Introduction
Patent ductus arteriosus (PDA) is a congenital heart
condition characterized by persistent patency between the proximal
left pulmonary artery and the descending aorta, which leads to
left-to-right shunting from the aorta to the pulmonary artery, and
a subsequent increase of left ventricular (LV) preload (1,2). Prior
to the commercialization of echocardiography, the incidence of PDA
was demonstrated to be approximately 1 in 2,000 births, with the
exception of complex defects with associated PDA (3,4).
Recently however, the incidence of PDA may be as high as 1 in 500
births as asymptomatic PDA is frequently identified by
echocardiography (2,3). PDA presents with a broad spectrum of
clinical manifestations ranging from asymptomatic cardiac murmur to
severe complications, including congestive heart failure or
Eisenmenger syndrome, which are predominantly associated with the
amount of left-to-right shunting and the size of PDA (3,5).
Treatment of the majority of PDAs is accomplished by surgical or
catheter closure with a complete closure rate of 90–95% (3,6–9). PDA closure is required in patients with
signs of LV volume overload, pulmonary arterial hypertension or
symptoms of heart failure (3).
Transient LV dysfunction following PDA closure has previously been
reported, although severe complications are rare (7–10). The
present study reported the case of an adult patient with PDA and
bicuspid aortic valve (AV) who experienced transient severe LV
dysfunction following percutaneous closure of PDA.
Case report
A 60-year-old woman underwent transthoracic
echocardiography at the Kyung Hee University Hospital at Gangdong
(Seoul, Korea) in October 2010 due to incidental systolic cardiac
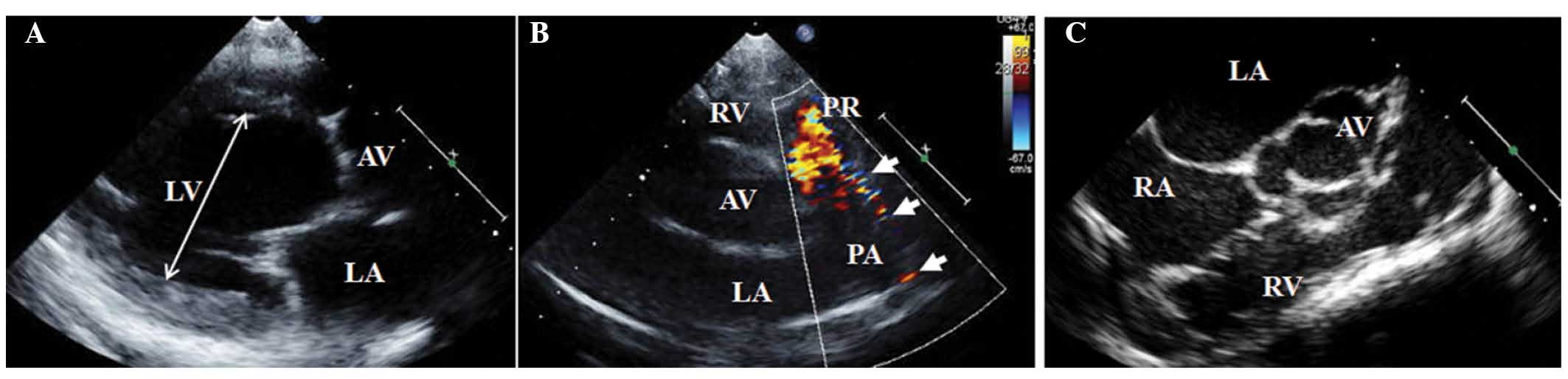
murmur. The patient was diagnosed with a PDA with LV dilatation
(Fig. 1A and B) and mild pulmonary
hypertension based on the following observations: (LV end-diastolic
dimension/body surface area) : (LV end-systolic dimension/body
surface area), 5.0:3.3 cm/m2 (reference value, ≤3.1:2.1
cm/m2); ejection fraction (EF), 60% (reference value,
≥55%); estimated systolic pulmonary arterial pressure (sPAP), 55
mmHg (reference value, <35 mmHg). Echocardiography also detected
bicuspid AV (Fig. 1C) without
significant flow obstruction [maximal velocity on AV, 2.2 m/s
(reference value <2 m/s); mean pressure gradient on AV, 10 mmHg
(reference value <20 mmHg)]. A normal sinus rhythm was
demonstrated via an electrocardiogram (ECG). The patient refused to
undergo PDA closure and was followed-up at an outpatient
clinic.
