Introduction
Kawasaki disease (KD) is a disease of unknown
etiology, identified primarily in children with systemic vasculitis
syndrome, that has become the leading cause of childhood acquired
heart disease (1). The main
pathological change of KD is systemic non-specific vasculitis,
possibly with severe cardiovascular complications. Coronary
arthritis can cause coronary artery dilatation, and coronary artery
stenosis, leading to myocardial ischemia and when severe leading to
myocardial infarction (2–5). KD pathogenesis is not clear, and is
associated with infection factors and superantigen-mediated immune
responses and the genetic susceptibility of children (6). Previous findings revealed that factors
such as nuclear factor-κB, nitric oxide, vascular endothelial
growth factor (VEGF), and matrix metalloproteinases are involved in
the occurrence and development of KD (7–10).
In the present study, intravenous bovine serum
albumin was used to replicate the experimental rabbit model of
vasculitis. VEGF was measured in the early, middle and recovery
stages of modeling. At the same time, the phosphatase and tensin
homolog (PTEN)/phosphoinositide 3-kinase (PI3K) signaling pathway
that regulates VEGF, was examined to determine the role of the
PTEN/PI3K/VEGF pathway in KD at different stages.
Materials and methods
Materials
Experimental animals
Twelve rabbits of 9–12 weeks old, were provided by
the Experimental Animal Department of China Medical University. The
rabbits were kept in an environment with a temperature of 26°C and
humidity of 70%. The animals were provided with food and water
ad libitum during the rearing period. Permission was
obtained from the Institutional Ethics Committee to conduct the
animal experiment.
Instruments
The Thermo Scientific Heraeus Biofuge Stratos
Centrifuge (Thermo Fisher Scientific, Waltham, MA, USA); RM2235
paraffin section machine (Leica, Mannheim, Germany); DH101 electric
heating constant temperature air blast drying box (Beijing Lee Kang
Science and Technology Development Co., Ltd., Beijing, China); BX43
microscope (Olympus Corporation, Tokyo, Japan); and MSHOT MC50
micro imaging system (Guangzhou Ming-Mei Technology Co., Ltd.,
Guangzhou, China), were used in the present study.
Reagents
The ELISA kit for VEGF, PTEN and PI3 rabbit
anti-mouse polyclonal antibodies were purchased from the Beijing
Boosen Biological Technology Co., Ltd. (cat: bs-0686R, bs-0128R,
Beijing, China). The General Type goat anti-rabbit monoclonal
secondary antibody and DAB color agent, were purchased from Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd. (cat: PV-6001, Beijing,
China). The creatine kinase (CK) kit was purchased from Beijing
Boding Biological Engineering Co., Ltd. (Beijing, China).
Methods
KD rabbit model
Twelve of 9 to 12-week-old rabbits were divided into
the experimental group (n=6) and control group (n=6).
Experimental group
The 6 rabbits in the experimental group, were
received 2.5 mg bovine serum/kg (in 2.5 ml volume/kg) as
intravenous injection (9). After 2
weeks, the experimental group was given another dose of 2.5 mg
bovine serum/kg intravenous slow bolus to induce arthritis, forming
vasculitis.
Control group
The 6 rabbits of the control group, were
intravenously injected with 2.5 ml/kg saline. Two weeks later, 2.5
ml/kg normal saline was intravenously injected.
Tissue pathology examination
Paraffin section hematoxylin and eosin (H&E)
staining
The two groups of rabbits, respectively 1, 7 and 30
days after modeling were sacrificed (n=2, at each time point). The
chest was opened to expose the heart, and the coronary artery was
isolated and immediately fixed in neutral-buffered formalin. The
fixed arteries were embedded in paraffin block, sectioned and
stained with H&E. Histological changes were observed under a
light microscope (CX31, Olympus, Tokyo, Japan).
