Introduction
Aging is associated with a wide range of impairments
of the regulatory systems, particularly systems that are associated
with surveillance and defence mechanisms (1). The skin, as the body's largest organ,
follows an oxidant/antioxidant defense path and abnormalities
leading to photoaging, chronoaging and inflammation are mediated by
reactive oxygen species (ROS) (2).
The true biological impact of the direct topical administration of
a wide variety of natural and synthetic compounds on skin health
remains unclear (3). Considering
that the hydrophobic nature of the upper layer of the skin is the
stratum corneum, specific oral phytonutrients have been proven to
improve certain clinical aspects of skin health (4–6).
Although the skin possesses a multifaceted antioxidant defense
structure, unbalanced and enduring exposure to ultraviolet (UV)
radiation can overwhelm the dermal antioxidant capability,
resulting in oxidative damage that may lead to skin immune system
dysregulation, premature aging and development of skin cancer
(7). Chronic sun exposure results in
photoaged skin (8), characterized by
the premature occurrence of aging signs on the skin, which displays
distinct morphological changes of the epidermal and dermal
compartments, the pigmentary system and the vasculature (9,10). These
changes are also accompanied by an increased skin cancer occurrence
(11). Furthermore, changes of
intrinsic aging have to be considered, and these are typically
observed in chronically sun-protected areas where aging is mainly
attributed to intrinsic factors, including genetics and changes in
the endocrine environment, reflecting the degradation processes of
the entire organism (12). Previous
in vitro data obtained from human epithelial skin cells have
shown that studies on this type of tissue may provide useful
information when investigating the same embryologic-derived
tissues, such as tissues from the central nervous system (13). More recently, a study has reported
that a resveratrol-procyanidin-based nutraceutical was able to
exert a beneficial skin antiaging effect (14). However, the study only partially
addressed skin clinicobiochemical evaluation and did not assess
gene expression.
A number of genes and associated proteins have
recently emerged as relevant for skin health (15). Among them, aquaporin-3 (AQP-3) is
expressed in keratinocytes and fibroblasts, and regulates water
movement across the plasma membrane via diffusion through the lipid
bilayer, as well as being involved in wound healing (16). The common upregulation of AQP-3 by
antioxidants has been suggested to beneficially modulate this
protein in order to maintain skin health, cell turnover and skin
hydration (15). Cyclophilin A
(CyPA), a member of the immunophilin family, has been recently
found to play an important role in impairing skin DNA repair
mechanisms (17), while also
affecting systemic oxidative stress-inflammatory response (18,19). The
transmembrane glycoprotein CD147, a cell surface receptor of CyPA
that belongs to the immunoglobulin superfamily, plays a relevant
role in CyPA-mediated signal transmission and chemotactic activity
(20). Finally, a recent study has
suggested that UVA may induce progerin mRNA and protein expression
in dermal fibroblasts as a novel mechanism of UV-accelerated skin
aging (21).
A fermented papaya preparation (FPP) has been shown
to possess effective antioxidant properties in previous in
vitro and in vivo studies (22–24).
Therefore, the aim of the current study was to determine whether
nutraceutical treatment with a supplement composed of GMP- and
ISO9001-certified FPP was able to improve the skin antioxidant
capacity and the expression of key skin genes, while promoting
clinically evident skin antiaging effects.
Materials and methods
Ethics
All procedures were approved by an independent
Ethical Committee for non-pharmacological research (ReGenera
Research Group for Aging Intervention, Milan, Italy). Each subject
recruited for the study was fully informed and treated in
compliance with the guidelines of the Declaration of Helsinki. All
skin samples were obtained under the written informed consent of
the donors.
FPP
The FPP used in the present study was obtained from
Carica papaya L. cultivated in Hawaii, following yeast
fermenting for 10 months and batch-to-batch checking at the Osato
Research Institute (Gifu, Japan). The final composition of FPP per
100 g is as follows: 90.7 g carbohydrates, 17 µg vitamin B6, 2 µg
folic acid, 2.5 mg calcium, 16.9 mg potassium, 240 µg niacin, 4.6
mg magnesium, 14 µg copper, 75 µg zinc, 16 mg arginine, 6 mg
lysine, 5 mg hystidine, 11 mg phenylalanine, 9 mg tyrosine, 18 mg
leucine, 9 mg isoleucine, 5 mg methionine, 13 mg valine, 11 mg
glycine, 8 mg proline, 37 mg gluthamic acid, 11 mg serine, 8 mg
treonine, 27 mg aspartic acid, and 2 mg triptophane.
Subjects and study design
The study enrolled 60 healthy non-smoker males and
females with an age of 40–65 years, all of whom showed clinical
signs of skin aging (including wrinkles, dull complexion and brown
spots). The inclusion criteria were as follows: Caucasian
ethnicity; Fitzpatrick skin type I–III (25); dull complexion; normal body mass
index; absence of current or prior skin diseases; compliance to
abstain during study period from any topical application of
compounds, with the exception of standard cleaning soap; and no
know food intolerances.
All subjects were subjected to a 2-week washout
period, during which they discontinued all moisturizer use and
substituted their body cleanser with a supplied mild body wash. The
subjects were not taking any vitamin/mineral supplements or
medication.
The healthy non-smoker subjects were randomly
recruited, divided into two groups matched based on their
life-style, alcohol use, body mass index and physical activity
(kcal/week), and then were assigned to a study group (active or
antioxidant-control group; n=30 each), avoiding any significant
differences between the groups. In the FPP-treated group, the test
product was administered as a 4.5 g sachet sublingually twice a day
(total dose, 9.0 g per day), 1 h after breakfast and lunch, and
then the subjects fasted for at least 30 min. In the
antioxidant-control group, a similar-flavored sugar was
administered along with antioxidants (10 mg trans-resveratrol, 60
µg selenium, 10 mg vitamin E and 50 mg vitamin C) in the same
manner as in the FPP-treated group. The subjects received
administration for 90 days and the study was designed as
double-blinded. A dietary questionnaire was used, and habitual
intake of macronutrient and micronutrient was also submitted using
a 7-day diet history model.
Skin moisturization
A skin capacitance instrument (Nova Dermal Phase
Meter 9003; Nova Technology Corporation, Portsmouth, NH, USA) was
used to measure skin moisturization. Using this corneometer, data
were obtained by integrating measurements at different frequencies
of the applied current at preselected frequencies up to 1 MHz.
Capacitance values were calculated from the phase delay of the
signal. The standard probe had two concentric brass ring electrodes
separated by a 1-mm isolator, and there was direct Galvanic contact
between the electrodes and the skin. The final values are provided
in arbitrary units (26).
Measurements were performed at five different sites
on the cheeks. The arithmetic mean for each volunteer and time
point were calculated.
Skin elasticity
Facial (malar area) skin elasticity was calculated
through a non-invasive suction skin elasticity device (Cutometer
dual MPA 580; Courage and Khazaka Electronic GmbH, Cologne,
Germany). This device generated a graph by measuring skin
extensibility (Ue), delayed distension (Uv), final deformation
(Uf), immediate retraction (Ur), total recovery (Ua) and residual
deformation at the end of measuring cycle (R). Using an optical
measuring probe, this device derives the aforementioned
measurements through the principle of suction skin-fold elongation
(27). A 2-mm measuring probe
applied a constant suction of 350 mbar for 18 sec followed by a
2-sec relaxation interval, and this was repeated twice.
