Introduction
Cystic tumors of the pancreas account for 5–15% of
pancreatic cystic lesions (1) and 5%
of pancreatic tumors (2,3). Due to the development of imaging
technology and an increase in health awareness, cystic pancreatic
tumor screening rates have improved (4). The treatment and prognosis of cystic
pancreatic tumors with distinct origins is variable, and due to the
lack of specific clinical symptoms and laboratory tests available
for cystic pancreatic tumors, imaging is particularly
important.
The spatial and density resolution of multi-slice
computed tomography (CT) is high (5). This technique has been used in
fractures (6,7), lymph node metastasis of
gastrointestinal cancer (8),
pre-operative evaluation of pancreatic cancer (9), and pre-operative staging of esophageal
cancer (10). However, the
literature on cystic pancreatic tumors is limited. Following image
acquisition, multi-slice spiral CT data can be processed to
generate multiplanar reformation and three-dimensional
(3D)-reconstructed images. With minimum intensity projection
(MinIP) and cured planar reformation (CPR), a bent duct can be
transformed into a straight duct, facilitating improved
visualization of the connection between the tumor and the
pancreatic duct. Furthermore, maximum intensity projection (MIP)
can help to visualize the relationship between tumor and adjacent
vessels. Overall, 3D reconstruction techniques may provide an
important source of information for the surgical team as they can
aid the visualization of: Tumor location, size and shape; the
pancreatic wall; cavity wall nodules; calcifications; the main
pancreatic duct; the connections between the tumor and the
pancreatic duct; and the associations between the tumor and the
adjacent organs and blood vessels (11). On the basis of these advantages,
multi-slice CT may become the standard diagnostic method for cystic
pancreatic tumors (12).
In the present study, imaging and clinical data
gathered from 30 patients with cystic pancreatic tumors were
retrospectively analyzed. The value of 3D reconstructions of
multi-slice spiral CT images for the diagnosis of cystic pancreatic
tumors is also discussed.
Materials and methods
Patients and data collection
CT and clinical data were collected from 30 patients
with pathologically confirmed cystic pancreatic tumors in Linyi
People's Hospital (Linyi, China) from August 2008 to July 2014.
Clinical data are presented in Table
I. The present study was approved by the Ethics Committee of
Linyi People's Hospital, in accordance with the Declaration of
Helsinki. Written informed consent was obtained from all
participants.
 | Table I.Clinical data of 30 cases of
pancreatic cystic lesions in patients with solid tumors. |
Table I.
Clinical data of 30 cases of
pancreatic cystic lesions in patients with solid tumors.
| Tumor category | No. of cases | Gender | Age (years) | Average tumor
diameter (cm) | Tumor site | Symptoms and
signs |
|---|
| Serous
cystadenoma | 6 | Female | 45–71 | 2.5–4.4 | Pancreatic head
(n=4), body and tail (n=2) | Abdominal pain (n=4)
and asymptomatic (n=2) |
| Mucinous
cystadenoma | 8 | Female | 38–63 |
5.5–10.5 | Pancreatic head
(n=2), body and tail (n=6) | Abdominal mass (n=6)
and jaundice (n=2) |
| Mucinous
cystadenocarcinoma | 9 | 1 male, 8 female | 41–69 | 3.1–8.7 | Pancreatic head
(n=2), body and tail (n=7) | Abdominal mass (n=3)
and nausea (n=6) |
| Solid
pseudopapilloma | 3 | Female | 16–29 |
5.6–10.8 | Pancreatic head
(n=2), body and tail (n=1) | Abdominal pain (n=1)
and asymptomatic (n=2) |
| Intraductal papillary
mucinous tumors | 4 | Male | 60–77 | 1.5–5.8 | Pancreatic head
(n=4) | Abdominal pain (n=2)
and jaundice (n=2) |
CT scan preparation
Following an 8-h fasting period, patients drank
600–800 ml warm water 30 min before checking in, plus 400–600 ml
warm water immediately prior to lying on the CT bed, in order to
fill up the stomach and the ileum.