PTEN and PI3K immunohistochemical staining of the
paraffin sections
The paraffin sections were extracted from the
coronary artery of the rabbits. Immunohistochemical staining
procedures were carried out as follows: the slides were kept at
65°C in an oven for 6 h, dewaxed in dimethylbenzene, dehydrated
with gradient ethanol and rinsed with double-distilled water to
block endogenous catalase. Following treatment with the primary and
secondary antibodies at 4°C, DAB was used to develop color and
observed under the microscope. Images were captured and analyzed
using IPP software (Image-Pro Plus) 6.0. (Media Cybernetics,
Baltimore, MD, USA).
Hematology test
Blood was collected for the white blood cell count
at 1, 7 and 30 days after modeling. The serum CK and VEGF levels
were measured according to the manufacturer's instructions.
Results
Immunohistochemical staining for PTEN,
PI3K and coronary H&E staining
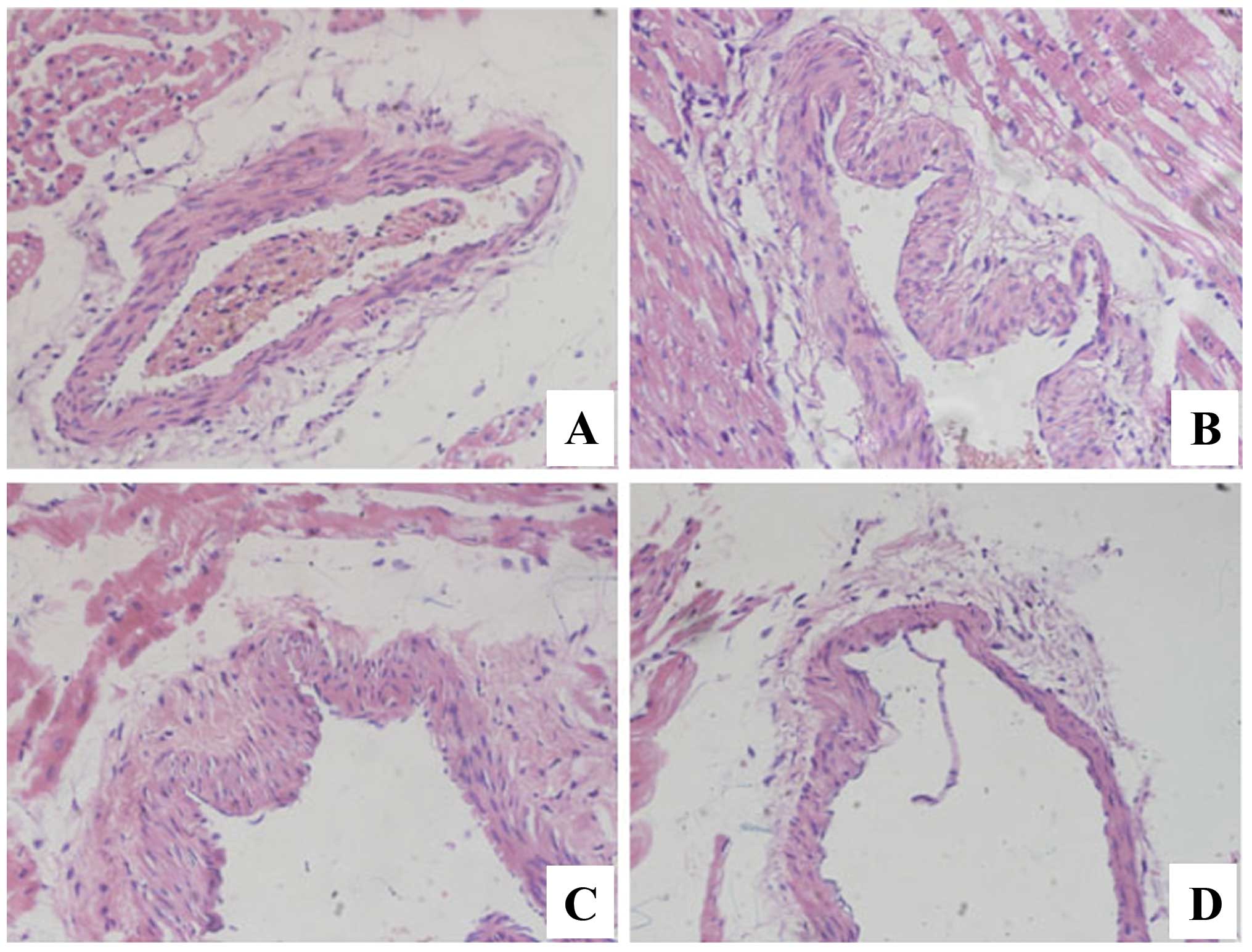
H&E staining (Fig.