Out of the measured R parameters retrieved by the
Cutometer, the values of R2, R5 and R6 were examined. R2 represents
the gross-elasticity of the skin, including the viscous
deformation, and it is expressed by the ratio of skin deformation
over the ultimate distension (Ua/Uf) (28). Values closer to 1 represented higher
elastic properties of the skin. R5, which is calculated by
immediate retraction (Ur)/skin extensibility (Ue), is associated
with the net skin elasticity without the interference of viscous
deformation (5,8). Similarly, values close to 1 were
associated with increased elastic properties of the skin. The ratio
of Ur/Ue is the most significant parameter for assessing skin
aging, since it mirrors the elastic recovery, which is known to
decline with aging (29), subsequent
to deformation and independently from the skin thickness. By
contrast, the factor R6 is the most reliable index of epidermal and
dermal water content (27),
representing the role of viscoelasticity and viscous deformation
components against the elastic deformation of the skin due to the
increased interstitial fluid through the fibrous mesh (28). R6 is represented by the ratio of
viscoelasticity over skin extensibility (Uv/Ue).
Skin surface and brown spot
intensity
The skin surface properties (including wrinkle depth
and roughness) and the brown spot intensity were assessed using
standardized digital photographs captured with the Visia-CR imaging
system (Canfield Scientific, Inc., Fairfield, NJ, USA). This is a
multi-spectral imaging device that uses a specialized software
detecting and analyzing dark spots directly on the captured
photographs (30). The analysis
allows the assessment of the color intensity and the size of
specific dark spots, as well as the assessment of the overall face
complexion (skin evenness). Evenness in skin color and texture was
identified based on gradations in skin color compared with the
surrounding skin tone. The skin evenness evaluation was performed
by an expert evaluator at specified study intervals. The evaluator
assessed each facial parameter using a modified 100 mm visual
analog scale (VAS) expressing the perception of unevenness, with
lower scores indicating a more even and less aged skin appearance
(31). The evaluator selected a
location on the VAS that corresponded with the perception of the
subject's skin in relation to the labelled vertical positions on
the scale. The distance between the mark recorded and the left
origin of the line was subsequently measured in millimeters to
allow for the assignment of a numerical score for the extent and/or
severity of the evaluated parameter.
Skin sampling
Following enrollment, the condition of all the
subjects was initially stabilized for 30 min, in a climate-
(22±2°C) and humidity-controlled (50±10%) room. Next, skin samples
were collected using Corneofix® foils (Courage and Khazaka
Electronic GmbH), and 3-ml punch biopsies were obtained from the
extensory side of the patient forearms. Samples were also collected
after 90 days.
Gene expression studies
Genes recently highlighted (18–20) as
playing a relevant role in aging and oxidative protection
(including AQP-3, CyPA and CD147) along with an intrinsic
aging-associated gene (progerin) were investigated. Reverse
transcription-polymerase chain reaction (RT-PCR) was used to
determine their expression at 0 days and after 90 days.
RNA extraction and RT-PCR
analysis
Briefly, cells were lysed by Ambion lysis buffer
(Ambion, Carlsbad, CA, USA) for 20 min and the lysates were mixed
with an equal volume of 64% ethanol. The lysates were transferred
to a mini-column and centrifuged at 10,000 × g for 1 min. The
column was then washed with 700 µl wash buffer 1 and twice with 500
µl wash buffer 2/3 (GenePharma Co., Ltd., Shanghai, China).
Following incubation with 50 µl elution buffer (GenePharma Co.,
Ltd.), the resulting flow was retrieved and 50 µl elution buffer
was added followed by centrifugation at 10,000 × g. Next, 1 µl
DNAse I was added to 20 µl of RNA solution with suitable DNAse I
buffer (GenePharma Co., Ltd.) and incubated at 37°C for 2 h. The
DNAse I was removed by adding DNAse removing reagent and the
purified RNA was retrieved by centrifugation at 10,000 × g for 1
min. The quantity of total RNA was first assessed by measuring the
optical density at 260 nm, and the quality of the total RNA was
estimated using 1.5% agarose gel electrophoresis. RT was performed
in a 20-ml reaction solution containing 3 µg total RNA using the
Revert cDNA Synthesis kit (Toyobo Biotech, Co., Ltd., Shanghai
China). PCR was performed in a thermal cycler with preliminary
denaturation at 94°C for 5 min, followed by amplification for 30
cycles of denaturation at 94°C for 40 sec, annealing at 65°C for 1
min and extension at 72°C for 1 min. Subsequently, 5 µl PCR product
was separated by electrophoresis on a 1.5% agarose gel, and
visualized using ethidium bromide staining under UV light. The
primers used were as follows: AQP3 forward,
5′-AGACAGCCCCTTCAGGATTT-3′, and reverse,
5′-TCCCTTGCCCTGAATATCTG-3′; CypA forward,
5′-GTCAACCCCACCGTGTTCTTC-3′, and reverse,
5′-TTTCTGCTGTCTTTGGGACCTTG-3′; CD147 forward,
5′-TCGCGCTGCTGGGCACC-3′, and reverse, 5′-TGGCGCTGTCATTCAAGGA-3′;
progerin forward, 5′-ACTGCAGCAGCTCGGGG-3′, and reverse,
5′-GGCTCTGGGCTCCTGAGCC-3′; and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) forward, 5′-ACCACAGTCCATGCCATCAC-3′, and
reverse, 5′-TCCACCACCCTGTTGCTGTA-3′. The PCR products were
subjected to electrophoresis on a 1.4% agarose gel using a Gel Doc
2000 fluorescence documentation system (Bio-Rad Laboratories Co.,
Ltd., Shanghai, China) and GeneScan analysis software (version 672;
Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The mRNA level of each sample was normalized to that of the
β-actin mRNA.
Dermal redox balance and nitrogen
oxide (NO) assessment
After the patients fasted overnight, the antioxidant
capacity of the skin was assessed on the third and fourth skin
strips (Corneofix® foils were used after discarding the first and
second layers).
Determination of lipid
peroxidation
The lipid peroxidation product was determined by
measuring the malondialdehyde (MDA) content in tissue homogenates.
Skin samples were immediately frozen to −70°C until assayed for
thiobarbituric acid reactive species (TBARS) formation. As an index
of oxidative stress, the formation of TBARS during an acid-heating
reaction was used. Briefly, the samples were mixed with 1 ml
trichloroacetic acid (10%) and 1 ml thiobarbituric acid (0.67%) and
then heated in a boiling water bath for 15 min. The TBARS content
was determined by measuring the absorbance at 535 nm, using
1,1,3,3-tetramethoxypropane as an external standard. The results
are expressed as malondialdehyde equivalents per milligram of
protein (Lowry assay), and values are expressed in units of nM/mg
protein.
Superoxide dismutase (SOD) activity
measurement
SOD production was evaluated using a
lucigenin-amplified chemiluminescence method. Skin samples were
placed in Krebs buffer (Sigma-Aldrich, St. Louis, MO, USA) at pH
7.40, for 20 min. The samples were then transferred to a counting
vial under light protection and immersed in a 5-µM lucigenin
solution (Sigma-Aldrich) in Krebs buffer (total volume, 1.0 ml)
mimicking tissue generation of superoxide. The emitted luminescence
was calculated for 5 min in a Berthold Biolumat luminometer
(Berthold Detection Systems GmbH, Pforzheim, Germany). Background
signals from the buffer and lucigenin were subtracted from the
tissue sample signals and the final value was normalized for dry
weight. Chemiluminescence was measured directly and continuously
for 5 min to obtain basal values. Data are expressed as counts per
min per mg of dry tissue.
