Introduction
Myocardial ischemia/reperfusion (I/R) injury is a
common pathological process in numerous clinical settings,
including aortic bypass surgery, cardiopulmonary surgery and heart
transplantation (1,2). The exact molecular mechanisms
underlying myocardial I/R injury are complicated and are yet to be
fully understood. However, there is considerable evidence that
inflammation has an important role in myocardial I/R injury
(3,4). Notably, the phosphoinositide 3-kinase
(PI3K)/AKT signaling pathway, which was originally recognized to
have a critical role in the regulation of cell growth and cell
survival in various systems (5,6), has
been shown to be involved in the protection of the kidneys and
liver against I/R injury by regulating the inflammatory response
(7,8).
The PI3K enzyme consists of a catalytic subunit p110
and a regulatory subunit p85, and its activation relies on p85
phosphorylation. Once p85 has been activated, it initiates the
phosphorylation of Akt (also known as protein kinase B), which
subsequently triggers signaling pathways in order to increase the
inflammatory response (9,10). Inhibiting the activation of the
PI3K/AKT signaling pathway has been demonstrated to attenuate
I/R-induced injury (7).
Berberine (BBR) is an isoquinoline alkaloid compound
that was originally isolated from the Chinese herb Coptis
chinensis (Huanglian) (11). It
is an antimicrobial drug routinely prescribed for the treatment of
diarrhea in various Asian countries (11). In addition to this well-known and
widely recognized effect, it has been demonstrated that BBR also
regulates the activity of transcription factors essential for the
inflammatory response (12). BBR has
been shown to provide intestinal and cerebral protection against
I/R-induced injury (13,14); however, the effects of BBR against
myocardial I/R injury are yet to be elucidated. Therefore, the
present study investigated whether BBR was able to prevent
myocardial I/R injury in a rat model.
Materials and methods
Animals
A total of 50 male Sprague Dawley (SD) rats (200–250
g) were purchased from the Hua Fukang Experimental Animal Center
(Beijing, China). The rats were housed in a specific pathogen-free
facility at 18–29°C under a 14 h light/10 h dark cycle, and were
fed with laboratory chow and water. After a minimum 7 days of
acclimation, the rats were randomly divided into five groups as
follows (10 rats/group): i) The I/R injury (IRI) group, in which
saline-treated rats were subjected to ischemia for 0.5 h followed
by reperfusion; ii) three BBR groups in which the rats were treated
with BBR at doses of 25, 50 or 100 mg/kg/day, respectively, by
gavage 14 days prior to the induction of I/R; and iii) a sham
group, in which saline-treated rats were subjected to sham surgery
without the induction of ischemia. The dosages administered to the
rats were determined according to a previous study (15). All experiments were approved by the
Institutional Animal Care and Use Committee at Weifang People's
Hospital (Weifang, China).
Induction of myocardial I/R
The rats were anesthetized with intraperitoneal
injection of 1% sodium pentobarbital solution (65 mg/kg) and their
temperature was regulated throughout the test by means of a heating
lamp. Myocardial I/R was induced as follows: The rats were placed
in the supine position and secured in a dissection tray. A left
thoracic incision was made to expose the heart, and myocardial
ischemia was induced by making a slipknot (4-0 silk) around the
left anterior descending coronary artery. After 30 min of ischemia,
the slipknot was released and the myocardium was reperfused for 4
h. The rats in the sham group underwent the same surgical
procedures with the exception of occlusion of the left anterior
descending coronary artery. Myocardial function was continuously
monitored prior to and during the I/R procedure and during the
ischemia and reperfusion period with an electrocardiogram (ECG)
recorder (ECG-9020p; Nihon Kohden, Tokyo, Japan). The total time of
recording was 4.5 h.
The incidence and count of premature ventricular
contraction (PVC) and the incidence and cumulative duration of
ventricular tachycardia (VT) and ventricular fibrillation (VF) were
recorded. Ventricular arrhythmia (VA) was scored using the criteria
described by Curtis and Walker (16)
and Ravingerova et al (17).
Histological analysis
At 3 h of reperfusion, the rats were anesthetized
with intraperitoneal injection of 1% sodium pentobarbital solution
(65 mg/kg), after which they were sacrificed by cervical
dislocation in order to collect serum and myocardial tissue. The
excised hearts were fixed with 10% formalin, embedded in paraffin,
and stained with hematoxylin and eosin (H&E). Myocardial I/R
injury was scored using published morphologic criteria (18): 0, no damage; 1 (mild), interstitial
edema and localized necrosis; 2 (moderate), widespread myocardial
cell swelling and necrosis; 3 (severe), necrosis with contraction
bands and compressed capillaries, or 4 (highly severe), diffuse
necrosis with contraction bands, compressed capillaries and
hemorrhage.