1) showed the changes of endothelial cell swelling,
osteoporosis, necrosis, and inflammatory cell infiltration in the
coronary artery tissue of the experimental group, which was
consistent with the pathological characteristics of KD, suggesting
that the model was successful.
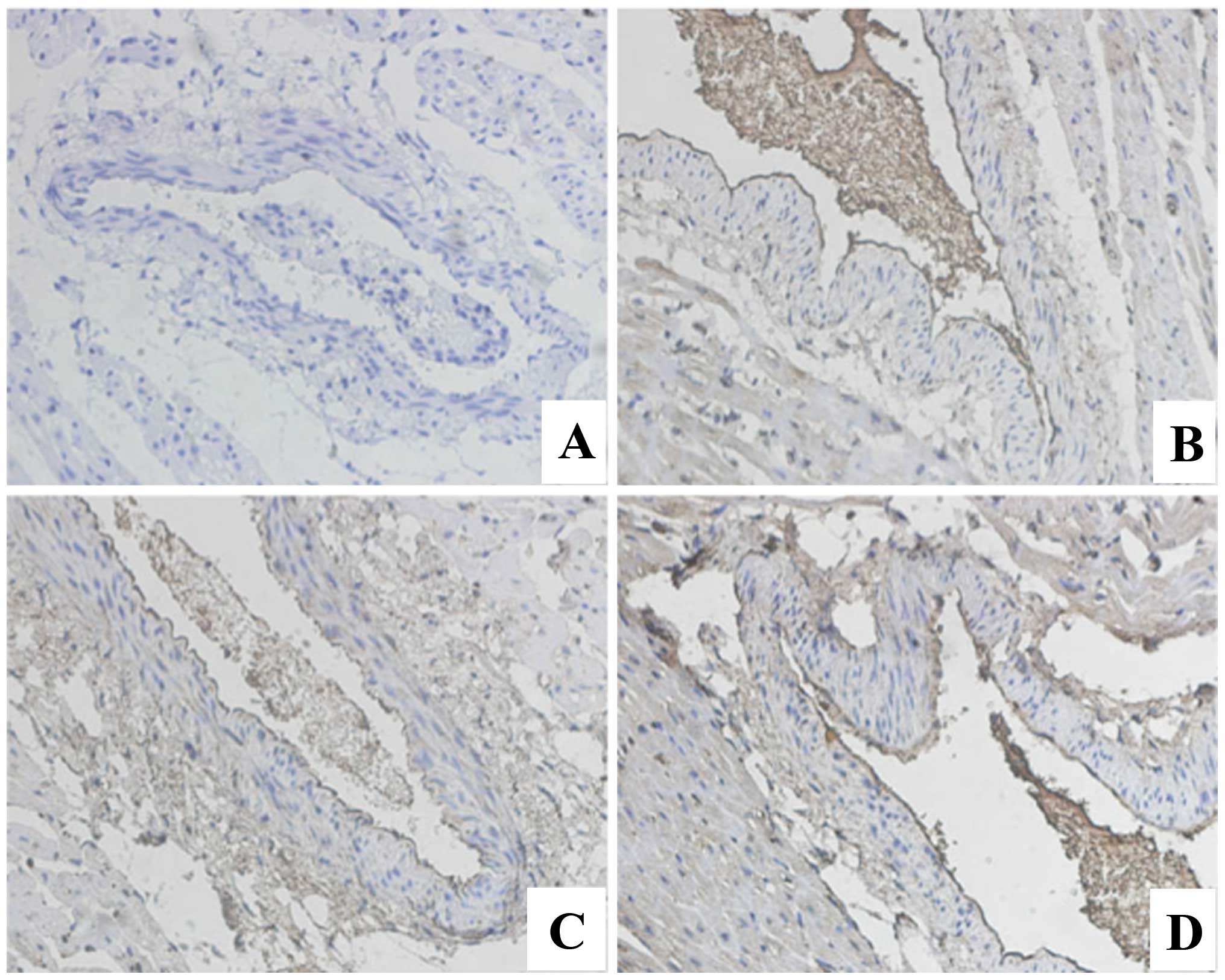
Immunohistochemical staining for PTEN
and PI3K
The expression of PTEN in the model group was
significantly higher than that of the control group. PTEN
expression of increased gradually with the increase in the number
of days after modeling (Fig. 2). The
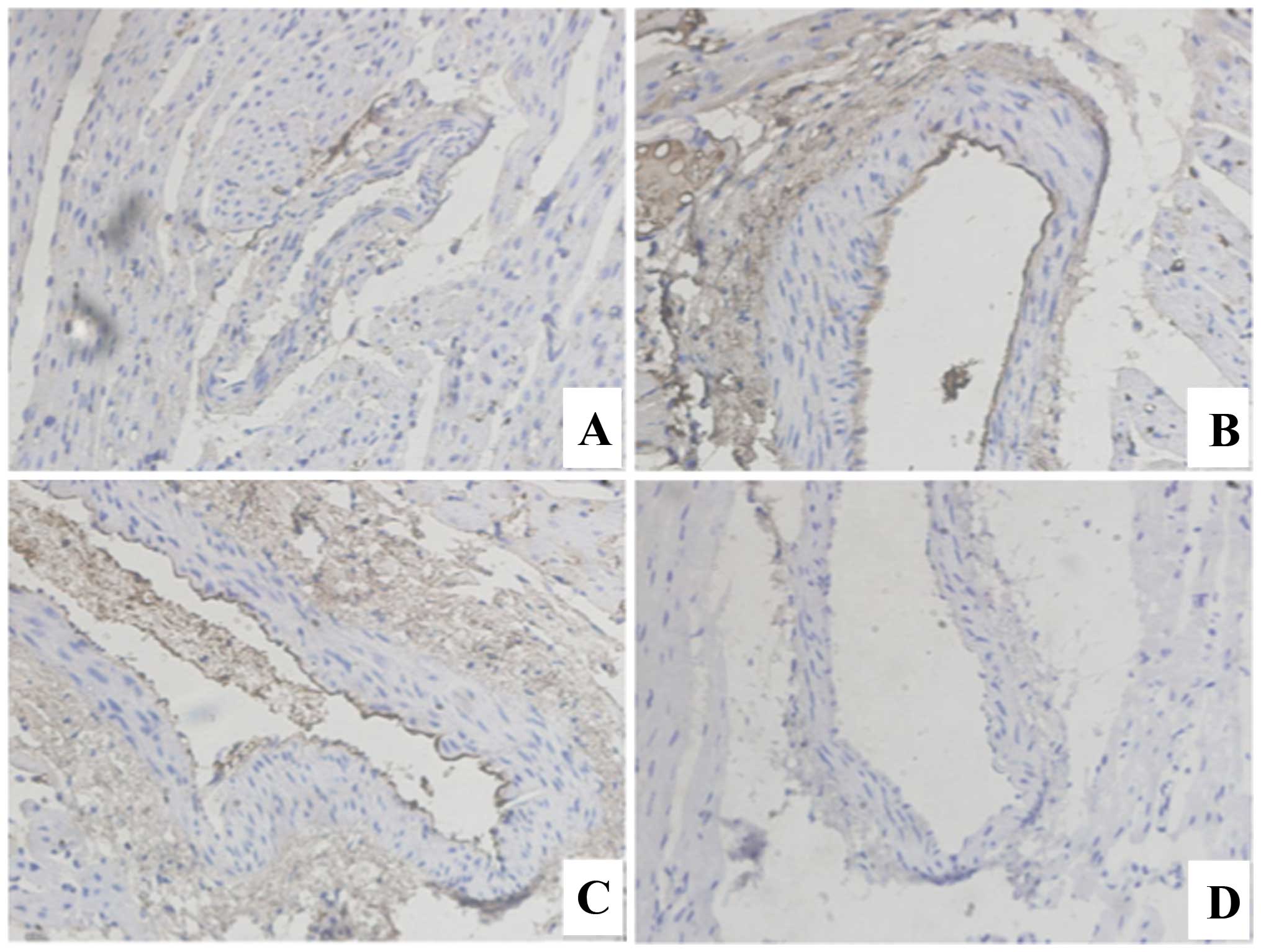
expression of PI3K showed the opposite trend. Compared with the
normal rabbit, the expression of PI3K in the coronary artery of
rabbits was lower. The expression of PI3K showed a gradually
decreasing trend following 1, 7 and 30 days of modeling (Fig. 3).
Changes in the blood parameters of
rabbits in the model group and control groups
Whole white blood cell count
The number of white blood cells of rabbits in the
