 | <0.01 | 0.632 | 0.607 to 0.930 |
Prediction of SE
APACHE II and SOFA scores, serum ALT and S-100β
protein levels, the percentage of CD4+ T lymphocytes and
NK cells and the CD4+/CD8+ ratio were
significantly different between the two groups and may be used as
predictive factors. However, subsequent to use of a binary logistic
regression analysis (using the forward conditional method),
performed with ‘SE presence or absence’ as a dependent variable and
all the predictors as independent variables, only the APACHE II
score and the percentage of CD4+ T-lymphocytes (P=0.012
and OR, 4.763; P=0.005 and OR, 0.810, respectively) entered the
final equation, demonstrating that they were independently
associated with SE (Table VI).
 | Table VI.Logistic regression analysis of risk
factors of septic encephalopathy. |
Table VI.
Logistic regression analysis of risk
factors of septic encephalopathy.
| Risk factor | B | SE | Wald | P-value | OR | 95% CI |
|---|
| APACHE II |
1.561 | 0.625 | 6.244 | 0.012 | 4.763 | 1.400–16.202 |
|
CD4+ | −0.211 | 0.076 | 7.731 | 0.005 | 0.810 |
0.698–0.940 |
The effectiveness of these variables in predicting
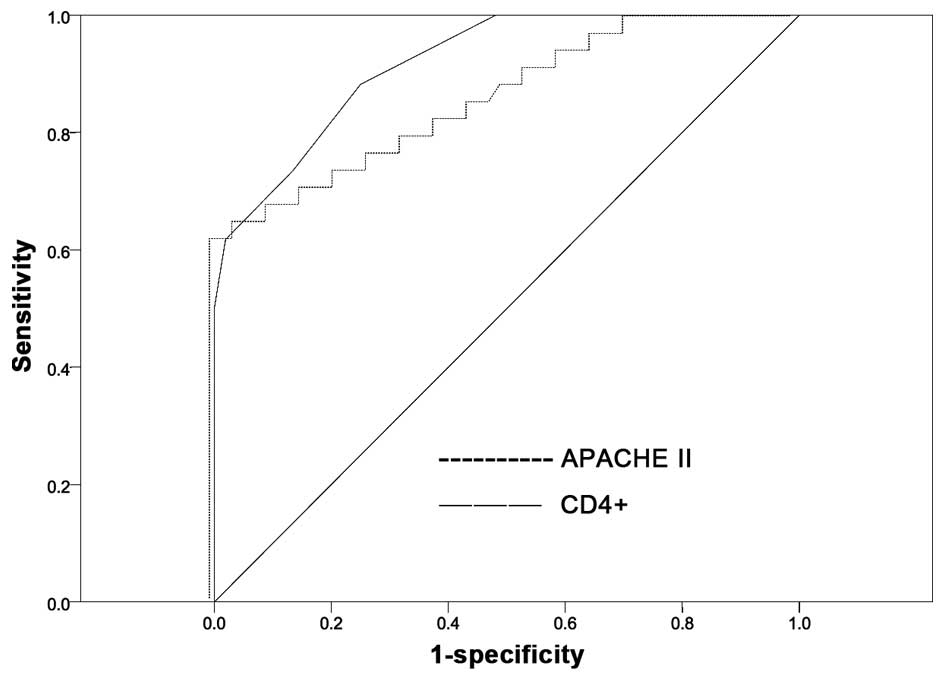
SE was evaluated by assessing the AUC of each ROC curve (Table VII; Fig.
1). The AUCs for the percentage of CD4+ T
lymphocytes and APACHE II score were 0.919 and 0.855, respectively
(P<0.001), reflecting good discrimination. A Z-test was
subsequently used to compare the predictive ability of these
variables, identifying no significant difference between the AUCs
of the percentage of CD4+ T lymphocytes and APACHE II
score (Z=1.247, P=0.212), revealing that these were equally
powerful measures in the prediction of SE (P>0.05). Based on the
ROC curve and the maximum Youden's index, the most appropriate
cut-off value was selected. With regard to the percentage of
CD4+ T lymphocytes, the most appropriate cut-off value
for predicting SE was 55.655%, corresponding to the sensitivity and
specificity values of 67.6 and 90.4%, respectively. With regard to
APACHE II score, the most appropriate cut-off value for predicting
SE was 18.500, corresponding to the sensitivity and specificity
values of 88.2 and 75.0%, respectively (Table VIII).
 | Table VII.Receiver operating characteristic
curve analysis of independent risk factors in diagnosing septic
encephalopathy. |
Table VII.