Western blot analysis
Myocardial samples (50 mg) were mechanically
homogenized in 1 ml hypotonic buffer, containing 200 µl
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.5), 25
mmol/l magnesium chloride, 5 mmol/l dithiothreitol, 5 mmol/l
phenylmethylsulfonyl fluoride, 2 mmol/l pepstatin A, 10 µg/ml
leupeptin, 5 mmol/l ethylenediamine tetraacetic acid, 10 µg/ml
aprotinin and 100 µl anti-phosphatases (Roche Diagnostics, Basel,
Switzerland). Protein concentrations were determined using a
bicinchoninic assay kit (Biyuntian Biotechnology, Wuhan, China),
according to the manufacturer's protocol. Protein samples (80 µg)
were resolved by 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to polyvinylidene fluoride
membranes (San Yin-tan, Beijing, China). The membranes were blocked
with 5% non-fat skimmed milk in Tris-buffered saline solution
containing Tween-20 (TBST; 10 mmol/l Tris, pH 7.5; 140 mmol/l
sodium chloride; 0.1% Tween-20) for 1 h at 37°C, washed and then
incubated with primary antibody in TBST containing 3% bovine serum
albumin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
for 12 h at 4°C. The primary antibodies were as follows: Rabbit
anti-p85 (1:1,000; 4292), rabbit anti-phosphorylated (p)-p85
(1:500; 4228), rabbit anti-Akt (1:1,000; 4691) and rabbit
anti-p-Akt (1:500; 4060) (Cell Signaling Technology, Inc., Danvers,
MA, USA). The membranes were then washed extensively with TBS,
prior to incubation with a horseradish peroxidase-conjugated
immunoglobulin G secondary antibody (1:4,000; J115005072; Jackson
ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h at 37°C.
β-actin (1:3,000; 113257; Abmart, Shanghai, China) was used for the
normalization of protein levels. The reactive bands were visualized
using the Enhanced Chemiluminescence-Plus reagent (GE Healthcare
Life Sciences, Piscataway, NJ, USA) according to the protocol
provided by the manufacturer. The density of each reactive band was
quantified using the LabWorks Image Acquisition platform (UVP,
Inc., Upland, CA, USA), and ImageJ (National Institutes of Health,
Bethesda, MA, USA).
Enzyme-linked immunosorbent assay
(ELISA) analysis
At 3 h of reperfusion, the rats were anesthetized
with intraperitoneal injection of 1% sodium pentobarbital solution
(65mg/kg), after which they were sacrificed for collection of serum
and myocardial tissue. Levels of the inflammatory mediators tumor
necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β in the serum
were quantified using specific ELISA kits for rats, according to
the manufacturer's protocol (BioSource™; Thermo Fisher Scientific,
Waltham, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from myocardial tissues using
TRIzol® reagent, according to the manufacturer's protocol (Thermo
Fisher Scientific, Inc.). Total RNA (4 µg) was reverse transcribed
into cDNA using the PrimeScript SYBR Premix Ex Taq™ II (PerfectReal
Time; Takara Bio, Inc., Otsu, Japan), as instructed by the
manufacturer. qPCR amplifications were conducted using the ABI 7500
Real Time PCR system (Applied Biosystems; Thermo Fisher
Scientific). PCR primers (Invitrogen; Thermo Fisher Scientific) for
all analyzed mRNA are presented in Table
I. PCR was conducted at 95°C for 30 sec, followed by 40 cycles
at 95°C for 5 sec, 60°C for 34 sec and 95°C for 15 sec. The amount
of mRNA for each gene was normalized using β-actin, and the
relative expression levels were calculated using the
2−ΔΔCq method, as previously reported (19).
 | Table I.Primers used for quantitative
polymerase chain reaction analysis. |
Table I.
Primers used for quantitative
polymerase chain reaction analysis.
| mRNA | Species | Forward | Reverse |
|---|
| TNF-α | Rat |
CTGAACTTCGGGGTGATCGG |
GGCTTGTCACTCGAATTTTGAGA |
| IL-6 | Rat |
AGCTTCCTTGTGCAAGTGTCT |
GACAGCCCAGGTCAAAGGTT |
| IL-1β | Rat |
CTGCAAGAGACTTCCATCCAG |
AGTGGTATAGACAGGTCTGTTGG |
| β-actin | Rat |
AGAGGGAAATCGTGCGTGAC |
CAATAGTGATGACCTGGCCGT |
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Comparisons between groups were performed using the
Student's t-test or one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
BBR attenuates the I/R-induced
incidence of ventricular arrhythmia
The impact of BBR on ventricular arrhythmia
following I/R insult was first investigated. For this purpose, the
incidence and count of PVC and the incidence and cumulative
duration of VT and VF were examined, and VA scores were determined.