In vivo NO assessment
Following a thorough skin cleansing, a sterilized
microdialysis probe was gently inserted into the forearm skin while
carefully avoiding fluid exudation. Next, 0.3 ml of 100 µM
4,4′-sulfonyldianiline in sterile normal saline (pH 5.8–6.0) was
fed to a microdialysis tube and maintained for 20 min to allow for
local absorption of NO. The total NOx−
concentration (NO2− and
NO3−) was calculated in the dialysate using
the ozone phase chemiluminescence method (NOA280i; GE Analytical
Instruments, Boulder, CO, USA), with a lowest sensitivity of 1.0
pM. A 5 µl sample of fresh dialysate was then reduced by a
vanadium(III)/HCl solution. The resulting NO gas was reacted with
ozone to generate a chemiluminescent reaction, and the nitrate
calibration curve was set using graded concentrations of
NaNO3 diluted in sterile nitrogen-free water. The whole
quantity of NO3 in each dialysate sample was measured by
correlating the signal peaks through nitrate curve calibration.
Triplicate measurements were performed. The presence of
(NO2−) in the dialysate samples was equalled
to the water basal level so that the final NOx
concentration in the dialysate was expressed in µM units (32).
Intraobserver and interobserver
variability
Intraobserver and interobserver variability for
clinical testing were estimated by calculating the mean and 95%
confidence interval (CI) of the arithmetic differences among three
consecutive measurements obtained by a single researcher on the
same volunteer. Variability was expressed the mean ± 1.96 (which
was the standard deviation value of the mean arithmetic
difference), according to Bland and Altman (33). When the differences were normally
distributed, 95% of the differences were within a range of SD of
the mean difference = 1.96.
Statistical analyses
Statistical analyses were performed using Prism 5
software (GraphPad Software, Inc., San Diego, CA, USA). Data are
expressed as the mean of three independent experiments, and were
analyzed by Student's t-test and analysis of variance (ANOVA). In
addition, ANOVA with Dunnett's test was used to compare VAS scores
for clinical grading of facial skin attributes or, more
specifically, to compare differences between the values at baseline
(day 0) and after 90 days of observation. Genes expression level
comparisons were tested by Kruskal-Wallis t-test, and the
correlation analysis was assessed with Spearman's rho method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
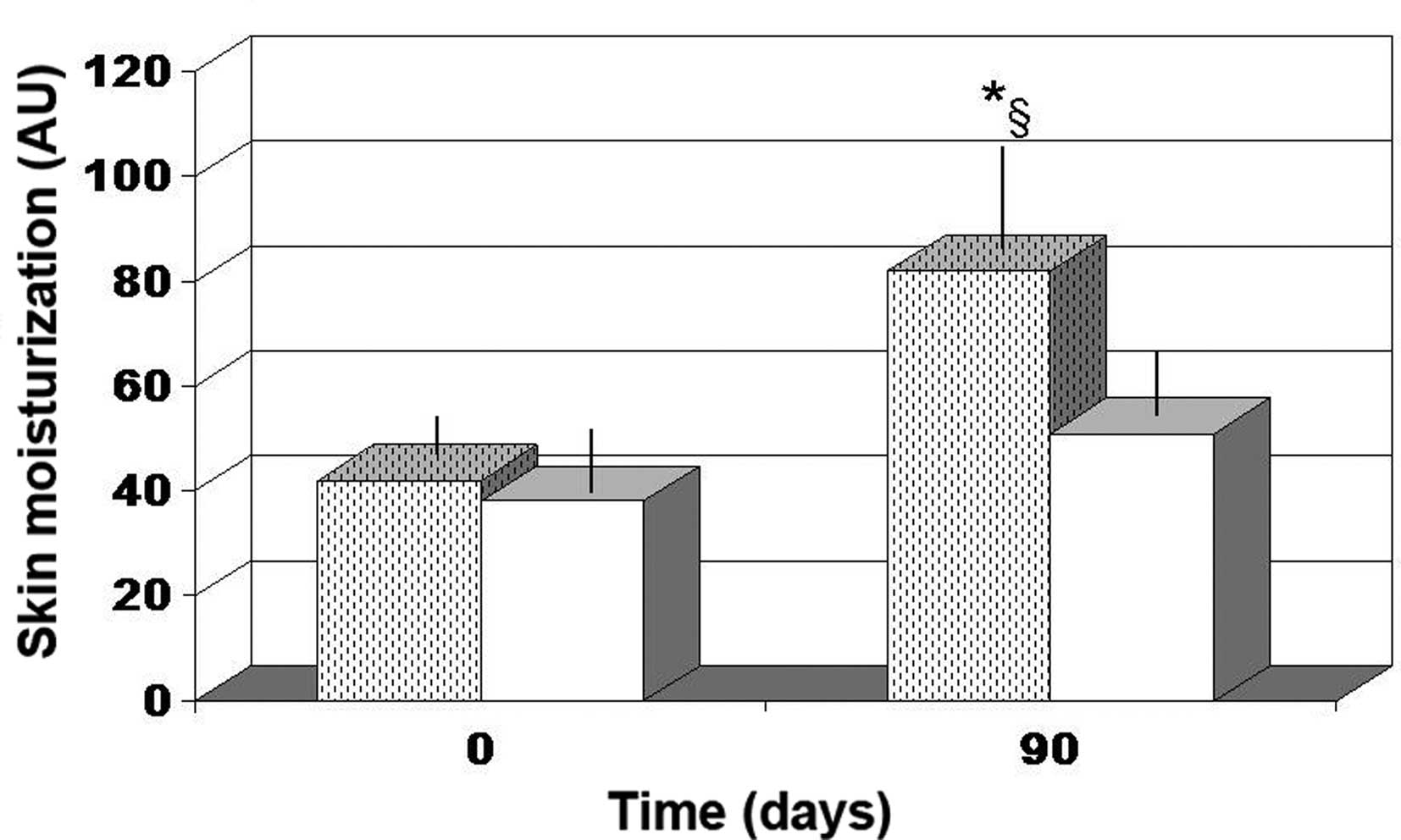
Skin moisturization
Antioxidant cocktail supplementation was not found
to affect skin moisturization. By contrast, FPP supplementation
resulted in a significant improvement in skin moisturization after
90 days (~95% increase; P<0.04; Fig.
1).
