Introduction
Acute aortic dissection (AAD) is a typical
characteristic of acute aortic syndrome, which was once a
potentially high-lethal clinical situation (1–3).
Currently, AAD is detectable within the early stages via minimally
invasive, cross-sectional imaging techniques, including multi-slice
computed tomography angiography (MSCTA) (4,5),
magnetic resonance angiography and transesophageal echocardiography
(6). In particular, the sensitivity
of MSCTA for the diagnosis of aortic dissection has been reported
to range between 83 and 100%, and the specificity is reported to be
100% (4,7). With the prevalent utilization of MSCTA
and advancements in image analysis software, patients with
suspicious symptoms may be rapidly examined to confirm or eliminate
the diagnosis of AAD. Notably, MSCTA may be used to evaluate the
original location and range of the intimal tearing and the size of
the false lumen and branch-vessel involvement, which are all
associated with the patient's management and prognosis.
Furthermore, AAD is currently a treatable disease due to recent
advances in medical and surgical therapeutic approaches, such as
endovascular aortic repair (7,8).
The variability in disease presentation may obscure
the diagnosis in certain cases (2,3), and
imaging modalities such as MSCTA remain prohibitive due to cost and
availability. Therefore, further research is required to develop a
rapid, inexpensive and accurate screening method for AAD. Limited
progress in the biochemical diagnosis of AAD has been made in the
last decade (9–11). Increases in a number of acute-phase
proteins and coagulation parameters have been identified in AAD
patients. However, the majority of these biomarkers are not
specific to AAD, as they may be aberrantly expressed in other
diseases (9,10).
Lumican is a member of the small leucine-rich
proteoglycan family that regulates the assembly and diameter of
collagen fibers in the extracellular matrix of various tissues
(12). Lumican expression correlates
with a number of pathological conditions, including skin fragility,
corneal opacification, and corneal and cardiac wound healing
(13). Lumican is overexpressed in
various tumor cell types (14), and
its expression correlates with the growth and metastasis of various
malignancies (15). To the best of
our knowledge, our team demonstrated for the first time that
lumican correlates with AAD (11).
In the present study, serum lumican levels were
compared with MSCTA manifestations to assess the association
between those two factors in AAD patients. In addition, a
semi-quantitative analysis was employed to evaluate the severity of
AAD in patients, based on MSCTA results.
Patients and methods
Patient population
Patients with a sharp and unbearable
recurrent/constant chest and/or back pain with a duration of at
least 5 min each time were recruited. A total of 70 patients with
AAD and 12 patients with aortic intramural hematoma (IMH) were
selected in a consecutive manner at the Zhongshan Hospital Fudan
University (Shanghai, China), and were matched with 30 healthy
volunteers. Patients with trauma, syphilis, a creatinine level of
>355 µmol/l, hemoglobin level of <60 g/l or systolic blood
pressure of <90 mmHg prior to treatment were excluded. The
diagnosis of AAD or IMH was confirmed using MSCTA. In cases with
AAD, an intimal flap showing a tear of the intimal layer was
observed. Unlike the false lumen in AAD cases, the crescent-shaped
area observed in IMH patients was not enhanced subsequent to
contrast agent administration, and no intimal tear was observed on
contrast-enhanced CT scans (16).
All study subjects provided their informed consent for
participation in the study. The study was approved by the Ethics
Committee of Zhongshan Hospital Fudan University. For each study
subject, whole blood samples were immediately collected in BD
Vacutainer SST tubes (BD Diagnostics, Plymouth, UK) following
admission and were centrifuged at 2,770 × g for 10 min at room
temperature. The serum was frozen and stored in aliquots at −80°C
until required for analysis.
The Stanford system was used to describe the aortic
dissection in the patients (17). If
the dissection involved the ascending aorta and/or aortic arch,
then patients were categorized as Stanford Type A. The type A
patients generally require surgical treatment, such as the Bentall
procedure which is performed to prevent the aorta from rupturing
and causing a fatal hemorrhage. This surgical procedure involves
replacing certain defective parts of the aorta, such as the valve
or the upper part (also known as the ascending aorta), with a
graft.
Enzyme-linked immunosorbent assay
(ELISA) for measurement of lumican in serum and aortic
sections
Serum levels of lumican were measured using ELISA
kits from Cusabio Biotech Co., Ltd. (Wuhan, China; cat. no.