As compared with the rats in the sham group, rats in the IRI group
exhibited an increased incidence and count of PVC, increased
incidence and cumulative duration of VT and VF and higher
arrhythmia scores (P<0.05), indicating that I/R insult induced
severe ventricular arrhythmia. Notably, rats treated with BBR had a
lower incidence and count/cumulative duration of PVC, VT and VF,
and reduced arrhythmia scores compared with those of the rats in
the IRI group, and the values were reduced in a dose-dependent
manner (P<0.05; Table II). These
data indicate that the administration of BBR protected the rats
against I/R-induced myocardial injury and the greatest protective
effect was observed at a BBR concentration of 100 mg/kg.
 | Table II.Effects of BBR on arrhythmia in IRI
model rats. |
Table II.
Effects of BBR on arrhythmia in IRI
model rats.
|
|
| PVC | VT | VF |
|
|---|
|
|
|
|
|
|
|
|---|
| Group | Number | Incidence | Count | Incidence | Duration (sec) | Incidence | Duration (sec) | VA score |
|---|
| Sham | 10 | 50 | 9±11 | 0 | 0 | 0 | 0 | 0.4±0.5 |
| IRI | 10 | 80 |
37±22a | 60 |
27.2±24.6a | 40 |
15.5±17.2a | 5.0±4.3a |
| BBR (25 mg/kg) | 10 | 80 |
32±21b | 50 |
22.7±26.6b | 40 |
11.5±14.7b | 4.2±4.1b |
| BBR (50 mg/kg) | 10 | 60 |
21±15b | 30 |
10.8±16.6b | 15 | 5.6±7.8b | 2.5±3.2b |
| BBR (100 mg/kg) | 10 | 60 |
15±12b | 20 | 3.4±7.0b | 5 | 1.8±4.2b |
1.5±3.0b |
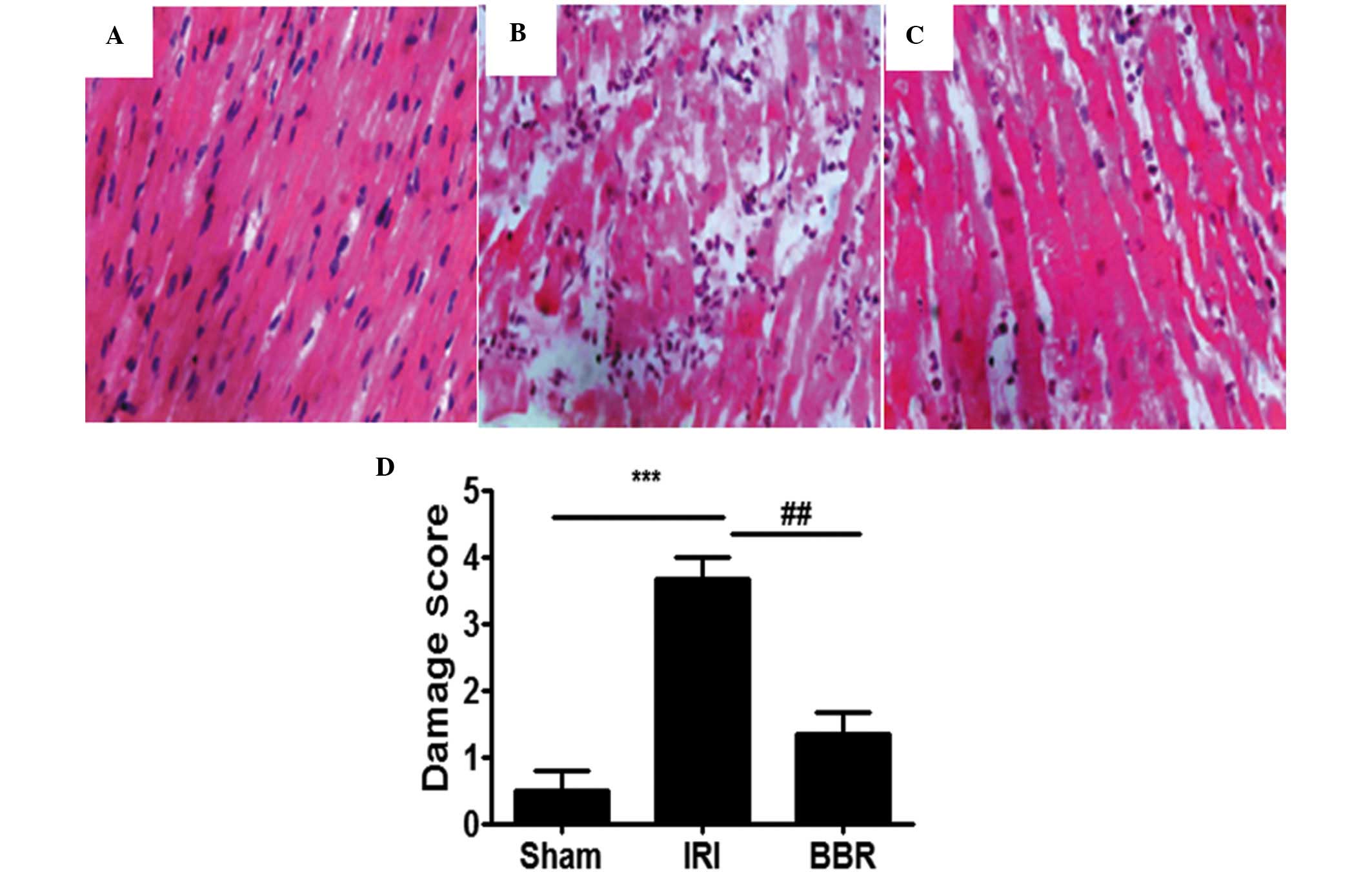