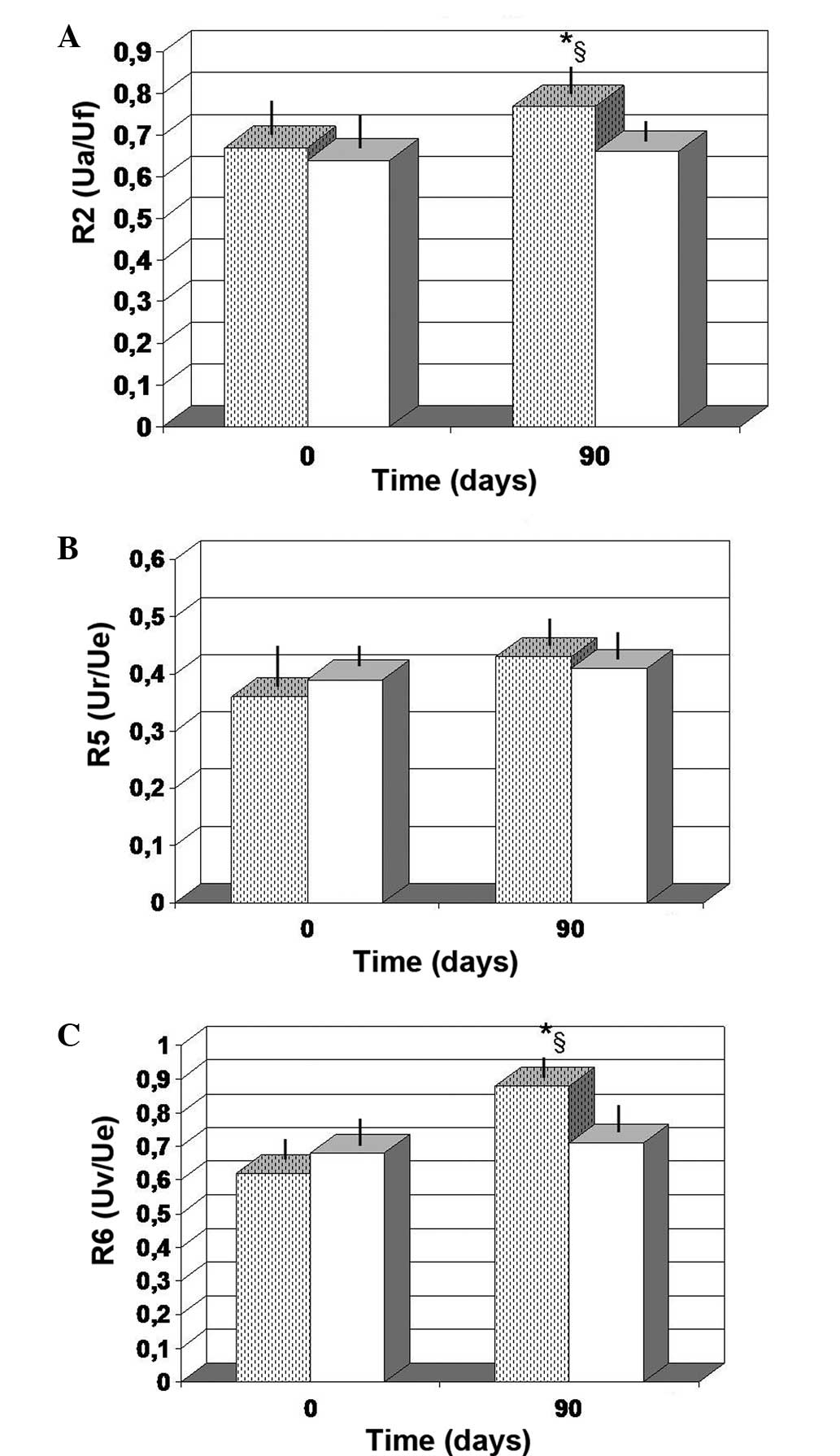
Skin elasticity
As compared to the baseline value, the FPP-treated
group showed a non-significant increase in the R2 values at 90 days
of observation (P<0.063; Fig.
2A). By contrast, the values obtained from the
antioxidant-treated group at 90 days were unchanged compared with
the baseline control values. Notably, the control values detected
after 90 days of observation for R6 (Uv/Ue) were significantly
lower compared with those obtained in the FPP-treated group
(P<0.05). For factor R5, no significant changes occurred,
regardless of the treatment employed (Fig. 2B). However, the FPP-treated group
showed a non-significant increase at 90 days compared with the
baseline value (P>0.05). Furthermore, FPP supplementation
maintained for 90 days resulted in a statistically significant
increase in the R6 values when compared with the baseline value
(P<0.01) and with the antioxidant-control group (P<0.05;
Fig. 2C).
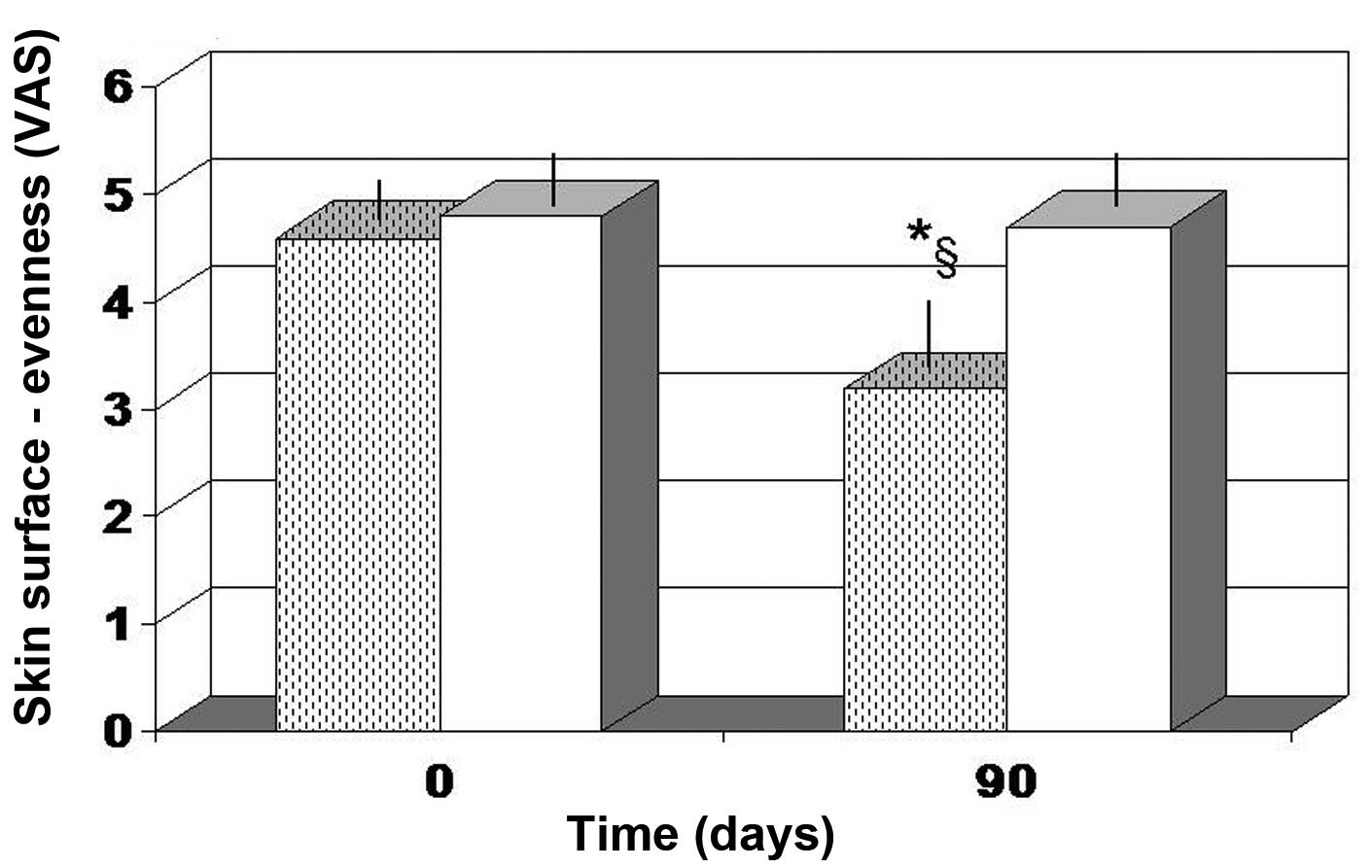
Skin surface and brown spot
intensity
The majority of the parameters tested, including
roughness, wrinkle depth and darkness of brown spots, did not show
a statistical significant change for the two treatment employed
(data not shown). However, the overall evenness (color variation in
the skin tone) was evaluated as significantly improved in the
FPP-treated group (P<0.05), but not in the antioxidant-control
group (Fig. 3).