Receiver operating characteristic
curve analysis of independent risk factors in diagnosing septic
encephalopathy.
| Risk factor | AUC | SE | 95% CI | P-value |
|---|
| APACHE II | 0.919 | 0.028 | 0.864–0.973 | <0.001 |
|
CD4+ | 0.855 | 0.043 | 0.771–0.939 | <0.001 |
 | Table VIII.Prediction of septic
encephalopathy. |
Table VIII.
Prediction of septic
encephalopathy.
| Risk factor | Maximum Youden's
index | Best cut-off
value | Sensitivity
(%) | Specificity
(%) |
|---|
| APACHE II | 1.632 | 18.500 | 88.2 | 75.0 |
|
CD4+ | 1.580 | 55.655 | 67.6 | 90.4 |
Discussion
SE is an acute neurological dysfunction that results
from sepsis and is associated with high morbidity and mortality.
During sepsis, the brain is vulnerable, and encephalopathy
frequently results but is not commonly identified (26,27).
According to previous studies, the incidence of SE following severe
sepsis varies from 9–71% with a mortality frequency of ~50%
(2,28), dependent on the method used to grade
altered mental status (3,29). In the present study, SE resulted in
40% of severe sepsis cases, with a mortality of 38%. Although the
incidence and mortality are inconsistent across studies, the brain
is sensitive to sepsis, and SE often has severe consequences
(2,28). SE should therefore be recognized as
an indicator of poor prognosis in patients with sepsis, inducing
prompt and aggressive therapy.
APACHE II and SOFA scores have been applied to
critically ill patients to evaluate the severity of SE and clinical
outcomes. Previous findings have shown that the severity of
encephalopathy was associated with the global severity of disease,
as assessed by APACHE II score or SOFA, and mortality rates
(1,2). In the present study, the mortality of
septic patients significantly increased with increased APACHE II
and SOFA scores, which is consistent with previous reports
(30), supporting an association of
SE with an increased mortality risk in patients with severe
sepsis.
The present study indicated that patients with SE
required a greater duration of ventilator treatment, revealing more
severe respiratory failure. In clinical practice, patients with
disturbance of consciousness are prone to respiratory failure due
to an inability to protect the airway and respiratory drive. During
a period of hypoxemia that occurs prior to respiratory failure, a
greater degree of brain injury may be generated. The present
analyses demonstrated a significantly increased level of ALT in
patients with SE. Sepsis is often associated with multiple organ
failure and numerous abnormal biochemical indicators, which
indicates there may be a complex inherent association between
individual organ failure and an amplification process that hastens
injury to other organs. This could be seen to explain the increased
mortality in the SE group.
The pathophysiology of SE is complex, and may
include activation of the inflammatory response, microglia and
astrocytes, alteration in the BBB, amino acid disruption, brain
hypoperfusion/ischemia and translocation of neurotoxic molecules
(7–10). An upregulated inflammatory response
is recognized as an integral contributor to SE. Previous studies
have comprehensively investigated the effect of infection on CNS.
However, the severity of SE is reportedly not associated with
infection by specific microorganisms, nor groups thereof (31). Furthermore, inflammatory mediators,
including interleukin-1 and tumor necrosis factor-α and oxidative
stress have a critical role in the abnormal neurotransmitter
composition and impaired neuronal and microglia function (32–34).
Reduced hepatic clearance and increased neurotoxic amino acids in
sepsis associated with muscle proteolysis also contribute to the
development of brain dysfunction (35,36). The
S-100β protein has been previously employed as an indicator of
astrocyte activation and injury, and as a marker for brain injury
in SE (37,38); however, not all studies concur with
this finding (39,40). In the present study, S-100β protein
was significantly higher in SE patients, but regression analysis
indicated that this is not the most reliable indicator for
predicting SE, a finding that was consistent with previously
conflicting findings (37–40). Additional evaluation of the direct
effect of brain injury to SE is required to clarify the most
effective markers.