BBR attenuates I/R-induced
pathological changes in the myocardial tissue
When the myocardial tissues from the IRI group were
compared with those from the sham group, significant pathological
changes were evident in the myocardial tissues from the IRI group
(Fig. 1A and B). The changes
observed in the IRI group included atrophy of myocardial fibers,
inflammatory cell infiltration, coagulative necrosis and
liquefactive necrosis. By contrast, the myocardial tissue in the
BBR group (100 mg/kg) exhibited fewer pathological changes, as
compared with the IRI group (Fig.
2C). Semi-quantitative assessment of the histological lesions
showed a significantly higher score in the rats of the IRI group
than in the rats of the sham and BBR groups (Fig. 2D).
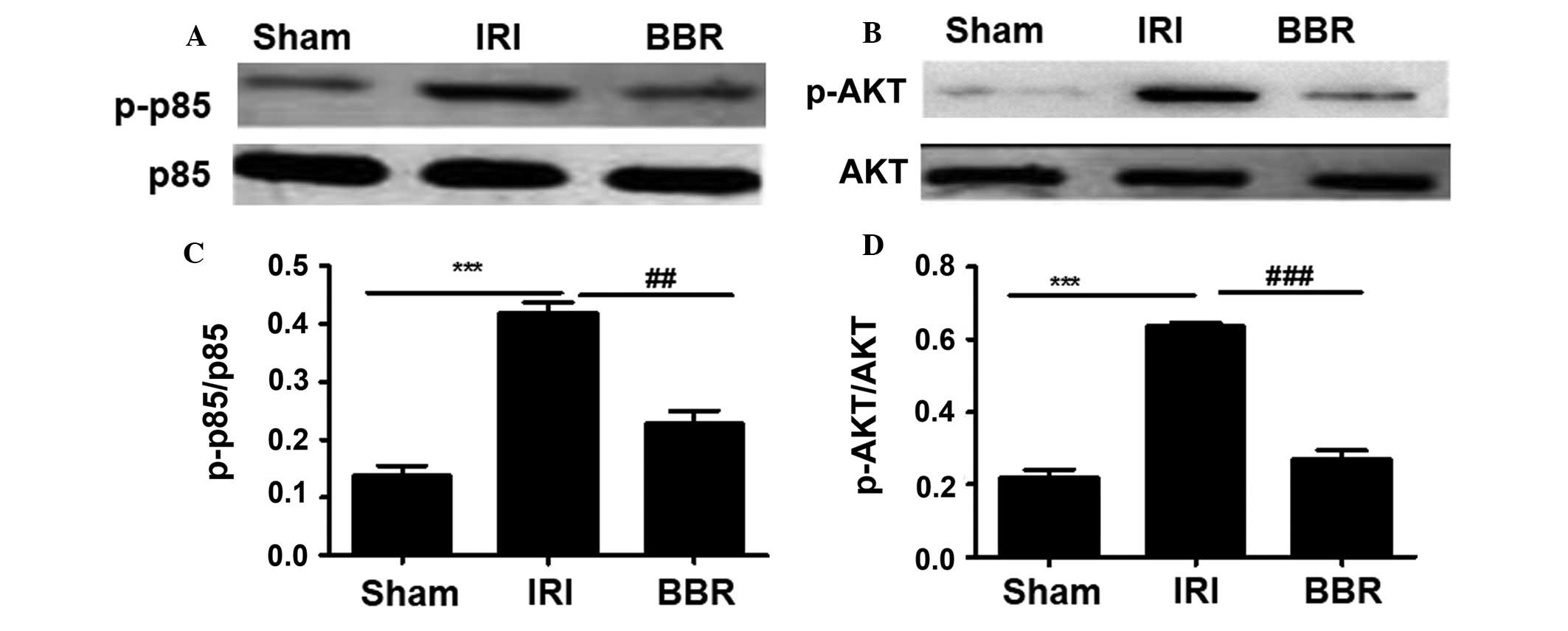
BBR suppresses the activation of
PI3K/AKT signaling
After ascertaining the concentration of BBR with the
strongest protective effect against myocardial I/R injury, the next
step was to explore the mechanisms underlying the protective effect
of BBR against I/R-induced myocardial injury. It has previously
been demonstrated that PI3K/AKT plays an important role in
myocardial injury (15) and that BBR
is able to regulate PI3K/AKT signaling (15). To this end, the activity of the PI3K
p85 regulatory subunit was first investigated. No significant
difference was detected in the expression of total p85 among the
three groups of rats (Fig. 2).