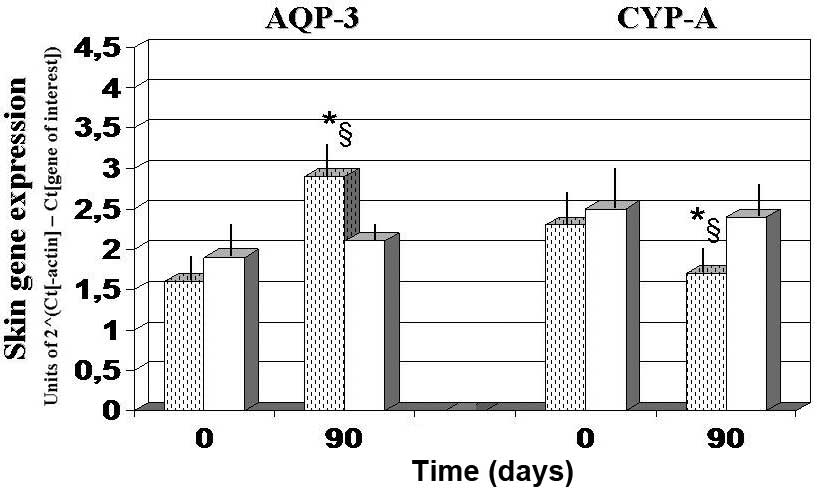
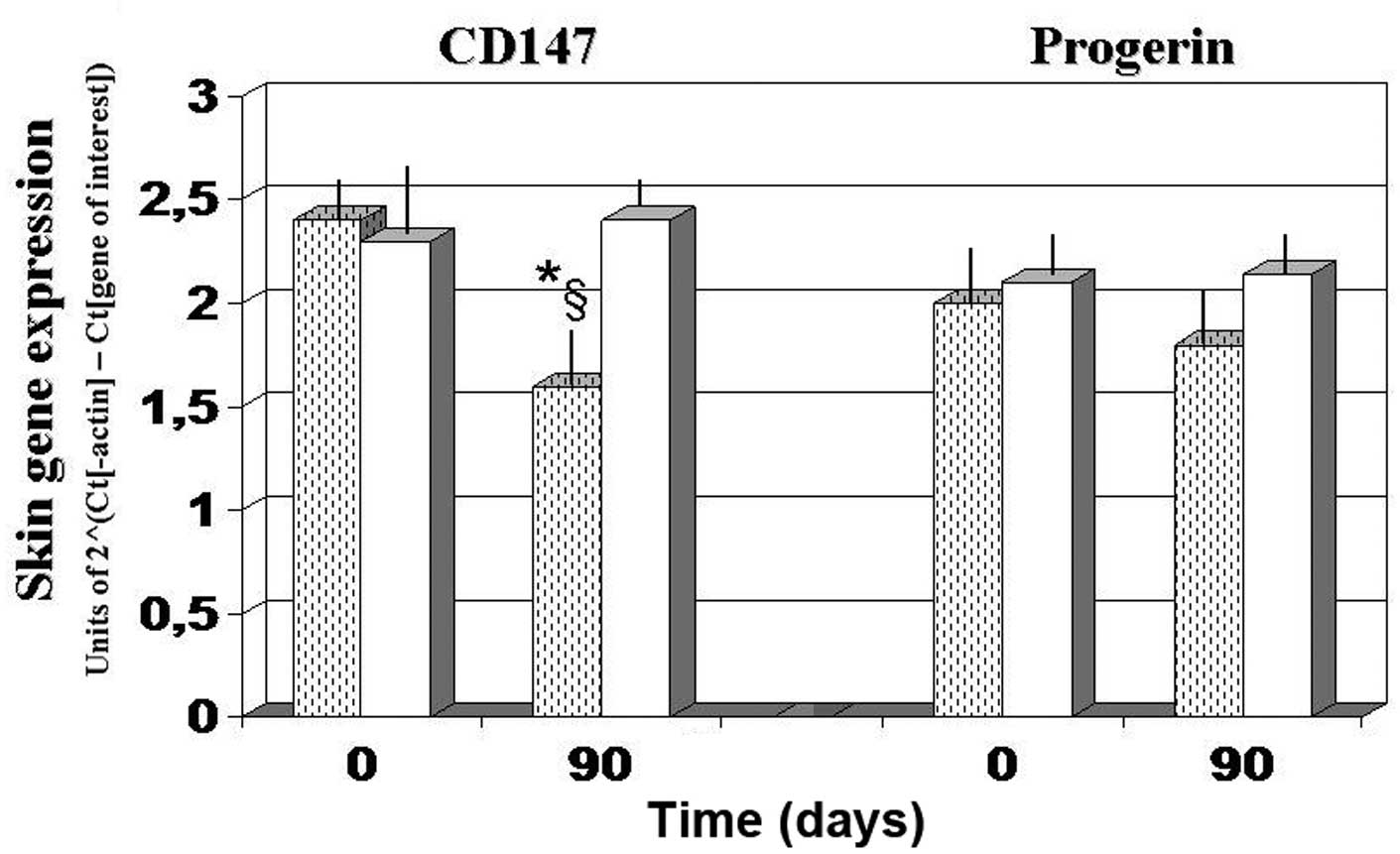
Skin gene expression experiments
Gene expression experiments demonstrated that, as
compared with the baseline and 90-day values observed in the
antioxidant-control group, AQP3 expression was significantly
upregulated after 90 days of FPP administration, whereas CyPA and
CD147 expression levels were significantly downregulated
(P<0.05; Figs. 4 and 5). By contrast, the
antioxidant-supplemented group did not show a significant
downregulation in the levels of CD147, after 90 days of treatment
(P>0.05 vs. baseline value). Progerin gene expression was
unaffected by the two treatments, although a trend towards
decreased expression was noted in the FPP-supplemented group
(P<0.068, non-significant change; Fig. 5).
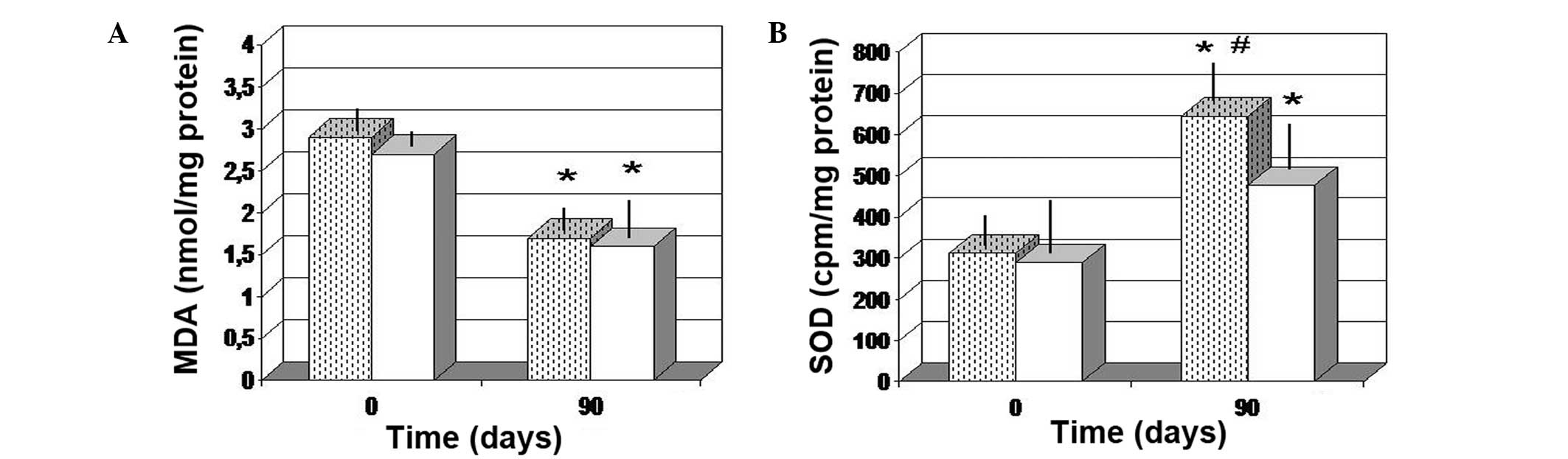
Dermal redox balance and NO
assessment
The two treatments were found to significantly
decrease the MDA level in the tissue, as compared with the baseline
values (P<0.05). In addition, there was no significant
difference between the two different treatment groups (Fig. 6A). A significant increase in the SOD
level was also noted after 90 days (P<0.01 vs. baseline values).