Lipopolysaccharide stimulation has previously been
reported to induce the release of proinflammatory and
anti-inflammatory cytokines, in addition to their receptors, from a
number of nervous system-associated cells, including neurones,
astrocytes and microglia (41,42).
This coexpression of proinflammatory and anti-inflammatory
cytokines indicates that the immune system is highly regulated
within the brain. Concordantly, in sepsis survivors, the initial
proinflammatory burst often develops into immune suppression,
characterized by T-cell dysfunction and adaptive immune suppression
accompanied by innate immune system activation (43–45) This
immune imbalance develops throughout sepsis and study in this area
has made significant progress (46).
Reduction in the number of circulating CD4+ T
lymphocytes and their shift to a Th2 phenotype are indicative of
aspects of sepsis-induced adaptive immunosuppression (47). The association between clinical
course of contradiction and poor prognosis of patients with sepsis
and the decline of peripheral blood CD4+ T lymphocytes
has been established in a majority of patients with sepsis
(48,49). Furthermore, NK cells, a type of
cytotoxic lymphocyte, are likely to be involved in the
antibacterial response of the innate immune system due to their
ability to recognize pathogen-associated molecular patterns
(50,51). Findings of a previous study have
shown that patients with the highest NK cell number have the lowest
probability of survival (52). In
the present study, the percentage of CD4+ T lymphocytes
and the CD4+/CD8+ ratio were significantly
lower and the percentage of NK cells was significantly higher in
the SE group than in the non-SE group, suggesting adaptive immune
suppression and innate immune activation of patients. The present
results are comparable with previous studies and indicate a highly
significant functional imbalance of immune cells in patients with
SE, which may be crucial in the development of encephalopathy.
CD4+ T lymphocytes are particularly
vulnerable to apoptotic death in polymicrobial sepsis models,
according to previous studies (43,53). In
addition, the response of T lymphocytes to continuously elevated
serum levels of anti-inflammatory cytokines was an improved
predictor of mortality than proinflammatory cytokines in patients
with severe sepsis (54), which are
typically used. In the current study, only the percentage of
CD4+ T lymphocytes and APACHE II score were determined
to be similarly accurate predictors of clinical outcome, based on
the regression analysis, when compared with the
CD4+/CD8+ ratio and percentage of NK cells.
These results indicate that the percentage of CD4+ T
lymphocytes is a promising biomarker in predicting SE occurrence
among patients with severe sepsis. The recirculation of
CD4+ T lymphocytes may be responsible for the percentage
of CD4+ T lymphocytes decreasing in the peripheral blood
of patients with sepsis. This recirculation may be associated with
the generation of stress hormones, cytokines and other humoral
factors, including prostaglandin E2-α, cortisol and interleukin-10
(55,56), which is supported by previous studies
revealing a rapid decrease in circulating CD4+ T
lymphocytes following the experimental administration of endotoxin
and an observed increase in the concentration of these cells in the
thoracic ducts of patients with systemic inflammatory response
syndrome (57,58).
The present study provides novel insights into the
role of CD4+ T lymphocytes during SE, but has several
limitations. Poor nutritional status was typical and varied among
patients with severe sepsis. Malnutrition has marked consequences
on the immune response that may affect results. Furthermore, the
diagnosis of SE may have been affected by a negative mood in
patients. In conclusion, the present study provides a unique
insight into the status of the immune system in SE. SE leads to
higher mortality rates in patients with severe sepsis, and immune
imbalance has an important role in this increase in mortality
rates. The current study indicates that the proportion of
CD4+ T lymphocytes present in the blood of patients with
severe sepsis is a powerful predictor of SE. However, additional
investigation is required to elucidate the pathogenesis of SE.
Acknowledgements
The authors of the current study thank all
biotechnicians of the clinical laboratories in Zhongshan Hospital
Xiamen University for their technical support. The present study
was supported by grants from the National Natural Science
Foundation of China (grant nos. 81071529 and 81272105).