However, much higher levels of activated p85 (p-p85) were noted in
the rats of the IRI group as compared with those in rats of either
the BBR group or the sham group (Fig.
2). Notably, rats in the BBR group showed very similar levels
of p-p85 to those in the sham group. Since p85 activation provides
signals for AKT phosphorylation, AKT activity was then examined.
Similar to p85, there was no difference in the levels of total AKT
among the groups (Fig. 2), but
activated AKT (p-AKT) levels were significantly higher in the rats
of the IRI group, and rats in the BBR group displayed p-AKT levels
very similar to those of the sham group (Fig. 2). Collectively, these data suggest
that BBR pretreatment can decrease PI3K p85 activity, which results
in the upregulation of AKT activation.
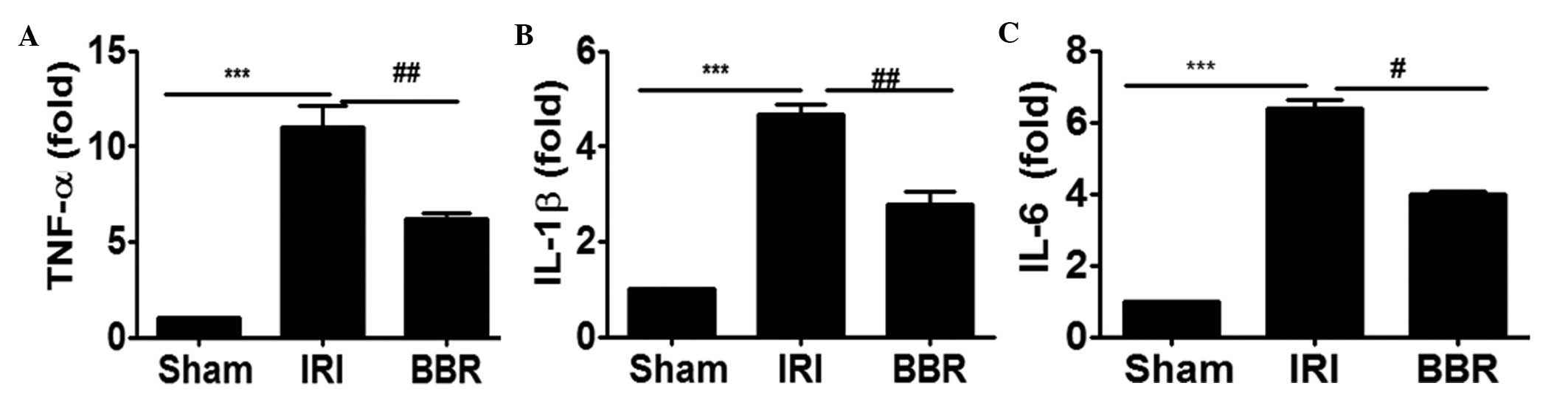
Blocking the activation of PI3K/AKT
signaling suppresses inflammatory cytokine expression
In order to investigate whether blocking the
activation of PI3K/AKT signaling suppressed the expression of
inflammatory cytokines in the heart, the expression of TNF-α, IL-6
and IL-1β in cardiac tissues after I/R insult was analyzed by
RT-qPCR. It was noted that I/R insult increased the expression
level of TNF-α by 9-fold (Fig. 3A),
IL-1β by 3.75-fold (Fig. 3B) and
IL-6 by 5-fold (Fig. 3C) as compared
with that of rats in the sham group. Notably, the administration of
BBR inhibited I/R-induced TNF-α expression by 49% (Fig. 3A), IL-1β by 40% (Fig. 3B) and IL-6 by 42% (Fig. 3C). These data support the hypothesis
that the attenuation of PI3K/AKT signaling by the administration of
BBR significantly suppressed I/R-induced inflammatory cytokine
expression in the myocardial tissues.