However, FPP treatment enabled a more significant increase in the
SOD tissue concentration (P<0.05 vs. antioxidant-control group;
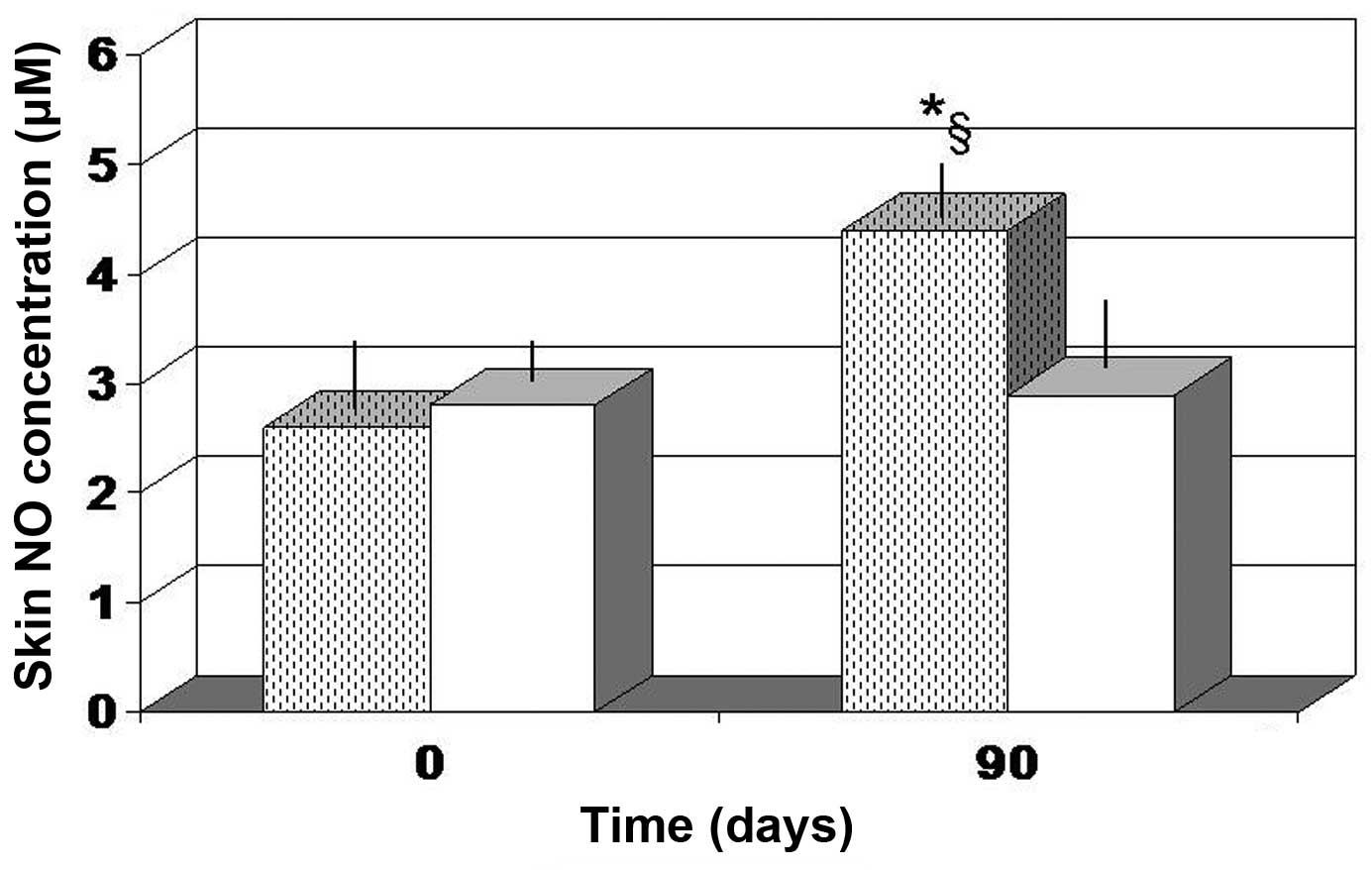
Fig. 6B). In addition, only FPP
showed a statistically significant increase in local NO production
(P<0.05 vs. baseline value and vs. antioxidant-control group;
Fig. 7).
Discussion
Aging is a complex process that underlies multiple
factors, with the involvement of heritable and several
environmental effects. In particular, aging is influenced by
large-scale epigenomic expression changes; however, the ways of
modulating such functional variations in order to achieve health
benefits in humans require further investigation. Skin cells are
constantly exposed to ROS and oxidative stress from exogenous and
endogenous sources (34–36). In addition, the skin is naturally
endowed with a variety of low molecular weight antioxidants and
antioxidant enzymes with ROS-scavenging activity, forming an
elaborate inducible/adaptive redox system (37). This system consists of enzymatic
antioxidants, including SOD, glutathione peroxidase and catalase,
as well as of non-enzymatic antioxidants, such as vitamin C,
vitamin E isoforms, ubiquinol, glutathione and uric acid. These
antioxidants act together with other factors, such as ascorbate,
carotenoids and sulfhydrils, to regulate the redox system. However,
this antioxidant machinery is not fully efficient; this becomes
more evident during the process of aging, in which an uncontrolled
oxidative damage to the proinflammatory cytokines occurs, which is
also the basis of carcinogenesis transformation (38). Certain abnormalities observed in
photoaging can be also found in chronoaging, and these include the
loss of dermal collagen that is observed at a lesser extent in
chronoaging. However, this also suggests that tissue injuries
initially triggered by environmental factors, such as sun exposure,
may indeed accelerate intrinsic aging mechanisms and help to
accumulate oxidized/degraded proteins (39).
From a macroscopic viewpoint, the present study
demonstrated that, unlike the antioxidant-control treatment, the
FPP nutraceutical resulted in a significant improvement in the skin
moisturization and elasticity, and namely in its viscous elastic
component. This finding is notable and can be tentatively linked to
the significant upregulation of AQP-3 expression that was exerted
only by this specific fermented nutraceutical. To add more
relevance to these findings, it is worth mentioning that Cao et
al (40) have demonstrated that
UV may induce AQP3 downregulation in human skin keratinocytes and
this is also likely to occur in vivo. Notably, a recent
study (41) suggested that
resveratrol treatment downregulated the expression of AQP-3, at
least in specific experimental set-ups, indicating that this
treatment may be more suitable for hyperplastic skin disorders,
rather than for the overall skin health.