CSB-E09797h). Briefly, each sample (100 µl) was added to a 96-well
microplate and incubated for 2 h at 37°C. The liquid was then
removed, and the biotin-antibody working solution (100 µl) was
added to each well and incubated for 1 h at 37°C, after which the
liquid was aspirated, and the wells were washed with 200 µl wash
buffer three times. Horseradish peroxidase-avidin working solution
(100 µl) was added to each well and incubated for 1 h at 37°C.
Subsequent to five washes, 90 µl 3,3′,5,5′-tetramethylbenzidine was
added to each well and incubated for 30 min at 37°C. The enzymatic
reaction was terminated by adding the stop solution. Thereafter,
the absorbance was measured at 450 nm using a Bio-Rad model 680
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Aortic sections
To confirm the localization of the lumican protein
in the human aortic sections, immunohistochemical staining and
western blot analysis were performed. The aortic sections were
obtained from 7 patients that had previously undergone the Bentall
procedure and from 2 heart transplant donors, after obtaining
patient consent.
Immunohistochemical staining
Immunohistochemical assay was performed according to
the protocol of the Vectastain ABC kit (Vector Laboratories,
Burlingame, CA, USA). Briefly, aortic tissue samples were fixed
with freshly-prepared 4% paraformaldehyde and then dehydrated. The
tissues were then embedded in molten paraffin and stored at room
temperature until ready for sectioning. A microtome (RM2235; Leica
Biosystems, Wetzlar, Germany) was used to cut the embedded tissue
into 5–20-µm sections, which were then floated in a 50°C water
bath. Next, the sections were mounted onto gelatin coated slides,
allowed dry overnight and stored at room temperature until ready
for staining. The sections were then deparaffinized, rehydrated and
washed twice for 10 min with 1% animal serum in phosphate-buffered
saline (PBS) with 0.4% Triton X-100 (PBS-T). Any non-specific
binding was blocked by incubating the tissue sections with 5% serum
in PBS-T for 30 min at room temperature, followed by incubation
with polyclonal rabbit anti-human lumican antibody (Novus
Biologicals, Littleton, CO, USA; cat. no. NBP1-87726; dilution,
1:200). Subsequently, the sections were incubated with ABC-HRP
reagent (part of the Vectastain ABC kit; formed by mixing avidin
with biotinylated horseradish peroxidase) for 1 h at room
temperature and then washed twice in PBS for 10 min each time. The
tissue sections were then counterstained with hematoxylin for 2 min
and dehydrated, and mounting media (neutral balsam; Sangon Biotech
Co. Ltd., Shanghai, China) was added.
Western blot analysis
For western blot analysis, the acute aortic
dissection samples were lysed with lysis buffer (Sigma-Aldrich, St.
Louis, MO, USA), total protein was extracted from the tissue
samples, and the protein concentrations were determined using a
protein assay kit (Bio-Rad Laboratories, Inc.). Next, 30 µg protein
was subjected to 10% SDS-PAGE and transferred to nitrocellulose
membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked
with 5% non-fat milk for 1.5 h. Subsequently, the samples were
incubated with polyclonal rabbit anti-human lumican antibody
(Abcam, Cambridge, UK; 1 µg/ml; cat. no. ab98067) for the detection
of lumican, followed by horseradish peroxidase-conjugated secondary
antibodies (Thermo Fisher Scientific, Inc., Waltham, MA, USA; cat.
no. A16104SAMPLE; dilution, 1:1,000). After incubation with
secondary antibody, the proteins were detected with enhanced
chemiluminescence reagents (Pierce Biotechnology, Rockford, IL,
USA).
MSCTA data acquisition and imaging
analysis
All patients and healthy volunteers underwent MSCTA
imaging. Whole blood samples were collected immediately after
admission; however, due to practical difficulties, MSCTA imaging
was performed at a later time (<3 h after admission). All CT
examinations were performed using a Somatom Definition AS MSCT
system (Siemens Medical Solutions, Forchheim, Germany) using a
standard clinical protocol. Scanning parameters were as follows:
Voltage, 140 kV; effective mAs value per rotation, 400 mAs; gantry
rotation time, 0.33-msec; collimation with z-flying focal spot for
each detector, 128×0.6 mm. The pitch varied between 0.2 and 0.43.