References
|
1
|
Sprung CL, Peduzzi PN, Shatney CH, Schein
RM, Wilson MF, Sheagren JN and Hinshaw LB: Impact of encephalopathy
on mortality in the sepsis syndrome. The Veterans Administration
Systemic Sepsis Cooperative Study Group. Crit Care Med. 18:801–806.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eidelman LA, Putterman D, Putterman C and
Sprung CL: The spectrum of septic encephalopathy. Definitions,
etiologies, and mortalities. JAMA. 275:470–473. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Straver JS, Keunen RW, Stam CJ, Tavy DL,
de Ruiter GR, Smith SJ, Thijs LG, Schellens RG and Gielen G:
Nonlinear analysis of EEG in septic encephalopathy. Neurol Res.
20:100–106. 1998.PubMed/NCBI
|
|
4
|
Schraag S: Studying septic encephalopathy:
What animal models can predict. Intensive Care Med. 29:667–668.
2003.PubMed/NCBI
|
|
5
|
Eggers V, Fügener K, Hein OV,
Rommelspacher H, Heyes MP, Kox WJ and Spies CD: Antibiotic-mediated
release of tumour necrosis factor alpha and norharman in patients
with hospital-acquired pneumonia and septic encephalopathy.
Intensive Care Med. 30:1544–1551. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Angel MJ and Young GB: Metabolic
encephalopathies. Neurol Clin. 29:837–882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koo DJ, Jackman D, Chaudry IH and Wang P:
Adrenal insufficiency during the late stage of polymicrobial
sepsis. Crit Care Med. 29:618–622. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsao N, Hsu HP, Wu CM, Liu CC and Lei HY:
Tumour necrosis factor-alpha causes an increase in blood-brain
barrier permeability during sepsis. J Med Microbiol. 50:812–821.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basler T, Meier-Hellmann A, Bredle D and
Reinhart K: Amino acid imbalance early in septic encephalopathy.
Intensive Care Med. 28:293–298. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deng YY, Fang M, Zhu GF, Zhou Y and Zeng
HK: Role of microglia in the pathogenesis of sepsis-associated
encephalopathy. CNS Neurol Disord Drug Targets. 12:720–725. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Munford RS and Pugin J: Normal responses
to injury prevent systemic inflammation and can be
immunosuppressive. Am J Respir Crit Care Med. 163:316–321. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oberholzer A, Oberholzer C and Moldawer
LL: Sepsis syndromes: Understanding the role of innate and acquired
immunity. Shock. 16:83–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abraham E and Singer M: Mechanisms of
sepsis-induced organ dysfunction. Crit Care Med. 35:2408–2416.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rittirsch D, Flierl MA and Ward PA:
Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 8:776–787.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luyt CE, Combes A, Deback C,
Aubriot-Lorton MH, Nieszkowska A, Trouillet JL, Capron F, Agut H,
Gibert C and Chastre J: Herpes simplex virus lung infection in
patients undergoing prolonged mechanical ventilation. Am J Respir
Crit Care Med. 175:935–942. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kollef KE, Schramm GE, Wills AR, Reichley
RM, Micek ST and Kollef MH: Predictors of 30-day mortality and
hospital costs in patients with ventilator-associated pneumonia
attributed to potentially antibiotic-resistant gram-negative
bacteria. Chest. 134:281–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Limaye AP, Kirby KA, Rubenfeld GD,
Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Hayes Santo
TK, Corey L and Boeckh M: Cytomegalovirus reactivation in
critically ill immunocompetent patients. JAMA. 300:413–422. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz M and Baruch K: The resolution of
neuroinflammation in neurodegeneration: Leukocyte recruitment via
the choroid plexus. EMBO J. 33:7–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tian L, Ma L, Kaarela T and Li Z:
Neuroimmune crosstalk in the central nervous system and its
significance for neurological diseases. J Neuroinflammation.
9:1552012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neumann H: Control of glial immune
function by neurons. Glia. 36:191–199. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levy MM, Fink MP, Marshall JC, Abraham E,
Angus D, Cook D, Cohen J, Opal SM, Vincent JL and Ramsay G:
International Sepsis Definitions Conference: 2001
SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions
Conference. Intensive Care Med. 29:530–538. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cotena S and Piazza O: Sepsis-associated
encephalopathy. Transl Med UniSa. 2:20–27. 2012.PubMed/NCBI
|
|
23
|
Dal-Pizzol F, Tomasi CD and Ritter C:
Septic encephalopathy: Does inflammation drive the brain crazy? Rev
Bras Psiquiatr. 36:251–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke
R, et al: Surviving Sepsis Campaign Guidelines Committee including
the Pediatric Subgroup: Surviving sepsis campaign: International
guidelines for management of severe sepsis and septic shock: 2012.