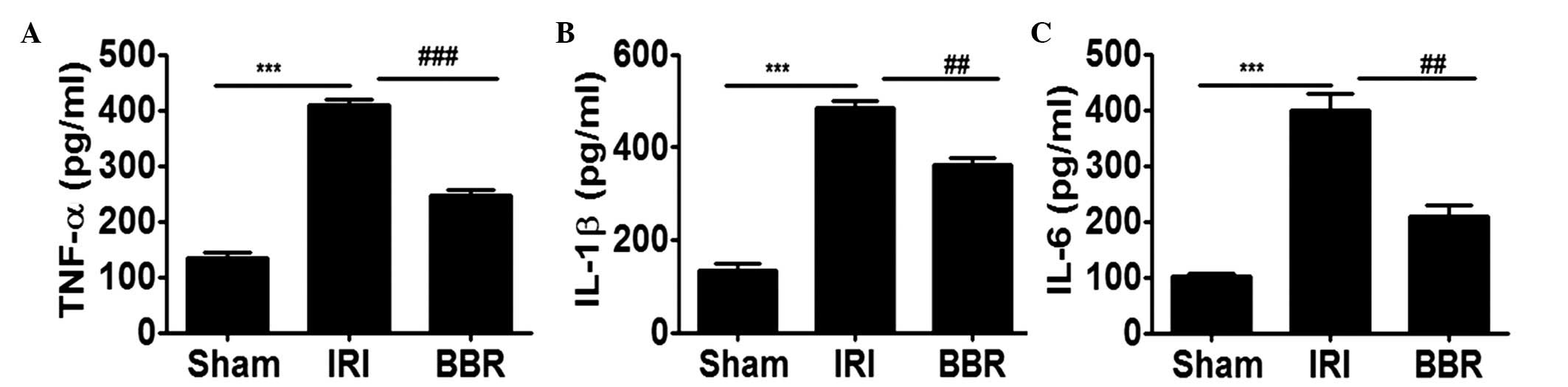
Blocking the activation of PI3K/AKT
signaling suppresses inflammatory cytokine secretion
In order to further elucidate the effect of BBR on
the expression of inflammatory cytokines following I/R insult, the
expression levels of TNF-α, IL-6 and IL-1β in the serum were
analyzed. It was noted that I/R insult increased the secretion of
TNF-α by 2-fold (Fig. 4A), IL-1β by
2.5-fold (Fig. 4B) and IL-6 by
3-fold (Fig. 4C) as compared with
that in the sham group. Notably, administration of BBR inhibited
I/R-induced TNF-α expression by 45% (Fig. 4A), IL-1β by 35% (Fig. 4B) and IL-6 by 45% (Fig. 4C), and. These data indicate that the
downregulation of PI3K/AKT signaling by BBR significantly
suppressed I/R-induced inflammatory cytokine secretion in the
serum.
Discussion
Although BBR has long been used for the treatment of
diarrhea in a number of Asian countries (11), its exact impact on myocardial I/R
injury, remains unclear and was thus investigated in the present
study. I/R injury was induced in rats by blocking the left
descending coronary artery for 30 min, followed by reperfusion for
4 h. ECG analysis demonstrated that myocardial I/R injury
significantly increased the incidence and count of PVC, the
incidence and cumulative duration of VT and VF, and arrhythmia
scores. However, pretreatment with BBR (25, 50 or 100 mg/kg/day) by
gavage for 14 days prior to the induction of I/R significantly
attenuated the changes in ECG results in a dose-dependent manner.
Moreover, pretreatment with BBR also decreased the IRI-induced
histological changes of the myocardium as manifested by reductions
in the atrophy of myocardial fibers, inflammatory cell
infiltration, coagulative necrosis and liquefactive necrosis in the
BBR group compared with those in the IRI group. These results are
consistent with those of previous studies, which suggest that the
treatment of rats with BBR can significantly decrease myocardial
I/R injury and the subsequent induction of ventricular arrhythmias
and myocardial histological changes (15,20,21). The
present data support the potential of BBR as a new therapeutic
agent for the prevention and treatment of myocardial I/R injury in
clinical practice.
To ascertain the molecular mechanisms by which BBR
provides protection against myocardial I/R injury in rats, the
impact of BBR on PI3K/AKT signaling was investigated. I/R insult
induced activation of the PI3K p85 regulatory subunit as manifested
by the significantly higher levels of p-p85 in rats of the IRI
group as compared with those in the sham control rats. BBR
pretreatment significantly decreased the activation of p85. This
result prompted the examination of AKT activity since the
phosphorylation of p85 would predispose AKT to activation.
Consistent with the aforementioned results, BBR significantly
attenuated AKT activity as manifested by a reduction in the levels
of p-AKT by more than half. To further investigate whether the
suppression of PI3K/AKT signaling activation by BBR inhibited the
inflammatory response, TNF-α, IL-6 and IL-1β expression in the
I/R-insulted myocardial tissues and serum were examined. Rats
preconditioned with BBR displayed significantly lower levels of
TNF-α, IL-6 and IL-1β in myocardial tissue and serum than were
observed in the saline-treated IRI group. These data suggest that
BBR suppressed the activation of PI3K/AKT signaling, which then
repressed the inflammatory response to mitigate myocardial I/R
injury.