Although the two nutraceuticals used in the current
study did not show any overt effect on the wrinkle and age spots
intensity, it was interesting to observe that FPP resulted in a
significant improvement in skin complexion (based on the skin
evenness, as evaluated by an expert investigator who was blinded to
the treatment). This clinical finding may be associated with the
obtained data of higher hydrated derma and possibly also due to the
higher concentration of NO. As a matter of fact, this unique
NO-modulating effect of FPP was demonstrated in an experimental
model established by Collard and Roy (42), while a recent study by our group
investigated the cardiovascular benefits of FPP in healthy subjects
(43). The two treatments seen in
the present study enabled a significant improvement of the redox
balance, and this may help counteract the increased MDA and
decreased SOD levels that were observed in skin injury caused by UV
and intrinsic aging (39). However,
unlike the antioxidant-control treatment, FPP exerted a significant
downregulating action on the gene expression levels of CyPA and
CD147. CyPA has been previously shown to be overexpressed in
several cancer cell lines (44). In
addition, Han et al (17)
recently provided convincing data in support of the oncogenic role
of CyPA in skin tumorigenesis, including squamous and basal cell
carcinomas, which are essentially caused by UV damage. The
transmembrane glycoprotein CD147 has been found to play a similar
role, since it is strictly connected to CyPA (45,46), and
therefore the CyPA-CD147 complex is suggested to be a novel
therapeutic target (47). Thus, the
aforementioned data provide further evidence that a systemic
redox-modulating approach (with FPP supplementation in the case of
the present study) is certainly noteworthy. In recent years, a
number of studies have investigated the brain-skin connection
(48,49), providing new insights from the
understanding and interventional perspective viewpoints.
Furthermore, a recent study reported that CyPA/CD147 modulation via
the extracellular regulated protein kinase signaling pathway may
play a relevant role in the brain physiopathology of dementia
(50). In fact, at a neurobiology
center in Italy, a study has demonstrated a significant improvement
in Parkinson patients using FPP, both biochemically and clinically
(51). In particular, among several
parameters, a significant decrease was observed in
8-hydroxy-2′-deoxyguanosine, which is know to increase also in the
skin of Parkinson patients (52).
Progerin can also be induced by UV, and its
accumulation is known to be involved in the process of DNA repair
and signaling during cellular injury (53). In the present study, none of the
supplemental interventions affected the progerin gene expression.
It is hypothesized that this fundamental biological parameter may
require a longer treatment period in order to show a response, but
also that oxidative stress-associated factors are only part of a
larger epigenomic environment to be advocated for its
modulation.
Although investigations involving dietary and
frequently high intake of a single antioxidant or vitamin may be
highly flawed due to the interactive nature of antioxidants in
vivo, large-scale nutrition and nutraceutical intervention
studies can aid the validation of nutrigenomic-dermagenomic
strategies for specific genes within an overall integrative health
medical approach. Nonetheless, the findings of the present study
findings have demonstrated that FPP possesses a distinct profile
among food supplements and functional foods by effectively acting
at a biochemical/epigenomic skin level. Furthermore, the importance
of well-designed topical formulations for skin protection maintains
its utmost importance and opens new avenues of treatment (54,55).
In conclusion, based on the present findings, orally
administered FPP appears to result in a consistent biological and
gene regulatory improvement in the skin, while common oral
antioxidants had only a minor effect.
References
|
1
|
Jones DP: Redox theory of aging. Redox
Biol. 5:71–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kammeyer A and Luiten RM: Oxidation events
and skin aging. Ageing Res Rev. 1:16–29. 2015. View Article : Google Scholar
|
|
3
|
Choi CM and Berson DS: Cosmeceuticals.
Semin Cutan Med Surg. 25:163–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katiyar SK: Green tea prevents
non-melanoma skin cancer by enhancing DNA repair. Arch Biochem
Biophys. 508:152–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Udompataikul M, Sripiroj P and
Palungwachira P: An oral nutra-ceutical containing antioxidants,
minerals and glycosaminoglycans improves skin roughness and fine
wrinkles. Int J Cosmet Sci. 31:427–435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Izumi T, Saito M, Obata A, Arii M,
Yamaguchi H and Matsuyama A: Oral intake of soy isoflavone aglycone
improves the aged skin in adult women. J Nutr Sci Vitaminol
(Tokyo). 53:57–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Natarajan VT, Ganju P, Ramkumar A, Grover
R and Gokhale R: Multifaceted pathways protect human skin from UV
radiation. Nat Chem Biol. 10:542–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uitto J: The role of elastin and collagen
in cutaneous aging: Intrinsic aging versus photoexposure. J Drugs
Dermatol. 7(Suppl 2): S12–S6. 2008.PubMed/NCBI
|
|
9
|
Rabe JH, Mamelak AJ, McElgunn PJ, Morison
WL and Sauder DN: Photoaging: Mechanisms and repair. J Am Acad
Dermatol. 55:1–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yaar M and Gilchrest BA: Photoageing:
mechanism, prevention and therapy. Br J Dermatol. 157:874–887.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melnikova VO and Ananthaswamy HN: Cellular
and molecular events leading to the development of skin cancer.
Mutat Res. 571:91–106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Makrantonaki E and Zouboulis CC: German
National Genome Research Network. 2 The skin as a mirror of the
aging process in the human organism-state of the art and results of
the aging research in the German National Genome Research Network 2
(NGFN-2). Exp Gerontol. 42:879–886. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Makrantonaki E, Schonknecht P, Hossini AM,
Kaiser E, Katsouli MM, Adjaye J, Schröder J and Zouboulis CC: Skin
and brain age together: The role of hormones in the ageing process.
Exp Gerontol. 45:801–813. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buonocore D, Lazzeretti A, Tocabens P,
Nobile V, Cestone E, Santin G, Bottone MG and Marzatico F:
Resveratrol-procyanidin blend: Nutraceutical and antiaging efficacy
evaluated in a placebo-controlled, double-blind study. Clin Cosmet
Investig Dermatol. 5:159–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gruber JV and Holtz R: Examining the
genomic influence of skin antioxidants in vitro. Mediators
Inflamm. 2010:2304502010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hara-Chikuma M and Verkman AS: Aquaporin-3
facilitates epidermal cell migration and proliferation during wound
healing. J Mol Med (Berl). 86:221–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han W, Soltani K, Ming M and He YY:
Deregulation of XPC and CypA by cyclosporin A. An
immunosuppression-independent mechanism of skin carcinogenesis.
Cancer Prev Res (Phila). 5:1155–1162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satoh K, Satoh T, Kikuchi N, Omura J,
Kurosawa R, Suzuki K, Sugimura K, Aoki T, Nochioka K, Tatebe S, et
al: Basigin mediates pulmonary hypertension by promoting
inflammation and vascular smooth muscle cell proliferation. Circ
Res. 115:738–750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ramachandran S, Venugopal A, Kutty VR, A
V, G D, Chitrasree V, Mullassari A, Pratapchandran NS, Santosh KR,
Pillai MR and Kartha CC: Plasma level of cyclophilin A is increased
in patients with type 2 diabetes mellitus and suggests presence of
vascular disease. Cardiovasc Diabetol. 13:382014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iacono KT, Brown AL, Greene MI and Saouaf
SJ: CD147 immunoglobulin superfamily receptor function and role in
pathology. Exp Mol Pathol. 83:283–295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takeuchi H and Rünger TM: Longwave UV
light induces the aging-associated progerin. J Invest Dermatol.
133:1857–1862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marotta F, Yoshida C, Barreto R, Naito Y
and Packer L: Oxidative-inflammatory damage in cirrhosis: Effect of
vitamin E and a fermented papaya preparation. J Gastroenterol
Hepatol. 22:697–703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aruoma OI, Colognato R, Fontana I, Gartlon
J, Migliore L, Koike K, Coecke S, Lamy E, Mersch-Sundermann V,
Laurenza I, et al: Molecular effects of fermented papaya
preparation on oxidative damage, MAP Kinase activation and
modulation of the benzo[a]pyrene mediated genotoxicity. Biofactors.
26:147–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bertuccelli G, Marotta F, Zerbinati N,
Cabeca A, He F, Jain S, Lorenzetti A, Yadav H, Milazzo M, Calabrese
F, et al: Iron supplementation in young iron-deficient females
causes gastrointestinal redox imbalance: Protective effect of a
fermented nutraceutical. J Biol Regul Homeost Agents. 28:53–63.