Crit Care Med. 41:580–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karmen A, Worblewski F and Landue JS:
Transaminases activity in human blood. J Clin Invest. 34:126–131.
1955. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milbrandt EB and Angus DC:
Bench-to-bedside review: Critical illness-associated cognitive
dysfunction - mechanisms, markers, and emerging therapeutics. Crit
Care. 10:2382006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ebersoldt M, Sharshar T and Annane D:
Sepsis-associated delirium. Intensive Care Med. 33:941–950. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang LN, Wang XT, Ai YH, Guo QL, Huang L,
Liu ZY and Yao B: Epidemiological features and risk factors of
sepsis-associated encephalopathy in intensive care unit patients:
2008–2011. Chin Med J (Engl). 125:828–831. 2012.PubMed/NCBI
|
|
29
|
Zauner C, Gendo A, Kramer L, Kranz A,
Grimm G and Madl C: Metabolic encephalopathy in critically ill
patients suffering from septic or nonseptic multiple organ failure.
Crit Care Med. 28:1310–1315. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taylor SL, Morgan DL, Denson KD, Lane MM
and Pennington LR: A comparison of the Ranson, Glasgow, and APACHE
II scoring systems to a multiple organ system score in predicting
patient outcome in pancreatitis. Am J Surg. 189:219–222. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bone RC, Sprung CL and Sibbald WJ:
Definitions for sepsis and organ failure. Crit Care Med.
20:724–726. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Babior BM: NADPH oxidase: An update.
Blood. 93:1464–1476. 1999.PubMed/NCBI
|
|
33
|
Wang CX and Shuaib A: Involvement of
inflammatory cytokines in central system injury. Prog Neurobiol.
67:161–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi SH, Lee DY, Kim SU and Jin BK:
Thrombin-induced oxidative stress contributes to the death of
hippocampal neurons in vivo: Role of microglial NADPH oxidase. J
Neurosci. 25:4082–4090. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sprung CL, Cerra FB, Freund HR, Schein RM,
Konstantinides FN, Marcial EH and Pena M: Amino acid alterations
and encephalopathy in the sepsis syndrome. Crit Care Med.
19:753–757. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kadoi Y and Saito S: An alteration in the
gamma-aminobutyric acid receptor system in experimentally induced
septic shock in rats. Crit Care Med. 24:298–305. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nguyen DN, Spapen H, Su F, Schiettecatte
J, Shi L, Hachimi-Idrissi S and Huyghens L: Elevated serum levels
of S-100beta protein and neuron-specific enolase are associated
with brain injury in patients with severe sepsis and septic shock.
Crit Care Med. 34:1967–1974. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Piazza O, Cotena S, De Robertis E, Caranci
F and Tufano R: Sepsis associated encephalopathy studied by MRI and
cerebral spinal fluid S100B measurement. Neurochem Res.
34:1289–1292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Piazza O, Russo E, Cotena S, Esposito G
and Tufano R: Elevated S100B levels do not correlate with the
severity of encephalopathy during sepsis. Br J Anaesth. 99:518–521.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
van den Boogaard M, Ramakers BP, van Alfen
N, van der Werf SP, Fick WF, Hoedemaekers CW, Verbeek MM,
Schoonhoven L, van der Hoeven JG and Pickkers P:
Endotoxemia-induced inflammation and the effect on the human brain.
Crit Care. 14:R812010. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Omari KM and Dorovini-Zis K: CD40
expressed by human brain endothelial cells regulates
CD4+ T cell adhesion to endothelium. J Neuroimmunol.
134:166–178. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sankowski R, Mader S and Valdés-Ferrer SI:
Systemic inflammation and the brain: novel roles of genetic,
molecular, and environmental cues as drivers of neurodegeneration.