The duration of ischemia and time at which
measurements are taken after reperfusion are critical factors
relevant to the severity of I/R injury in rats (1). Published data describe variations in
results when experimental conditions such as temperature and
duration of ischemia are changed. In particular, the results may
vary depending on the strain of rats employed, for example SD
versus diabetic rats (21). In the
experimental model used in the present study, SD rats were
employed, and a time period of 30 min was used for ischemic insult
and I/R injury was examined 4 h after reperfusion. The degree of
severity of the myocardial injuries in this model was noted to be
similar to that in a previous study (15). As aforementioned, evidence for
myocardial injury was strongly supported by the chamges in the
electrocardiograms along with inflammatory infiltration. These data
support the hypothesis that I/R initiates a complex cascade of
events, which eventually result in myocardial injury characterized
by changes in ECG results and inflammatory infiltration.
The PI3K/AKT pathway is known to be important in
regulating the adaptive immune response. For example, PI3K
heterodimers control cell survival, proliferation, B- and T-cell
receptor signaling, and chemotaxis in B and T lymphocytes (21,22). The
PI3K/AKT signaling pathway also has a variety of roles in innate
immune cells, including neutrophils, mast cells, monocytes,
macrophages and myeloid as well as plasmacytoid dendritic cells.
For example, the migration of innate immune cells into sites of
injury in tissues or organs involves the dynamic reorganization of
cytoskeletons and membrane structures, while PI3K signaling is
essential for this process by providing cell polarity and
pseudopodia extension (22). The
hypothesis that the inhibition of PI3K attenuates I/R-induced
myocardial injury has been investigated previously (23). Therefore, in the current study, no
further experiments to demonstrate that the suppression of PI3K/AKT
signaling attenuated the I/R-induced immune response in the
myocardial tissue were conducted. Given the capacity of BBR
preconditioning to prevent myocardial I/R injury, it is worthy of
note that BBR may have an effect on other pathways in addition to
the PI3K/AKT signaling pathway, for example, the MAPK kinase
cascade (24). Further studies are
required to investigate the pathways associated with I/R
insult.
In conclusion, the present study provides evidence
that the preconditioning of rats with BBR protects against
I/R-induced myocardial injury in a dose-dependent manner, as
manifested by reduced ventricular arrhythmia and suppressed
inflammatory infiltration. The mechanistic investigation
demonstrated that BBR inhibits the activation of PI3K/AKT
signaling, which then suppresses inflammatory infiltration and
protects against myocardial I/R injury. These results support the
use of BBR as an effective alternative therapy for the prevention
and treatment of myocardial I/R injury in clinical practice.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81100164).
References
|
1
|
Chen K, Li G, Geng F, Zhang Z, Li J, Yang
M, Dong L and Gao F: Berberine reduces ischemia/reperfusion-induced
myocardial apoptosis via activating AMPK and PI3K-Akt signaling in
diabetic rats. Apoptosis. 19:946–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan X, Qiu W, Jia B, Zhong H, Li X and
Chen Z: Myocardial protection by interferon-γ late preconditioning
during cardiopulmonary bypass-associated myocardial
ischemia-reperfusion in pigs. Oncol Rep. 30:2145–2152.
2013.PubMed/NCBI
|
|
3
|
Lassaletta AD, Elmadhun NY, Zanetti AV,
Feng J, Anduaga J, Gohh RY, Sellke FW and Bianchi C: Rapamycin
treatment of healthy pigs subjected to acute myocardial
ischemia-reperfusion injury attenuates cardiac functions and
increases myocardial necrosis. Ann Thorac Surg. 97:901–907. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du X, Hu X and Wei J: Anti-inflammatory
effect of exendin-4 postconditioning during myocardial ischemia and
reperfusion. Mol Biol Rep. 41:3853–3857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu B, Meng K, Ji Q, Cheng M, Yu K, Zhao X,
Tony H, Liu Y, Zhou Y, Chang C, et al: Interleukin-37 ameliorates
myocardial ischaemia/reperfusion injury in mice. Clin Exp Immunol.