model group was significantly increased compared to that of the
control group (Table I), which is
consistent with the hematological manifestation of arthritis. At 7
days after modeling, the number of white blood cells increased
compared to 1 day after modeling. At 30 days after modeling, the
number of white blood cells decreased, although their number was
higher than that of the control group.
 | Table I.Effects of the number of white blood
cells of the rabbit model with Kawasaki disease (KD) in 1, 7 and 30
days after modeling. |
Table I.
Effects of the number of white blood
cells of the rabbit model with Kawasaki disease (KD) in 1, 7 and 30
days after modeling.
| White blood cell
count (109/l) | Group 1 day
(n=2) | Group 7 days
(n=2) | Group 30 days
(n=2) |
|---|
| Control group | 5.9±1.2 | 5.7±1.3 | 6.0±1.3 |
| Group KD | 14.6±2.3a | 21.4±2.2a | 11.1±1.9a |
Serum VEGF results
Serum VEGF levels in rabbits on the day of modeling
increased significantly compared with the control group (Table II). At 1 week after modeling the
serum level of VEGF initially increase but was then decreased.
However, this serum level was higher than that of the control
group. At 30 days after modeling, the VEGF levels were
significantly decreased, and lower than those of the control
group.
 | Table II.Effects of serum VEGF of rabbit model
with Kawasaki disease (KD) in 1, 7 and 30 days after modeling. |
Table II.
Effects of serum VEGF of rabbit model
with Kawasaki disease (KD) in 1, 7 and 30 days after modeling.
| VEGF (ng/l) | Group 1 day
(n=2) | Group 7 days
(n=2) | Group 30 days
(n=2) |
|---|
| Control group | 33.9±6.7 | 35.9±7.3 | 34.5±5.9 |
| Group KD |
89.1±15.5a | 76.9±9.9a | 19.8±4.4a |
Serum CK results
On the day of modeling, serum CK exhibited no
obvious change (Table III).
However, 1 week to 1 month after modeling, serum CK increased
significantly compared with that of the control group.
 | Table III.The effect of serum CK of the rabbit
model with Kawasaki disease (KD) in 1, 7 and 30 days after
modeling. |
Table III.