Front Cell Neurosci. 9:282015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hotchkiss RS, Tinsley KW, Swanson PE,
Schmieg RE Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP,
Buchman TG and Karl IE: Sepsis-induced apoptosis causes progressive
profound depletion of B and CD4+ T lymphocytes in
humans. J Immunol. 166:6952–6963. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roth G, Moser B, Krenn C, Brunner M,
Haisjackl M, Almer G, Gerlitz S, Wolner E, Boltz-Nitulescu G and
Ankersmit HJ: Susceptibility to programmed cell death in
T-lymphocytes from septic patients: A mechanism for lymphopenia and
Th2 predominance. Biochem Biophys Res Commun. 308:840–846. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boomer JS, To K, Chang KC, Takasu O,
Osborne DF, Walton AH, Bricker TL, Jarman SD II, Kreisel D,
Krupnick AS, et al: Immunosuppression in patients who die of sepsis
and multiple organ failure. JAMA. 306:2594–2605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sunkara B, Bheemreddy S, Lorber B, Lephart
PR, Hayakawa K, Sobel JD, Kaye KS and Marchaim D: Group B
Streptococcus infections in non-pregnant adults: The role of
immunosuppression. Int J Infect Dis. 16:e182–e186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ferguson NR, Galley HF and Webster NR: T
helper cell subset ratios in patients with severe sepsis. Intensive
Care Med. 25:106–109. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cheadle WG, Pemberton RM, Robinson D,
Livingston DH, Rodriguez JL and Polk HC Jr: Lymphocyte subset
responses to trauma and sepsis. J Trauma. 35:844–849. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wakefield CH, Carey PD, Foulds S, Monson
JR and Guillou PJ: Changes in major histocompatibility complex
class II expression in monocytes and T cells of patients developing
infection after surgery. Br J Surg. 80:205–209. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chalifour A, Jeannin P, Gauchat JF,
Blaecke A, Malissard M, N'Guyen T, Thieblemont N and Delneste Y:
Direct bacterial protein PAMP recognition by human NK cells
involves TLRs and triggers alpha-defensin production. Blood.
104:1778–1783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Andaluz-Ojeda D, Iglesias V, Bobillo F,
Almansa R, Rico L, Gandía F, Loma AM, Nieto C, Diego R, Ramos E, et
al: Early natural killer cell counts in blood predict mortality in
severe sepsis. Crit Care. 15:R2432011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Anduluz-Ojeda D, Iglesias V, Bobillo F,
Almansa R, Rico L, Gandía F, Loma AM, Nieto C, Diego R, Ramos E, et
al: Early natural killer cell counts in blood predict mortality in
severe sepsis. Crit Care. 15:R2432011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Markwart R, Condotta SA, Requardt RP,
Borken F, Schubert K, Weigel C, Bauer M, Griffith TS, Förster M,
Brunkhorst FM, et al: Immunosuppression after sepsis: Systemic
inflammation and sepsis induce a loss of naïve T-cells but no
enduring cell-autonomous defects in T-cell function. PLoS One.
9:e1150942014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
de Pablo R, Monserrat J, Reyes E,
Diaz-Martin D, Zapata Rodriguez M, Carballo F, de la Hera A, Prieto
A and Alvarez-Mon M: Mortality in patients with septic shock
correlates with anti-inflammatory but not proinflammatory
immunomodulatory molecules. J Intensive Care Med. 26:125–132. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Menges T, Engel J, Welters I, Wagner RM,
Little S, Ruwoldt R, Wollbrueck M and Hempelmann G: Changes in
blood lymphocyte populations after multiple trauma: Association
with posttraumatic complications. Crit Care Med. 27:733–740. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sarlis NJ, Chanock SJ and Nieman LK:
Cortisolemic indices predict severe infections in Cushing syndrome
due to ectopic production of adrenocorticotropin. J Clin Endocrinol
Metab. 85:42–7. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Toft P, Hokland M, Hansen TG and Tønnesen
E: Changes in lymphocyte subpopulations and adhesion/activation
molecules following endotoxemia and major surgery. APMIS.
103:261–266. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lemaire LC, van Deventer SJ, van Lanschot
JJ, Meenan J and Gouma DJ: Phenotypical characterization of cells
in the thoracic duct of patients with and without systemic
inflammatory response syndrome and multiple organ failure. Scand J
Immunol. 47:69–75. 1998. View Article : Google Scholar : PubMed/NCBI
|