For dose reduction, an automatic tube current modulation algorithm
was used. A standard volume of 85 ml contrast material (Isovue 370;
Bracco Diagnostics, Inc., Seattle, WA, USA) was administered at 3
ml/sec followed by a 50-ml saline flush. The automatic detection of
peak enhancement in the descending aorta with a detection threshold
of 120 Hounsfield units was used, while the helical scan was
initiated automatically after the bolus detection. CT data sets
were reconstructed at a slice thickness of 1 mm.
Reconstructed images were transferred to a remote
workstation and a cardiac function analysis software package
(GE/Siemens Workstation: ADW 4.2, General Electric, Schenectady,
NY, USA; Syngo MMWP VE36A, Siemens Healthcare, Erlangen, Germany)
was used. The CT data sets were analyzed in a consensus reading by
two experienced cardiovascular radiologists.
Data evaluation methods
Due to the complexity and inaccuracy of direct
measurements, the vertical range of the false lumen was measured
and we determined whether vital vessels were affected to estimate
its surface area and the severity of AAD. Thus, a ‘SCORE X, RANGE
Y’ system was designed to measure the number of groups of affected
vital arteries and the vertical range of the false lumen. This
system was designed by our group, based on the fact that the extent
of the dissection and branch-vessel involvement may increase the
morbidity and mortality (18).
SCORE grading depended on the number of affected
vital vessels. The different groups of affected vital vessels were
as follows: Group 1, including the brachiocephalic trunk, left
common carotid artery and left subclavian artery; group 2,
including the celiac trunk and superior mesenteric artery; and
group 3, including the bilateral renal artery and bilateral iliac
artery. If the affected vessel fell within one of the groups, the
SCORE was marked as ‘1’. If the affected vessel fell within two of
the groups, the SCORE was marked as ‘2’. If no vital vessels were
affected, the SCORE was marked as ‘0’. The maximal and minimal
SCORE were 3 and 0, respectively.
RANGE grading depended on the number of aortic
segments that were affected by the false lumen. The aorta was
divided into the following segments: i) Ascending to arch; ii) arch
to celiac trunk level; iii) celiac trunk level to the level of the
renal arteries; and iv) level of the renal arteries to the iliac
arteries. If one segment was affected, RANGE was marked as ‘1’. If
two segments were affected, RANGE was marked as ‘2’, If three
segments were affected, RANGE was marked as ‘3’. If all segments
were affected, RANGE was marked as ‘4’.
Statistical analysis
Data were collected and inputted in Excel (Microsoft
Corporation, Redmond, WA, USA). All statistical analyses were
performed using SPSS software, version 17.0 (SPSS, Inc., Chicago,
IL, USA) and GraphPad Prism 5 software (GraphPad Software, Inc.,
San Diego, CA, USA), and the results were presented as mean ±
standard deviation. A comparative analysis of multiple groups was
performed with a one-way analysis of variance or
Mann-Whitney/Kruskal-Wallis test. Spearman's correlation
coefficients were used to quantify the correlations. Bivariate
correlation analyses were used to present the results, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical features
In total, 70 patients with AAD, 12 patients with IMH
and 30 healthy volunteers were enrolled in the present study. The
clinical features of all subjects are summarized in Table I. No statistically significant
differences in age distribution, gender composition and past
medical history (hypertension and Marfan syndrome, which is a
genetic disorder of connective tissue resulting in defects of the
heart valves and aorta, and occasionally aortic dissection) were
observed among the three groups (P=0.148, 0.275, 0.620 and 0.252,
respectively). Furthermore, there was no differences in the time
from onset to admission between the AAD and IMH groups (P=0.331).
However, systolic blood pressure was increased in the AAD and IMH
patients compared with the healthy volunteers (P=0.002).
 | Table I.Clinical features of all subjects. |
Table I.