CT data were acquired at 5-mm intervals from the top
of the diaphragm to the bottom of the uncinate process using a
LightSpeed® Pro 64-slice CT scanner (GE Healthcare Life Sciences,
Waukesha, WI, USA). A high-pressure syringe was used to inject a
bolus of 80–100 ml Ultravist® 300 contrast agent (Bayer AG,
Leverkusen, Germany) into the cubital vein (injection speed, 2.5–3
ml/sec). Following injection of the contrast agent, arterial,
portal venous and parenchymal phase scans were obtained at 28, 60
and 150 sec, respectively. 3D reconstruction was performed by
constructing two- and three-dimensional images using the original
thin-layer (0.625mm) volume data, which was subsequently used to
assess pathological changes in the pancreatic duct. The average
tumor diameter was calculated as the average of the maximum
diameter of the tumor cross-section. The diagnostic accuracy (DA)
of the 3D-reconstructed images of cystic pancreatic tumors was
calculated as: Patients pathologically confirmed following
operation/number of patients diagnosed using CT prior to operation
× 100%.
Results
Imaging of mucinous cystadenoma
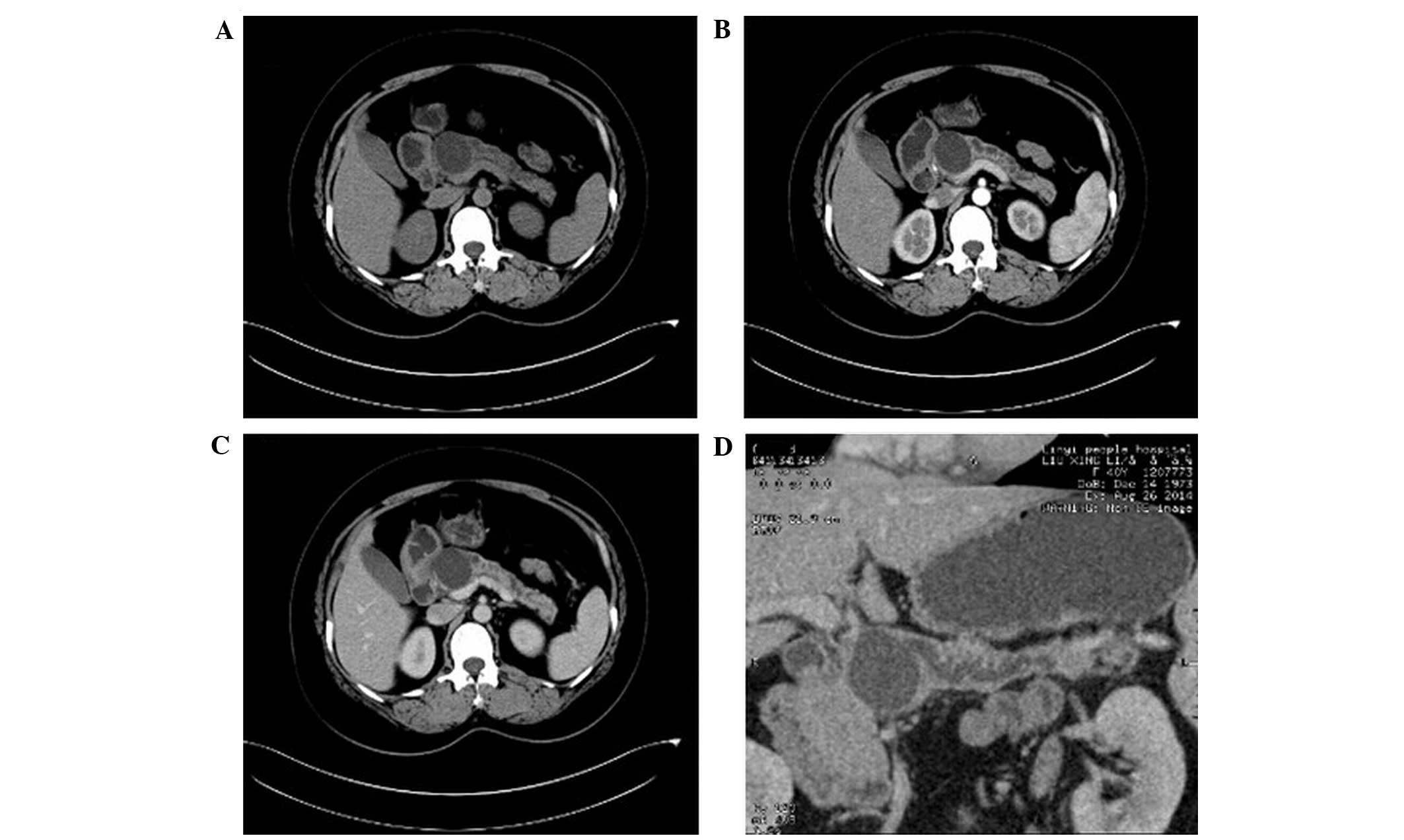
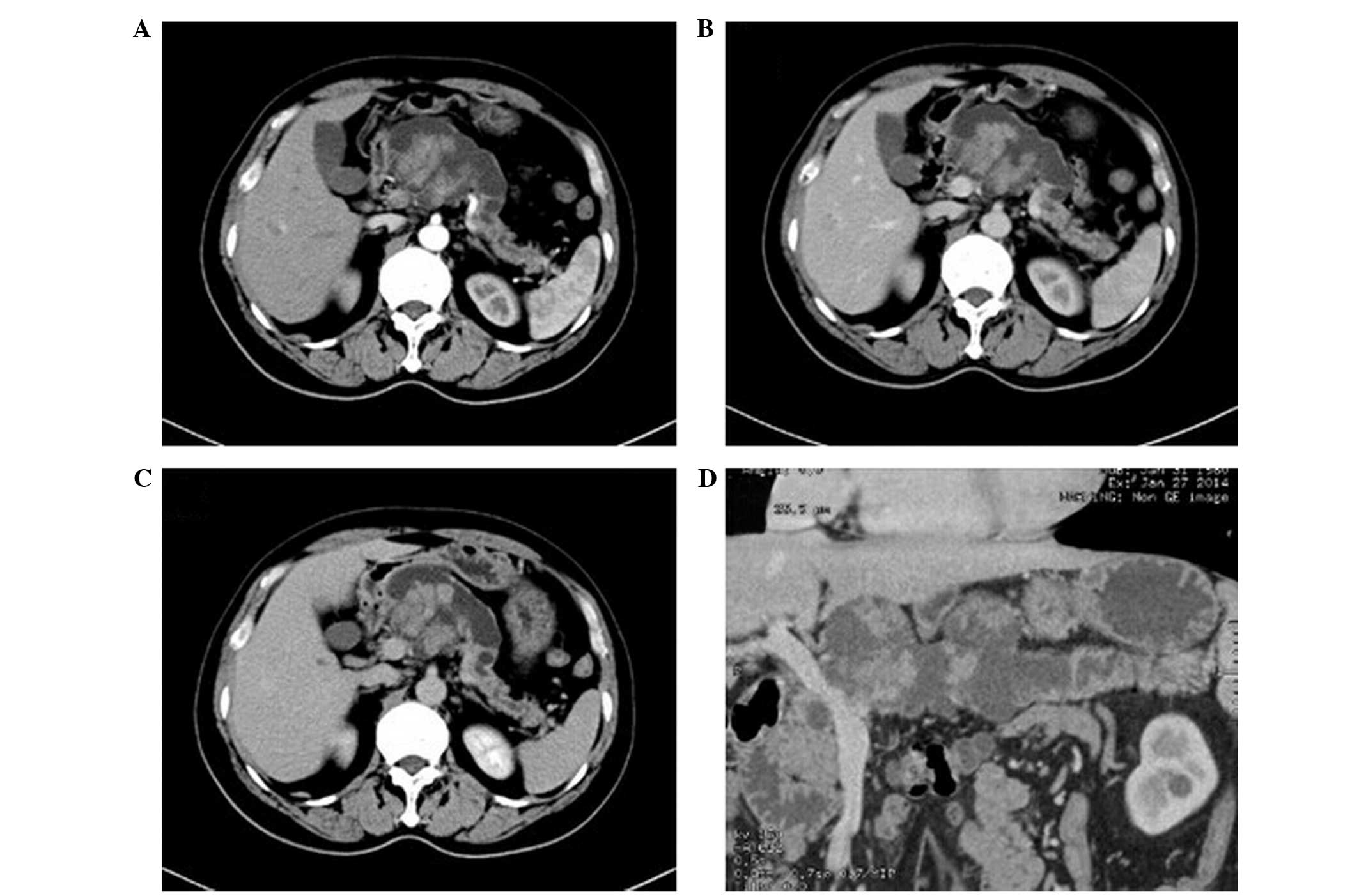
Among the 30 patients, there were 8 cases of
mucinous cystadenoma (DA, 80%; Fig.