176:438–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pourrajab F, Zarch Babaei M, Baghi Yazdi
M, Rahimi Zarchi A and Vakili Zarch A: Application of stem
cell/growth factor system, as a multimodal therapy approach in
regenerative medicine to improve cell therapy yields. Int J
Cardiol. 173:12–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Westin JR: Status of PI3K/Akt/mTOR pathway
inhibitors in lymphoma. Clin Lymphoma Myeloma Leuk. 14:335–342.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Yao Y, Xiao F, Lan X, Yu C, Zhang
Y, Jiang C, Yang J, Pei G, Li Y, et al: Administration of
dexamethasone protects mice against ischemia/reperfusion induced
renal injury by suppressing PI3K/AKT signaling. Int J Clin Exp
Pathol. 6:2366–2375. 2013.PubMed/NCBI
|
|
9
|
Kim HJ, Joe Y, Kong JS, Jeong SO, Cho GJ,
Ryter SW and Chung HT: Carbon monoxide protects against hepatic
ischemia/reperfusion injury via ROS-dependent Akt signaling and
inhibition of glycogen synthase kinase 3β. Oxid Med Cell Longev.
2013:306–421. 2013. View Article : Google Scholar
|
|
10
|
Hofmann BT and Jucker M: Activation of
PI3K/Akt signaling by n-terminal SH2 domain mutants of the p85α
regulatory subunit of PI3K is enhanced by deletion of its
c-terminal SH2 domain. Cell Signal. 24:1950–1954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tie G, Yan J, Yang Y, Park BD, Messina JA,
Raffai RL, Nowicki PT and Messina LM: Oxidized low-density
lipoprotein induces apoptosis in endothelial progenitor cells by
inactivating the phosphoinositide 3-kinase/Akt pathway. J Vasc Res.
47:519–530. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang F, Yang Y, Ma LL, Tian XJ and He YQ:
Berberine ameliorates renal interstitial fibrosis induced by
unilateral ureteral obstruction in rats. Nephrology (Carlton).
19:542–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui G, Qin X, Zhang Y, Gong Z, Ge B and
Zang YQ: Berberine differentially modulates the activities of ERK,
p38 MAPK and JNK to suppress Th17 and Th1 T cell differentiation in
type 1 diabetic mice. J Biol Chem. 284:28420–28429. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu L, Li N, Yu W, Gong J, Li Q, Zhu W and
Li J: Berberine reduces rat intestinal tight junction injury
induced by ischemia-reperfusion associated with the suppression of
inducible nitric oxide synthesis. Am J Chin Med. 41:1297–1312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang W, Zhang M, Li J, Meng Z, Xiao D,
Wei S, Chen L, Wang C and Hatch GM: Berberine attenuates
ischemia-reperfusion injury via regulation of
adenosine-5′-monophosphate kinase activity in both non-ischemic and
ischemic areas of the rat heart. Cardiovasc Drugs Ther. 26:467–478.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Curtis MJ and Walker MJ: Quantification of
arrhythmias using scoring systems: An examination of seven scores
in an in vivo model of regional myocardial ischaemia. Cardiovasc
Res. 22:656–665. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ravingerova T, Tribulova N, Slezak J and
Curtis MJ: Brief, intermediate and prolonged ischemia in the
isolated crystalloid perfused rat heart: Relationship between
susceptibility to arrhythmias and degree of ultrastructural injury.
J Mol Cell Cardio. 27:1937–1951. 1995. View Article : Google Scholar
|
|
18
|
Jia Y, Mo SJ, Feng QQ, Zhan ML, OuYang LS,
Chen JC, Ma YX, Wu JJ and Lei WL: EPO-dependent activation of
PI3K/Akt/FoxO3a signalling mediates neuroprotection in in vitro and
in vivo models of Parkinson's disease. J Mol Neurosci. 53:117–124.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Lv JW and Yang P: Loss of dicer
exacerbates cyclophosphamide-induced bladder overactivity by
enhancing purinergic signaling. Am J Pathol. 181:937–946. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun X, Zhong J, Wang D, Xu J, Su H, An C,
Zhu H and Yan J: Increasing glutamate promotes
ischemia-reperfusion-induced ventricular arrhythmias in rats in
vivo. Pharmacology. 93:4–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Briest F and Grabowski P:
PI3K-AKT-mTOR-signaling and beyond: The complex network in
gastroenteropancreatic neuroendocrine neoplasms. Theranostics.
4:336–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shrimali D, Shanmugam MK, Kumar AP, Zhang
J, Tan BK, Ahn KS and Sethi G: Targeted abrogation of diverse
signal transduction cascades by emodin for the treatment of
inflammatory disorders and cancer. Cancer Lett. 341:139–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin X, Zheng Y, Zhai X, Zhao X and Cai L:
Diabetic inhibition of preconditioning- and
postconditioning-mediated myocardial protection against
ischemia/reperfusion injury. Exp Diabetes Res. 2012:1980482012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong LY, Li S, Zhen YL, Wang YN, Shao X
and Luo ZG: Cardioprotection of vitexin on myocardial
ischemia/reperfusion injury in rat via regulating inflammatory
cytokines and MAPK pathway. Am J Chin Med. 41:1251–1266. 2013.
View Article : Google Scholar : PubMed/NCBI
|