Clinical features of all subjects.
| Clinical feature | AAD patients | IMH patients | Healthy
volunteers | P-value |
|---|
| Patients, n | 70 | 12 | 30 | – |
| Age, years | 53.99±15.37 | 59.17±8.17 | 59.50±12.65 | 0.148a |
| Male gender, n
(%) | 49 (70.00) | 8 (66.67) | 16 (53.33) | 0.275b |
| Hypertension, n
(%) | 36 (51.42) | 10 (83.33) | 19 (63.33) | 0.620b |
| Marfan syndrome, n
(%) | 7 (10.00) | 0 (0.00) | 0 (0.00) | 0.252b |
| Time from onset to
admission, h | 55.55±128.25 | 41.08±65.03 | – | 0.444c |
| Systolic blood
pressure, mmHg |
150.44±28.88d |
163.50±34.96d | 133.50±19.42 | 0.002a |
| Diastolic blood
pressure, mmHg | 75.93±19.35 | 86.17±10.71 | 77.07±13.34 | 0.167a |
| In-hospital
mortality, n (%) | 8 (11.42) | 0 (0.00) | – | 0.218b |
Serum levels of lumican
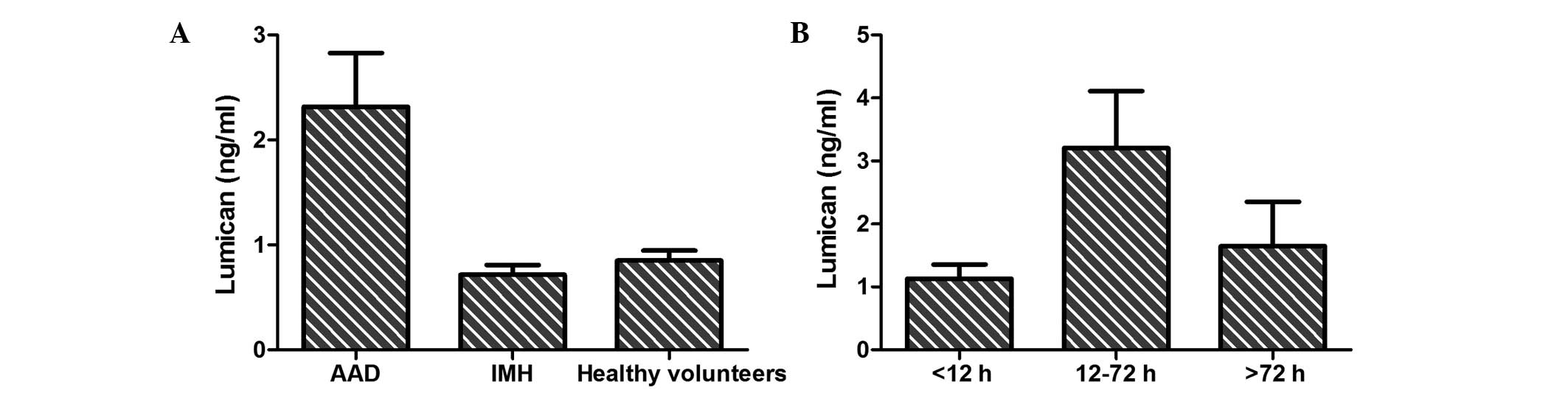
As shown in Fig. 1A,
a statistically significant difference in serum lumican levels was
detected among AAD patients (2.32±4.29 ng/ml), IMH patients
(0.72±0.32 ng/ml) and healthy volunteers (0.85±0.53 ng/ml;
P=0.003). Furthermore, the association between the level of serum
lumican and the time from symptom onset (Fig. 1B) in AAD patients was assessed, and a
significant difference was observed between patients presenting at
the hospital within 12–72 h (3.21±5.57 ng/ml) and those presenting
within <12 h (1.13±1.10 ng/ml) or >72 h (1.65±1.99 ng/ml;
P=0.005) from the symptom onset.
Correlation of lumican levels with
SCORE and RANGE
In total, 8 patients presented at >72 h,
including 4 patients with Stanford Type A disease, of which 1
patient underwent the Bentall procedure, and 4 patients with
Stanford Type B disease, of which 1 patient received endovascular
graft exclusion treatment. Among the 24 patients that presented at
<12 h, 7/14 Type A and 7/10 Type B patients were treated
surgically. As the serum lumican levels are significantly
associated with the time from symptom onset, further analysis was
only performed in 38 patients that presented within 12–72 h after
an episode of chest/back pain (of these, 9/25 Type A and 8/13 Type
B patients were treated surgically). In total, 9 subjects were
excluded as their diagnosis was not confirmed by MSCTA or MSCTA
data acquisition was obtained at >3 h following admission. Among
the remaining 29 AAD patients, 7/16 Stanford Type A patients had
undergone the Bentall procedure, and 8/13 Stanford Type B patients
had received endovascular graft exclusion treatment.