1). Four of these cases demonstrated multiple cystic lesions,
of which 2 cases demonstrated uniform wall and cyst spacing and the
other 2 cases exhibited uneven wall and cyst spacing with mural
nodules. The remaining 4 cases demonstrated a single cystic lesion
with uniform thickness. All cysts were large (diameter, >2 cm)
and of uneven density with CT values slightly higher than that of
water. In 1 case, the cysts contained flocculent material.
Following contrast-enhancement, the CT values for the density of
the walls increased from 20 to 25 HU. No enhancement was detected
in the cavity. No tumor invasion into the adjacent tissues or
connection to the expanded main pancreatic duct were detected. Two
cases with mural nodules were misdiagnosed as cystic adenocarcinoma
prior to surgery.
Imaging of mucinous
cystadenocarcinoma
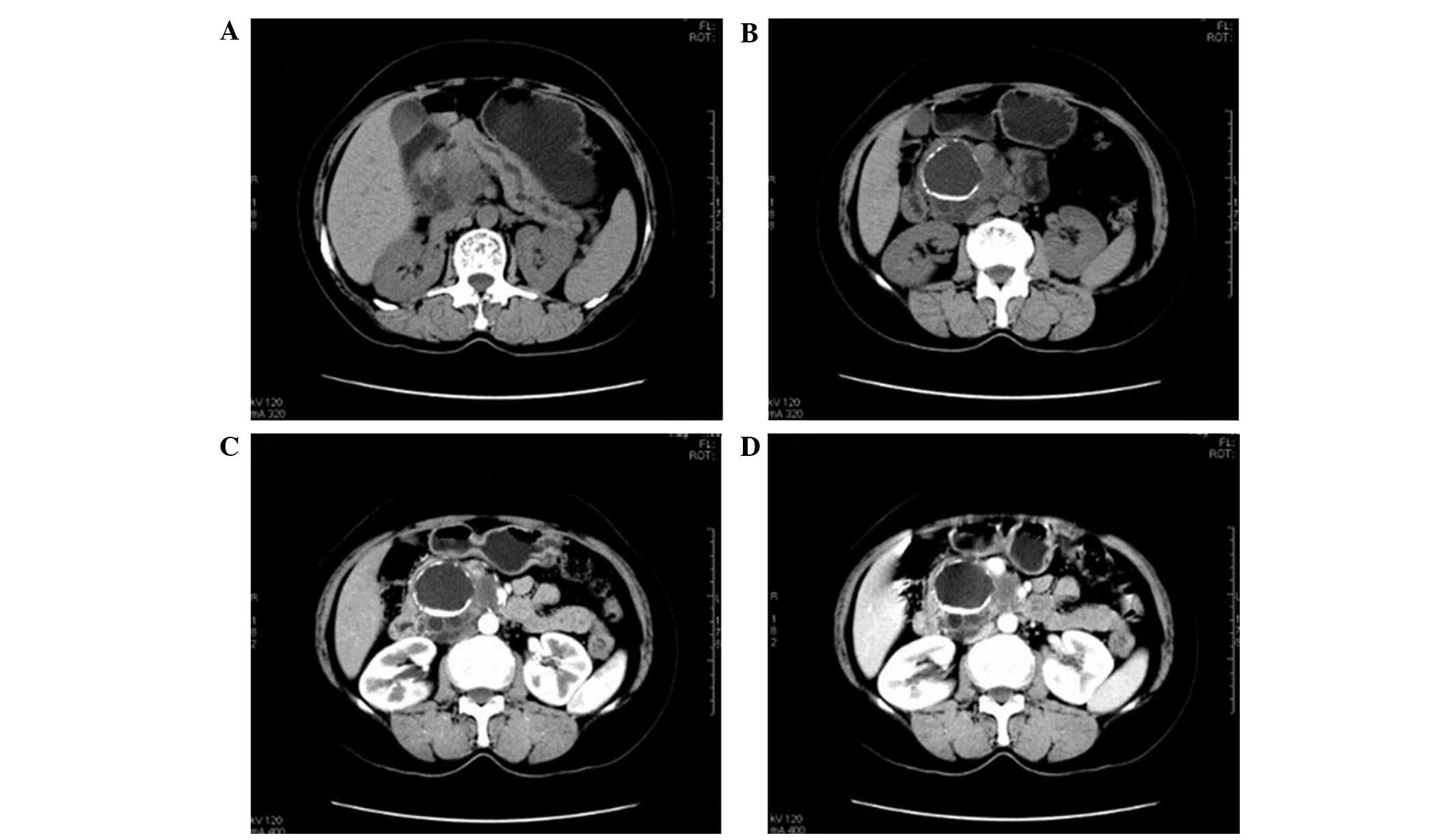
The 30 patients in the present study included 9
cases of mucinous cystadenocarcinoma (DA, 84%; Fig. 2). Five cases demonstrated multiple
sac-like cystic lesions with varying sizes, whereas a single cystic
lesion was detected in the remaining 4 cases. In all 9 cases, the
walls were thick and uneven, including 2 cases with mural nodules;
2 cases with blotchy walls, crude linear calcification, enhanced