The effect of serum CK of the rabbit
model with Kawasaki disease (KD) in 1, 7 and 30 days after
modeling.
| CK (U/l) | Group 1 day
(n=2) | Group 7 days
(n=2) | Group 30 days
(n=2) |
|---|
| Control group | 635.7±169.3 | 640.5±174.7 | 629.4±163.8 |
| Group KD | 637.6±127.4 |
1441.9±637.3a |
1165.68±256.4a |
Discussion
The pathological changes of KD mainly show as a
systemic non-specific vasculitis, which mainly involves small and
medium-sized arteries, especially coronary arteries (2–5). The
present study produced a KD model as previously described, using
bovine serum albumin to immunize rabbits (11). The pathological changes of the
coronary artery were similar to the vascular lesions of KD in this
model. At 1, 7 and 30 days after injection of bovine serum albumin,
the wall of the coronary artery gradually became thinner, deformed
and enlarged. Serum white blood cells increased gradually and the
amount of WBC reached their maximum after 21 days. The CK level was
the highest on day 21, which indicated segment cardiac damage at
different time points of the model.
VEGF is mainly generated by vascular smooth muscle
cells and released during vascular inflammation. The latter process
can induce fractures, collagenase and metalloprotease synthesis,
accelerate small veins and capillary cracks, express endothelial
cell adhesion of molecules expression and cause peripheral vascular
edema, during the pathogenesis of KD (12). Previous findings have shown that
serum VEGF level in children with KD was significantly higher than
that in remission and normal children (13). The current findings show that serum
VEGF significantly increased on days 1 and 7, but on day 30 the
level of VEGF was decreased. This result may be due to the fact
that the animal model and Kawasaki patients are different, due to
the sampling time.
In order to determine the causes of VEGF change, we
detected changes in the PTEN/PI3K pathway. The expression of PI3K
and VEGF with active mutation in tumor cells is associated with an
increased expression of angiogenesis (14–16). The
overexpression of PI3K and AKT also induces VEGF transcription and
promotes the formation of new blood vessels.
PI3K can produce PIP3, phosphorylate the Ser473,
Thr308 site of AKT, activate AKT, participate in the transcription
and translation of intracellular-associated genes and promote the
development of normal blood vessels. Previous studies have found
that LY294002, a PI3K inhibitor, has a role of anti-angiogenesis in
quality microenvironment and retinopathy of tumor tissue (17,18),
while PTEN is a phosphatase, which is a major negative regulator of
PIP3, by dephosphorylating and thus antagonizing the PI3K/AKT
pathway. If PTEN is inactivated, PI3K/AKT sustains activation,
resulting in cell division, increased volume, apoptosis and tumor
angiogenesis (19–21), suggesting that the PTEN/PI3K/VEGF
signaling pathway plays an important role in vascular injury.
In conclucion, immunohistochemistry was used in the
current study to show that in the rabbit model, the expression of
PTEN/PI3K was different at different stages. In addition, the
expression of PTEN gradually increased, whereas the expression of
PI3K was gradually reduced. There was a negative correlation
between PTEN and PI3K. Changes in the serum VEGF level suggest that
the PTEN/PI3K/VEGF signaling pathway plays an important role in the
development of KD.
References
|
1
|
Patel RM and Shulman ST: Kawasaki disease:
a comprehensive review of treatment options. J Clin Pharm Ther.
40:620–625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanaka N: Kawasaki disease (acute febrile
infantile muco-cutaneous lymph node syndrome) in Japan;
relationship with infantile periarteritis nodosa. Pathol Microbiol
(Basel). 43(2-O): 204–218. 1975.PubMed/NCBI
|
|
3
|
Fujiwara H and Hamashima Y: Pathology of
the heart in Kawasaki disease. Pediatrics. 61:100–107.
1978.PubMed/NCBI
|
|
4
|
Newburger JW, Takahashi M, Gerber MA,
Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P,
Baltimore RS, et al: Committee on Rheumatic Fever, Endocarditis,
and Kawasaki Disease, Council on Cardiovascular Disease in the
Young, American Heart Association: Diagnosis, treatment, and
long-term management of Kawasaki disease: a statement for health
professionals from the Committee on Rheumatic Fever, Endocarditis,
and Kawasaki Disease, Council on Cardiovascular Disease in the
Young, American Heart Association. Pediatrics. 114:1708–1733. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dajani AS, Taubert KA, Gerber MA, Shulman
ST, Ferrieri P, Freed M, Takahashi M, Bierman FZ, Karchmer AW and
Wilson W: Diagnosis and therapy of Kawasaki disease in children.