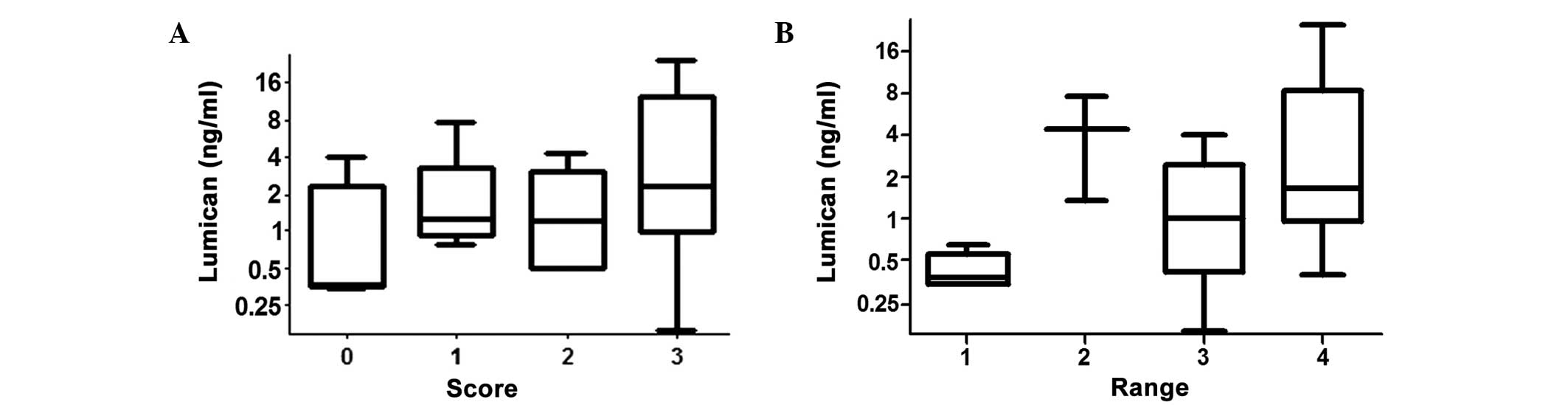
As shown in Fig. 2A,
the lumican levels in patients with SCORE values of 0, 1, 2 and 3
were 1.15±1.61, 2.31±2.61, 1.64±1.57 and 6.63±8.43 ng/ml,
respectively. The Spearman's rho correlation coefficient between
lumican levels and SCORE was 0.373 (P=0.046), which indicated that
the level of lumican was correlated with the number of affected
vital vessels. In patients with RANGE values of 1, 2, 3 and 4
(Fig. 2B), the serum lumican levels
were 0.43±0.14, 4.46±4.41, 1.45±1.40 and 5.57±7.58 ng/ml,
respectively. In addition, the correlation coefficient between
lumican levels and RANGE was 0.468 (P=0.010), which indicated that
lumican levels were correlated with the range of the false
lumen.
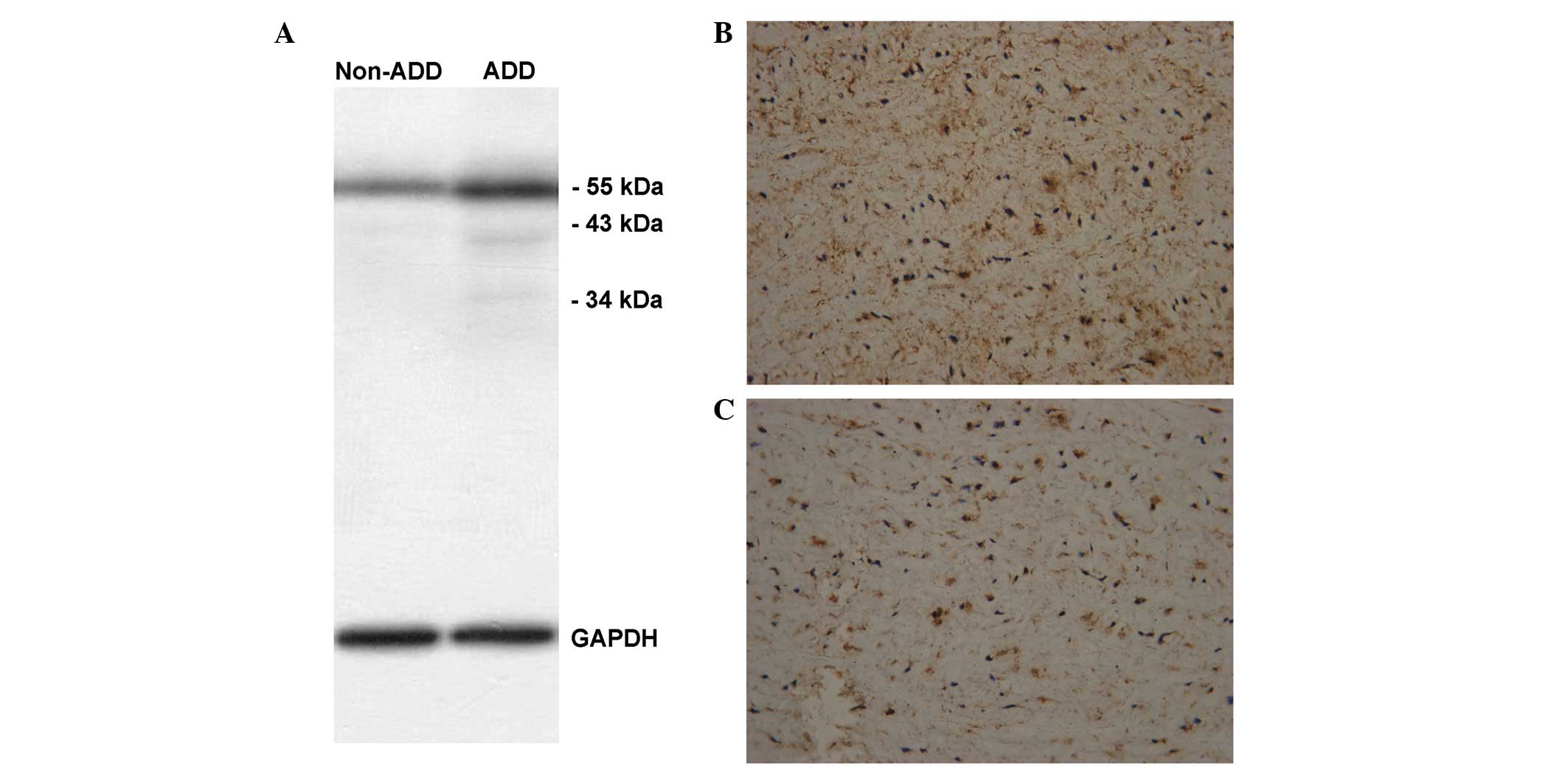
Immunohistochemical staining and
western blot analysis
Among the 29 eligible AAD patients presenting within
12–72 h after onset, 7 Standford Type A patients had received the
Bentall procedure. Immunohistochemical staining and western blot
analysis of lumican were performed in the aortic sections from the
7 patients that had previously undergone the Bentall procedure and
from 2 heart transplant donors. Western blot analysis (Fig. 3A) and immunohistochemical staining
(Fig. 3B and C) were performed to
detect the expression of lumican in the aortic sections of AAD
patients and donors.
Typical case
Images demonstrating the expression of lumican from
the aortic sections of a 37-year-old male patient are shown in
Fig. 3B. This patient had no history
of hypertension or family history of aortic dissection, and