The patient was hospitalized two years later on
October 2012 with diarrhea and dyspnea, and atrial fibrillation was
detected following an ECG. Echocardiography demonstrated moderate
pulmonary hypertension (sPAP, 77 mmHg) without LV dysfunction (EF,
62%). Following electric cardioversion, the ECG was normalized to a
sinus rhythm without T wave inversion. The patient completely
recovered following fluid therapy for acute gastritis. The patient
agreed to a transcatheter procedure for PDA closure prior to
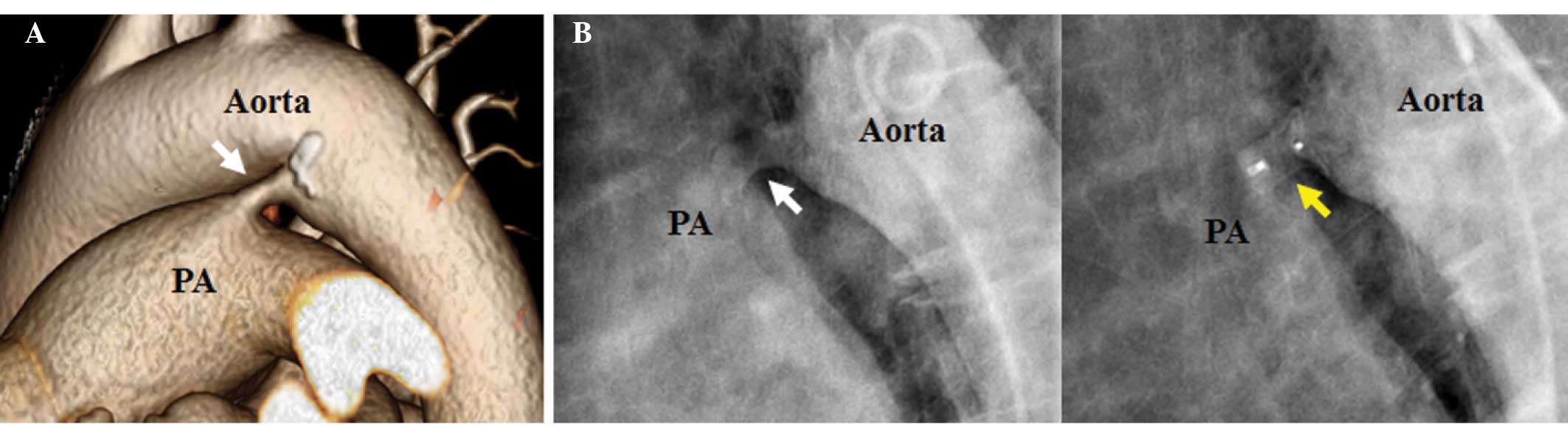
discharge. Thoracic aortic computed tomography revealed that the
PDA width was 10×6 mm and length was 7 mm (Fig. 2A). Coronary arteries were normal, as
detected using angiography. Subsequent cardiac catheterization
demonstrated systolic, diastolic and mean PAP of 52, 29 and 40
mmHg, respectively (reference value, ≤30, 12, and 19 mmHg,
respectively), and a Qp/Qs ratio of 3.9 (reference value, 1:1).
Following aortography, an Amplatzer duct occluder (AGA Medical
Corp., Golden Valley, MN, USA) was successfully implanted (Fig. 2B). The following day, the patient
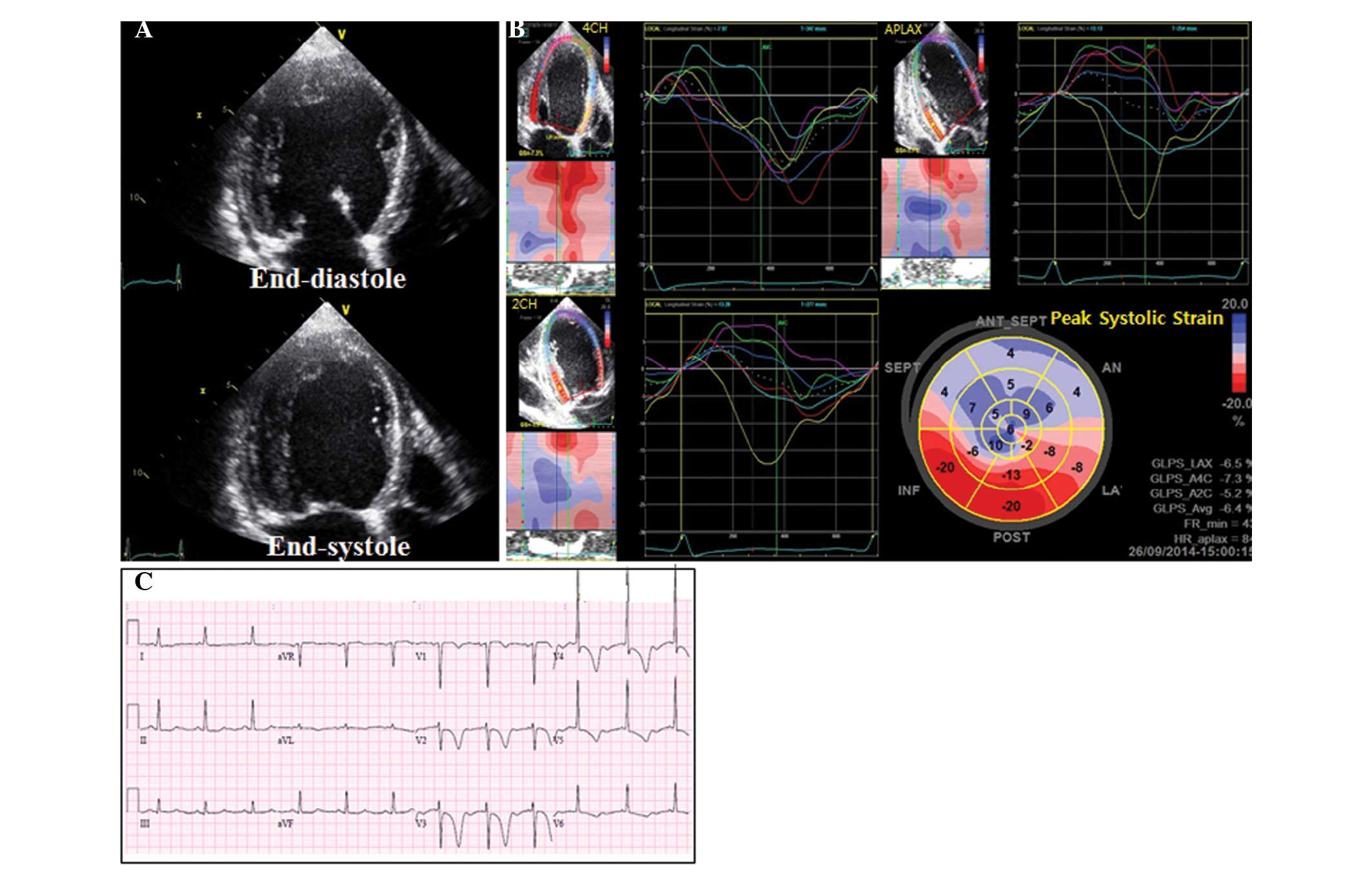
complained of mild dyspnea. Conventional echocardiography
demonstrated global LV hypokinesia with decreased LV systolic
function (EF, 32%; Fig. 3A).
Abnormal shunt flow was not detected and the occluder was
well-positioned, as detected by echocardiography. Speckle-tracking
echocardiography demonstrated dyskinetic movement in the septal and
anterior segments with low global longitudinal peak systolic strain