wall and wall nodules, significantly enhanced solid tissues
(contrast-enhanced CT value was increased to 40–45 HU), and unclear
wall boundaries; and 2 cases with dilated main pancreatic ducts,
one of which exhibited an enlarged para-aortic lymph node. Two
cases were misdiagnosed as mucinous cystadenoma as a result of
unclear enhancement of the wall.
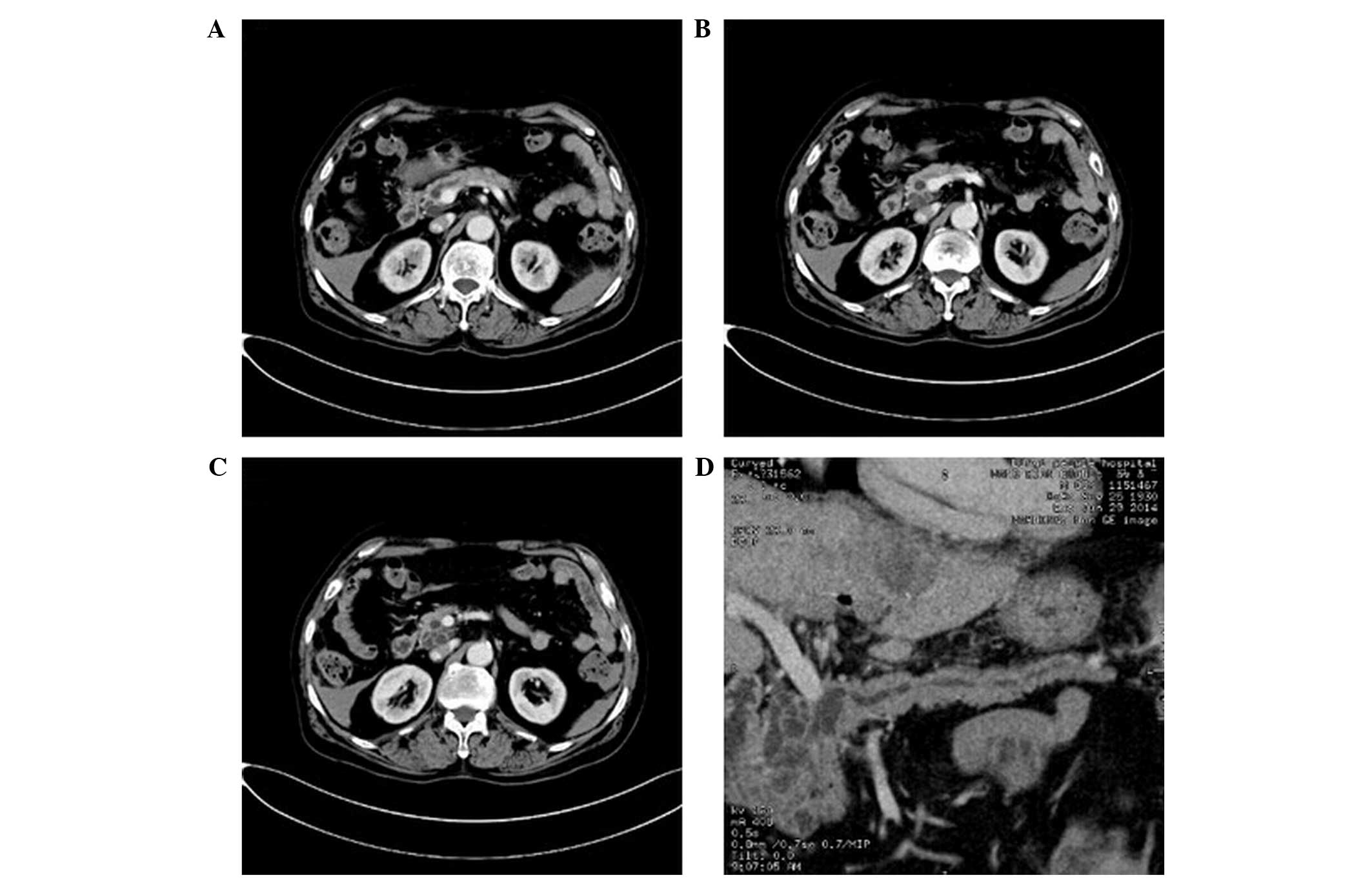
Imaging of serous cystadenoma
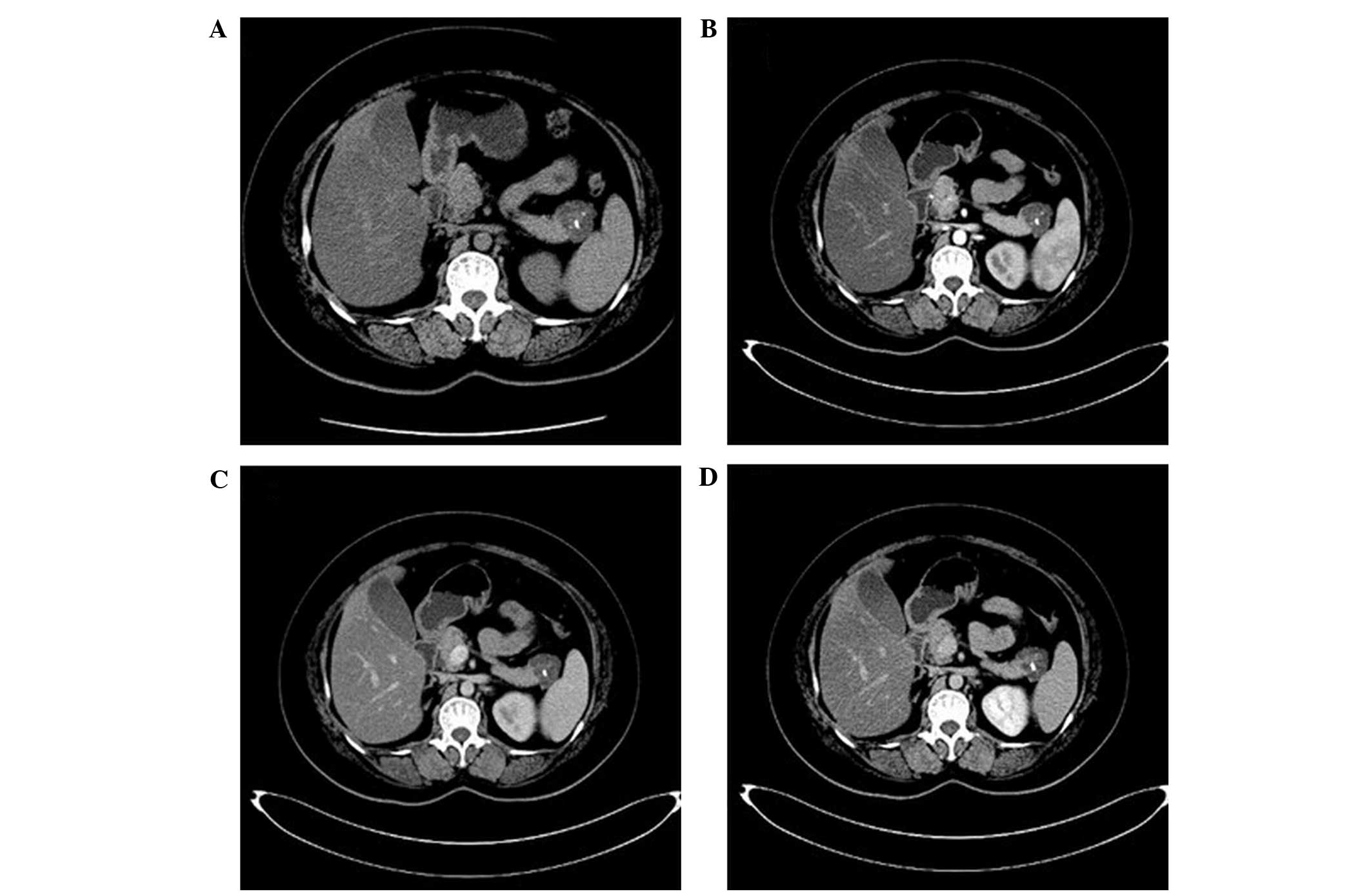
The study group included 6 cases of serous
cystadenoma (DA, 100%; Fig. 3).
Three cases exhibited multiple cystic lesions; 2 cases had
honeycomb-like cysts; and 1 case had a single cystic lesion. Wall
thickness and wall and cyst spacing were uniform in all 6 cases and
no wall nodules were detected. Eggshell-like punctate
calcifications were detected in 2 cases. The density of the cysts
was consistent, with CT values similar to that of water. Following
enhanced CT, the density of the walls and the capsule interval were
mildly enhanced and the CT value increased to 12–16 HU, however
there was no enhancement detected in the cavity. Furthermore, the
main pancreatic duct was not dilated and there was no invasion into
the adjacent tissues. 3D-reconstructed images demonstrated that the
tumors were not connected to the main pancreatic duct (DA,
86.7%).