presented with sharp chest pain for 72 h, following which he
underwent a Bentall procedure and survived. The evaluation for this
patient was SCORE 3 and RANGE 4, and the serum lumican level was
12.54 ng/ml. Fig. 3B shows the
expression of lumican (brown) in the aortic medial layer
(magnification, ×200). The expression of lumican in the aortic
medial layer of a 24-year old male heart transplant donor is shown
in Fig. 3C (magnification, ×200).
Although lumican was expressed in the aortic medial layer of AAD
patients and healthy donors, the serum levels of lumican were
elevated in AAD patients, but not in the healthy donors, which may
reflect our hypothesis that the medial layer released more lumican
into the circulation via the blood flow from the intimal tear.
Discussion
Although AAD is uncommon, it may rapidly evolve into
a serious cardiovascular condition if not detected rapidly. Thus, a
specific biomarker test or array that can be used as a diagnostic
tool to reliably include or exclude AAD is required. As AAD affects
the aortic medial layer, the search for biomarkers has focused on
the markers that are associated with the vascular smooth muscle
(myosin), vascular interstitium (calponin), elastic laminae
(soluble elastin fragments) of the aorta, or secondary phenomena
due to the exposure of blood to nonintimal vascular surfaces
(D-dimers) (19). In 2004, a study
demonstrated that extracellular matrix components of the vessel
walls, such as elastin, may be elevated in aortic dissections
(10); however, these are
nonspecific biomarkers for AAD (9).