(GLS) at −6.4% (Fig. 3B). The brain
natriuretic peptide measured using a Triage BNP assay (Alere, Inc.,
San Diego, CA, USA) and a Beckman Coulter DXI 800 analyzer (Beckman
Coulter, Inc., Brea, CA, USA) was found to be 821 pg/ml (reference
value, <100 pg/ml) and creatine-kinase MB and cardiac troponin I
measured using a Beckman Coulter DC-800 analyzer (Beckman Coulter,
Inc.) were within the reference range. An ECG revealed new deep
T-wave inversions in the precordial leads V2-V6 (Fig. 3C). Subsequently, the patient received
medical treatment, including angiotensin-converting enzyme
inhibitor perindopril (4mg acertil®; Servier Inc.,
Neuilly-sur-Seine, France) and was then discharged one day
later.
Echocardiography performed 3 weeks after PDA closure
demonstrated improved LV function (EF, 60%; GLS, −15%; Fig. 4A and B). ST-segments and T-wave
abnormalities appeared to be completely resolved after 9 months, as
detected by an ECG (Fig. 4C).
The patient interrupted perindopril medication one
year following the procedure as her LV function was preserved and
her blood pressure was low (systolic and diastolic blood pressure,
95 and 55 mmHg). At the three-year follow up following the
procedure (October 2015), echocardiography revealed that the
patient's LV systolic function (EF, 66%) was normal, and LV chamber
size (LV end-systolic dimension/body surface area, 4.1: 2.7
cm/m2) and pulmonary hypertension (sPAP, 38 mmHg) were
decreased. At a follow up on December 2015 the patient showed
normal sinus rhythm following an ECG.