2014.PubMed/NCBI
|
|
25
|
Sachdeva S: Fitzpatrick skin typing:
Applications in dermatology. Indian J Dermatol Venereol Leprol.
75:93–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clarys P, Barel AO and Gabard B:
Non-invasive electrical measurements for the evaluation of the
hydration state of the skin: Comparison between three conventional
instruments - the Corneometer, the Skicon, and the Nova DPM. Skin
Res Technol. 5:14–20. 1999. View Article : Google Scholar
|
|
27
|
Courage W: Hardware and measuring
principle: corneometer. Bioengineering of the Skin: Water and the
Stratum Corneum. Elsner P, Berardesca E and Maibach HI: (Boca
Raton, FL). CRC Press. 1994.
|
|
28
|
Barel AO, Courage W and Clarys P: Suction
method for measurement of skin mechanical properties: The
cutometer. Handbook of Non-invasive methods and the Skin. Serup J
and Jemec GBE: (Boca Raton, FL). CRC Press. 1995.
|
|
29
|
Han A, Chien AL and Kang S: Photoaging.
Dermatol Clin. 32:291–299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilhelm KP, Elsner P, Berardesca E and
Maibach HI: Bioengineering of the skin: Skin surface imaging and
analysis. Boca Raton, FL: CRC Press. 1996.
|
|
31
|
Luebberding S, Krueger N and Kerscher M:
Comparison of Validated Assessment Scales and 3D digital fringe
projection method to assess lifetime development of wrinkles in
men. Skin Res Technol. 20:30–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ding TM, Chen J, Zhang ZX and Zhang YH:
The methods for determination of nitric oxide in vivo and
their applications. Yao Xue Jin Zhan. 29:221–226. 2005.(In
Chinese).
|
|
33
|
Bland JM and Altman DG: Applying the right
statistics: Analyses of measurement studies. Ultrasound Obstet
Gynecol. 22:85–93. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Indo HP, Yen HC, Nakanishi I, Matsumoto K,
Tamura M, Nagano Y, Matsui H, Gusev O, Cornette R, Okuda T, et al:
A mitochondrial superoxide theory for oxidative stress diseases and
aging. J Clin Biochem Nutr. 56:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vajapey R, Rini D, Walston J and Abadir P:
The impact of age-related dysregulation of the angiotensin system
on mitochondrial redox balance. Front Physiol. 5:4392014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fulop T, Witkowski JM, Pawelec G, Alan C
and Larbi A: On the immunological theory of aging. Interdiscip Top
Gerontol. 39:163–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Poljšak B, Dahmane RG and Godić A:
Intrinsic skin aging: The role of oxidative stress. Acta
Dermatovenerol Alp Pannonica Adriat. 21:33–36. 2012.PubMed/NCBI
|
|
38
|
Godic A, Poljšak B, Adamic M and Dahmane
R: The role of antioxidants in skin cancer prevention and
treatment. Oxid Med Cell Longev. 2014:8604792014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu CY, Lee HC, Fahn HJ and Wei YH:
Oxidative damage elicited by imbalance of free radical scavenging
enzymes is associated with large-scale mtDNA deletions in aging
human skin. Mutat Res. 423:11–21. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cao C, Wan S, Jiang Q, Amaral A, Lu S, Hu
G, Bi Z, Kouttab N, Chu W and Wan Y: All-trans retinoic acid
attenuates ultraviolet radiation-induced down-regulation of
aquaporin-3 and water permeability in human keratinocytes. J Cell
Physiol. 215:506–516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu Z, Uchi H, Morino-Koga S, Shi W and
Furue M: Resveratrol inhibition of human keratinocyte proliferation
via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J
Dermatol Sci. 75:16–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Collard E and Roy S: Improved function of
diabetic wound-site macrophages and accelerated wound closure in
response to oral supplementation of a fermented papaya preparation.
Antioxid Redox Signal. 13:599–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marotta F, Yadav H, Kumari A, Catanzaro R,
Jain S, Polimeni A, Lorenzetti A and Soresi V: Cardioprotective
effect of a biofermented nutraceutical on endothelial function in
healthy middle-aged subjects. Rejuvenation Res. 15:178–181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee J and Kim SS: An overview of
cyclophilins in human cancers. J Int Med Res. 38:1561–1574. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ayva SK, Karabulut AA, Akatli AN, Atasoy P
and Bozdogan O: Epithelial expression of extracellular matrix
metalloproteinase inducer/CD147 and matrix metalloproteinase-2 in
neoplasms and precursor lesions derived from cutaneous squamous
cells: An immunohistochemical study. Pathol Res Pract. 209:627–634.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang CH, Rong MY, Wang L, Ren Z, Chen LN,
Jia JF, Li XY, Wu ZB, Chen ZN and Zhu P: CD147 up-regulates
calcium-induced chemotaxis, adhesion ability and invasiveness of
human neutrophils via a TRPM-7-mediated mechanism. Rheumatology
(Oxford). 53:2288–2296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yurchenko V, Constant S, Eisenmesser E and
Bukrinsky M: Cyclophilin-CD147 interactions: A new target for
anti-inflammatory therapeutics. Clin Exp Immunol. 160:305–317.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen Y and Lyga J: Brain-skin connection:
Stress, inflammation and skin aging. Inflamm Allergy Drug Targets.
13:177–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Doppler K, Ebert S, Uçeyler N, Trenkwalder
C, Ebentheuer J, Volkmann J and Sommer C: Cutaneous neuropathy in
Parkinson's disease: A window into brain pathology. Acta
Neuropathol. 128:99–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kanyenda LJ, Verdile G, Martins R, Meloni
BP, Chieng J, Mastaglia F, Laws SM, Anderton RS and Boulos S: Is
cholesterol and amyloid-β stress induced CD147 expression a
protective response? Evidence that extracellular cyclophilin a
mediated neuroprotection is reliant on CD147. J Alzheimers Dis.
39:545–556. 2014.PubMed/NCBI
|
|
51
|
Mantello M, Catanzaro R, He F, Bissi L,
Milazzo M, Lorenzetti A and Marotta F: Novel nutrigenomics avenues
in nutraceuticals use: The current status of fermented papaya
preparation. Bioactive Compounds: At the Frontier Between Nutrition
and Parmacology. Aguilar Villas MV and Otero C: Bentham Science.
2015.(in press).
|
|
52
|
del Hoyo P, García-Redondo A, de Bustos F,
Molina JA, Sayed Y, Alonso-Navarro H, Caballero L, Arenas J,
Agúndez JA and Jiménez-Jiménez FJ: Oxidative stress in skin
fibroblasts cultures from patients with Parkinson's disease. BMC
Neurol. 10:952010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Musich PR and Zou Y: Genomic instability
and DNA damage responses in progeria arising from defective
maturation of prelamin A. Aging (Albany NY). 1:28–37.
2009.PubMed/NCBI
|
|
54
|
Martins RM, Siqueira S, Fonseca MJ and
Freitas LA: Skin penetration and photoprotection of topical
formulations containing benzophenone-3 solid lipid microparticles
prepared by the solvent-free spray-congealing technique. J
Microencapsul. 31:644–653. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Scalia S, Marchetti N and Bianchi A:
Comparative evaluation of different co-antioxidants on the
photochemical- and functional-stability of
epigallocatechin-3-gallate in topical creams exposed to simulated
sunlight. Molecules. 18:574–587. 2013. View Article : Google Scholar : PubMed/NCBI
|