Lumican is distributed in interstitial collagenous
matrices (ICMs) throughout the body. In coronary artery ischemic
lesions, lumican is overexpressed by vascular smooth muscle cells
(20) and is synthesized in aortic
smooth muscle cells (21). The
results of our previous proteomic study indicated that lumican may
be a potentially specific marker for AAD (13). In the present study, the results
demonstrated that the level of lumican in patients with AAD was
significant higher compared with that in patients with IMH and
healthy volunteers, and the levels were 2.32±4.29, 0.72±0.32 and
0.85±0.53 ng/ml, respectively (P=0.003).
Furthermore, in the present study, the expression of
lumican was detected in the aortic sections of healthy donors and
AAD patients, and the serum level of lumican did not significantly
differ between IMH patients without an intimal tear and healthy
volunteers. These results suggest that an increased number of ICMs
are exposed to the circulation in cases featuring an intimal tear,
which may result in a false lumen. Due to the exposure of ICMs to
the circulation, lumican is released into the circulation, thus
allowing for it to be identified by laboratory tests.
Theoretically, the level of serum lumican is
correlated with the surface area of exposed ICMs. Therefore, we
hypothesize that the level of serum lumican may be used to predict
the size and range of lesions, in addition to the severity of AAD
in afflicted patients.
Currently, >70% of AAD patients are diagnosed
following evaluation using MSCTA, since these patients show typical
signs of aortic dissection, including false lumen and the intima
tear (16). Notably, these signs may
additionally indicate other pathological entities, such as IMH. As
the extent of the dissection and branch-vessel involvement may
increase morbidity and mortality (18), the current study evaluated the extent
of the dissection and the branch-vessel involvement. Lumican levels
were elevated in AAD patients, but not in IMH patients, which may
reflect the pathological process by which the blood enters through
the intimal tear, thereby releasing lumican into the circulation.
Furthermore, the level of lumican in the serum was correlated with
the SCORE (r=0.373, P=0.046) or RANGE (r=0.468, P=0.010) values,
thus indicating that patients with higher levels of lumican exhibit
increased disruption of the aortic media layer and more marked
branch-vessel involvement.
As an initial step, the results of the current study
indicated that lumican may be a potential novel serum biomarker for
AAD. However, the ultimate development of biomarkers that provide
sufficient sensitivity or specificity for the diagnosis of AAD may
require multiple validations and clinical studies. The current
study is merely a perspective study due to the limited number of
samples, which is the primary limitation of the study. Due to the
insufficient recruitment of patients, any association between
lumican and the quantity of intra-false lumen thrombus formation
cannot be confirmed, while the diagnostic value is also uncertain.
An expanded patient population is required in order to confirm the
present results and conduct further investigations. However, the
present study provided preliminary indications of the diagnostic
capacity of lumican, which require validation.
In conclusion, the present results demonstrated that
serum lumican levels were higher in AAD patients compared with
those in IMH patients or healthy volunteers. Therefore, lumican may
be a useful marker for the diagnosis of and screening for AAD. In
addition, the level of lumican in the serum may be a potential
predictor of the severity of AAD, as it was associated with the
lesion range, as confirmed by MSCTA.
Acknowledgements
This study was supported by a grant from the
Shanghai Committee of Science and Technology (no. 114119A9000).
References
|
1
|
Mészáros I, Mórocz J, Szlávi J, Schmidt J,
Tornóci L, Nagy L and Szép L: Epidemiology and clinicopathology of
aortic dissection. Chest. 117:1271–1278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hagan PG, Nienaber CA, Isselbacher EM,
Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R,
Suzuki T, Oh JK, et al: The international registry of acute aortic
dissection (IRAD): New insights into an old disease. JAMA.