Discussion
Abrupt interruption of pulmonary recirculation
following PDA closure induces a decrease in LV end-diastolic
volume. If decreased LV end-diastolic volume does not accompany a
decrease in LV end-systolic volume, the EF will decrease via
several possible mechanisms including decreased myocardial
contractility or increased afterload (3,10).
Previous studies have demonstrated that an overall transient
decrease of LV function following PDA closure occurs in 50–70% of
patients, whereas significant transient decrease of LV ejection
fraction of <55% or fractional shortening of <29% was
observed in 8–43% of patients (5–7). In
addition, transient LV dysfunction following PDA closure was found
to be associated with large PDAs (7–9), large
amount of shunting (9), presence of
high pulmonary hypertension (9) or
low EF (8–10) prior to PDA closure, and an increased
age of patients (8). Furthermore,
the presence of anomalies associated with PDA, such as mitral
regurgitation or aortic coarctation, has been reported to be a risk
factor that can provoke or deteriorate LV dysfunction following PDA
closure (8).
In the present case, the patient presented a large
PDA, a high Qp/Qs ratio and increased pulmonary hypertension. The
patient's prolonged LV volume overload is suggested to have caused
the LV remodeling into a spherical shape. Furthermore, combined
bicuspid AV anomaly may have contributed to the deterioration of LV
function, as it is a potential risk factor for increased
afterload.
Speckle-tracking echocardiography data gathered from
the present patient following PDA closure was consistent with a
previous study in infants, which observed a global reduction of
peak systolic strain following PDA closure, thus mimicking systolic
heart failure (11). However, the
dyskinetic movement in the septal and anterior segments accompanied
by abnormal LV geometry and severe LV systolic dysfunction in the
present case was not concordant with the results from a previous
study conducted on infants (11).
In conclusion, the combination of large PDA size,
large amount of shunting, LV remodeling and bicuspid aortic valve
may have induced severe deterioration of LV function following PDA
closure in the present case. Speckle-tracking echocardiography may
also be a useful tool in the detection of decreased LV myocardial
function and altered LV strain pattern following PDA closure.
References
|
1
|
Daniel B: Congenital heart disease: Patent
ductus arteriosus. Nelson Textbook of Pediatrics. Kliegman RM,
Stanton BF, St. Geme JW, Schor NF and Behrman RE: (19th).
(Philadelphia, PA). Elsevier Saunders. 1559–1560. 2011.
|
|
2
|
Lloyd TR and Beekman RH III: Clinically
silent patent ductus arteriosus. Am Heart J. 127:1664–1665. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schneider DJ and Moore JW: Patent ductus
arteriosus. Circulation. 114:1873–1882. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitchell SC, Korones SB and Berendes HW:
Congenital heart disease in 56,109 births: Incidence and natural
history. Circulation. 43:323–332. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schneider DJ: The patent ductus arteriosus
in term infants, children and adults. Semin Perinatol. 36:146–153.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rao PS and Kern MJ: Summary and comparison
of patent ductus arteriosus closure methods. Catheter Based devices
for the Treatment of Non-Coronary Cardiovascular Disease in Adults
and Children (Philadelphia, PA). Lippincott, Williams, and Wilkins.
219–228. 2003.
|
|
7
|
Galal MO, Amin M, Hussein A, Kouatli A,
Al-Ata J and Jamjoom A: Left ventricular dysfunction after closure
of large patent ductus arteriosus. Asian Cardiovasc Thorac Ann.
13:24–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tilahun B and Tefera E: Transient left
ventricular systolic dysfunction following surgical closure of
large patent ductus arteriosus among children and adolescents
operated at the cardiac centre, Ethiopia. J Cardiothorac Surg.
8:1392013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YH, Choi HJ, Cho Y, Lee SB and Hyun
MC: Transient left ventricular dysfunction after percutaneous
patent ductus arteriosus closure in children. Korean Circ J.
38:596–600. 2008. View Article : Google Scholar
|
|
10
|
Jeong YH, Yun TJ, Song JM, Park JJ, Seo
DM, Koh JK, Lee SW, Kim MJ, Kang DH and Song JK: Left ventricular
remodeling and change of systolic function after closure of patent
ductus arteriosus in adults: Device and surgical closure. Am Heart
J. 154:436–440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Khuffash AF, Jain A, Dragulescu A,
McNamara PJ and Mertens L: Acute changes in myocardial systolic
function in preterm infants undergoing patent ductus arteriosus
ligation: A tissue Doppler and myocardial deformation study. J Am
Soc Echocardiogr. 25:1058–1067. 2012. View Article : Google Scholar : PubMed/NCBI
|