Circulation. 87:1776–1780. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsubara K and Fukaya T: The role of
superantigens of group A streptococcus and staphylococcus aureus in
Kawasaki disease. Curr Opin Infect Dis. 20:298–303. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ichiyama T, Yoshitomi T, Nishikawa M,
Fujiwara M, Matsubara T, Hayashi T and Furukawa S: NF-kappaB
activation in peripheral blood monocytes/macrophages and T cells
during acute Kawasaki disease. Clin Immunol. 99:373–377. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu X, Hirono KI, Ichida F, Uese K, Rui C,
Watanabe S, Watanabe K, Hashimoto I, Kumada T, Okada E, et al:
Enhanced iNOS expression in leukocytes and circulating endothelial
cells is associated with the progression of coronary artery lesions
in acute Kawasaki disease. Pediatr Res. 55:688–694. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kariyazono H, Ohno T, Khajoee V, Ihara K,
Kusuhara K, Kinukawa N, Mizuno Y and Hara T: Association of
vascular endothelial growth factor (VEGF) and VEGF receptor gene
polymorphisms with coronary artery lesions of Kawasaki disease.
Pediatr Res. 56:953–959. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng Q, Zhou TF, Chen CH, Hua YM, Liu HM,
Hong H, Zhang LY and Wu Q: Clinical value of serum matrix
metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 for
the prediction and early diagnosis of coronary artery lesion in
patients with Kawasaki disease. Zhonghua Er Ke Za Zhi. 43:676–680.
2005.(In Chinese). PubMed/NCBI
|
|
11
|
Onouchi Z, Ikuta K, Nagamatsu K, Tamiya H,
Sakakibara Y and Ando M: Coronary artery aneurysms develop in
weanling rabbits with serum sickness but not in mature rabbits. An
experimental model for Kawasaki disease in humans. Angiology.
46:679–687. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shulman ST and Rowley AH: Kawasaki
disease: insights into pathogenesis and approaches to treatment[J].
Nat Rev Rheumatol. 11:475–482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamei H, Zaifang J and Futang Z: Pract
Paidonosology. 2012.16472012.
|
|
14
|
Qi Z, Limin X and Shuxia D: Changes of
serum VEGF and NO levels in children with Kawasaki disease and its
clinical significance. J Med Res. 36:100–102. 2007.
|
|
15
|
Dey N, Crosswell HE, De P, Parsons R, Peng
Q, Su JD and Durden DL: The protein phosphatase activity of PTEN
regulates SRC family kinases and controls glioma migration. Cancer
Res. 68:1862–1871. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang BH, Zheng JZ, Aoki M and Vogt PK:
Phosphatidylinositol 3-kinase signaling mediates angiogenesis and
expression of vascular endothelial growth factor in endothelial
cells. Proc Natl Acad Sci USA. 97:1749–1753. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garlich JR, De P, Dey N, Su JD, Peng X,
Miller A, Murali R, Lu Y, Mills GB, Kundra V, et al: A vascular
targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126,
with antitumor and antiangiogenic activity. Cancer Res. 68:206–215.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alvarez Y, Astudillo O, Jensen L, Reynolds
AL, Waghorne N, Brazil DP, Cao Y, O'Connor JJ and Kennedy BN:
Selective inhibition of retinal angiogenesis by targeting PI3
kinase. PLoS One. 4:e78672009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carracedo A and Pandolfi PP: The PTEN-PI3K
pathway: of feedbacks and cross-talks. Oncogene. 27:5527–5541.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodríguez-Escudero I, Roelants FM, Thorner
J, Nombela C, Molina M and Cid VJ: Reconstitution of the mammalian
PI3K/PTEN/Akt pathway in yeast. Biochem J. 390:613–623. 2005.
View Article : Google Scholar : PubMed/NCBI
|