283:897–903. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elefteriades JA, Barrett PW and Kopf GS:
Litigation in nontraumatic aortic diseases-a tempest in the
malpractice maelstrom. Cardiology. 109:263–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayter RG, Rhea JT, Small A, Tafazoli FS
and Novelline RA: Suspected aortic dissection and other aortic
disorders: Multi-detector row CT in 373 cases in the emergency
setting. Radiology. 238:841–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stein PD, Fowler SE, Goodman LR,
Gottschalk A, Hales CA, Hull RD, Leeper KV Jr, Popovich J Jr, Quinn
DA, Sos TA, et al: Multidetector computed tomography for acute
pulmonary embolism. N Engl J Med. 354:2317–2327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Macura KJ, Szarf G, Fishman EK and Bluemke
DA: Role of computed tomography and magnetic resonance imaging in
assessment of acute aortic syndromes. Semin Ultrasound CT MR.
24:232–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iezzi R and Cotroneo AR: Endovascular
repair of abdominal aortic aneurysms: CTA evaluation of
contraindications. Abdom Imaging. 31:722–731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rousseau H, Chabbert V, Maracher MA, El
Aassar O, Auriol J, Massabuau P and Moreno R: The importance of
imaging assessment before endovascular repair of thoracic aorta.
Eur J Vasc Endovasc Surg. 38:408–421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki T, Distante A and Eagle K:
Biomarker-assisted diagnosis of acute aortic dissection: How far we
have come and what to expect. Curr Opin Cardiol. 25:541–545. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shinohara T, Suzuki K, Okada M, Shigai M,
Shimizu M, Maehara T and Ohsuzu F: Soluble elastin fragments in
serum are elevated in acute aortic dissection. J Cardiol. 43:96–97.
2004.(In Japanese). PubMed/NCBI
|
|
11
|
Gu G, Cheng W, Yao C, Yin J, Tong C, Rao
A, Yen L, Ku M and Rao J: Quantitative proteomics analysis by
isobaric tags for relative and absolute quantitation identified
lumican as a potential marker for acute aortic dissection. J Biomed
Biotechnol. 2011:9207632011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takayama R, Ishiwata T, Ansai S, Yamamoto
T, Matsuda Y, Naito Z and Kawana S: Lumican as a novel marker for
differential diagnosis of Bowen disease and actinic keratosis. Am J
Dermatopathol. 35:827–832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Engebretsen KV, Lunde IG, Strand ME,
Waehre A, Sjaastad I, Marstein HS, Skrbic B, Dahl CP, Askevold ET,
Christensen G, et al: Lumican is increased in experimental and
clinical heart failure and its production by cardiac fibroblasts is
induced by mechanical and proinflammatory stimuli. FEBS J.
280:2382–2398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang ZX, Lu CY, Yang YL, Dou KF and Tao
KS: Lumican expression in pancreatic ductal adenocarcinoma.
Hepatogastroenterology. 60:349–353. 2013.PubMed/NCBI
|
|
15
|
Nikitovic D, Papoutsidakis A, Karamanos NK
and Tzanakakis GN: Lumican affects tumor cell functions, tumor-ECM
interactions, angiogenesis and inflammatory response. Matrix Biol.
35:206–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Erbel R, Alfonso F, Boileau C, Dirsch O,
Eber B, Haverich A, Rakowski H, Struyven J, Radegran K, Sechtem U,
et al: Diagnosis and management of aortic dissection. Eur Heart J.
22:1642–1681. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sheikh AS, Ali K and Mazhar S: Acute
aortic syndrome. Circulation. 128:1122–1127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Castañer E, Andreu M, Gallardo X, Mata JM,
Cabezuelo MA and Pallardó Y: CT in nontraumatic acute thoracic
aortic disease: Typical and atypical features and complications.
Radiographics. 23:S93–S110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nazerian P, Morello F, Vanni S, Bono A,
Castelli M, Forno D, Gigli C, Soardo F, Carbone F, Lupia E and
Grifoni S: Combined use of aortic dissection detection risk score
and D-dimer in the diagnostic workup of suspected acute aortic
dissection. Int J Cardiol. 175:78–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin H, Ishiwata T and Asano G: Effects of
the extracellular matrix on lumican expression in rat aortic smooth
muscle cells in vitro. J Pathol. 195:604–608. 2011. View Article : Google Scholar
|
|
21
|
Naito Z: Role of small leucine-rich
proteoglycan (SLRP) family in pathological lesions and cancer cell
growth. J Nippon Med Sch. 72:137–145. 2005. View Article : Google Scholar : PubMed/NCBI
|