Imaging of solid pseudopapilloma
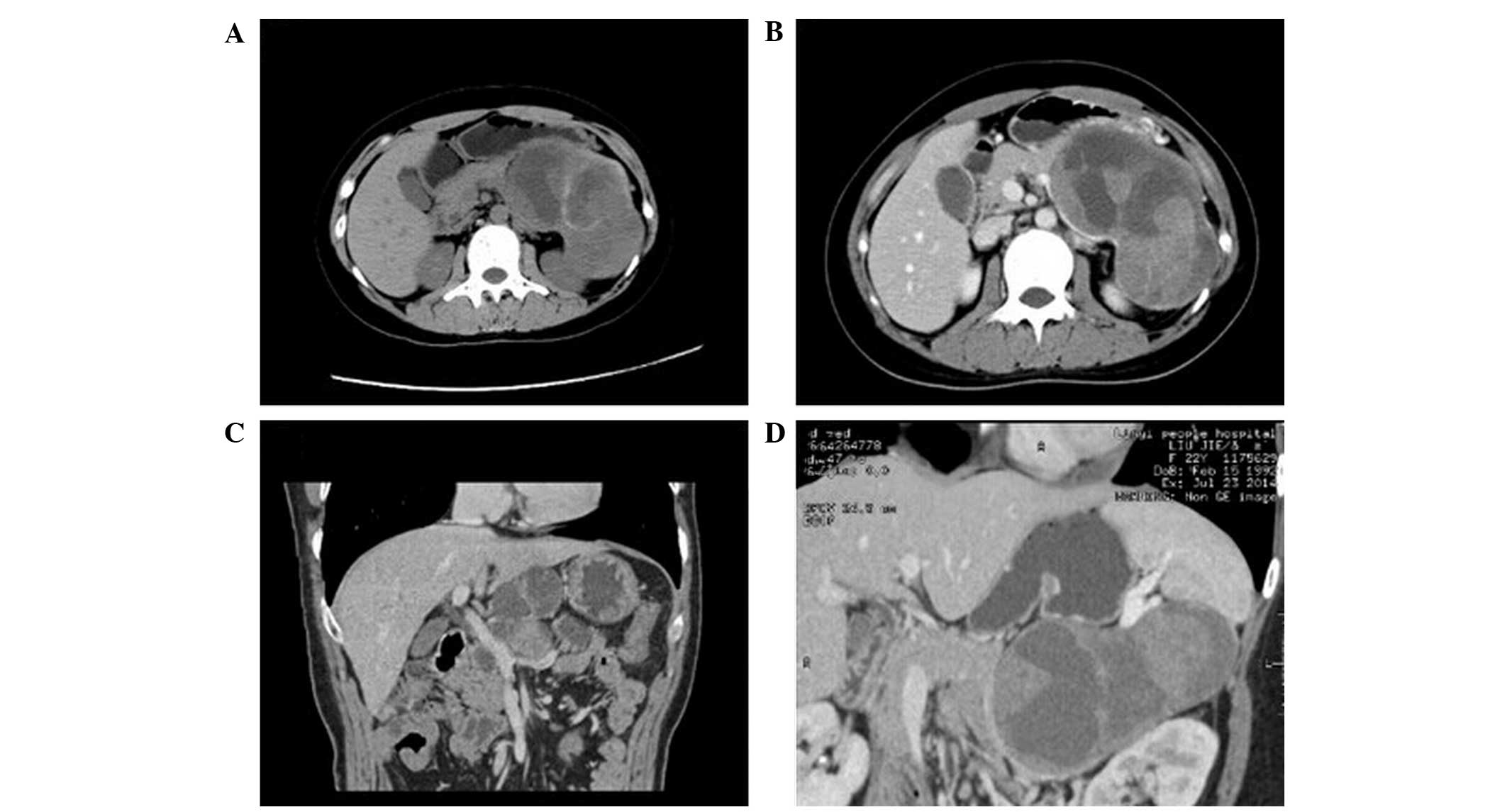
Three cases of solid pseudopapilloma (DA, 100%;
Fig. 4) were investigated in the
present study. Two cases demonstrated cystic masses and patchy
calcification in the solid tissues, and 1 case exhibited a single
cystic lesion, a thicker wall and flocculent material in the cysts.
Following enhanced CT, 1 case demonstrated enhancement of the CT
value in the wall and the solid tissues of the portal venous phase
were highly enhanced in 2 cases. The CT values increased to 15 and
23 HU, respectively.
Imaging of intraductal papillary
mucinous tumors
The remaining 4 patients had intraductal papillary
mucinous tumors (DA, 100%; Figs. 5
and 6). In 2 cases, a single cystic
lesion was detected, the walls were not thick, multiple low-density
tissue nodules were present on the wall and the cysts were
connected to the expanded main pancreatic duct. The other 2 cases
demonstrated grape-like cysts, which were connected to the expanded
main pancreatic duct. Contrast-enhanced CT images of the wall and
the nodules demonstrated persistent enhancement.
Discussion
Although the incidence of cystic pancreatic tumors
is low, these tumors are perceived as increasingly more important
among clinicians. According to the World Health Organization (WHO),
cystic pancreatic tumors are divided into three categories based on
their biological behavior: Benign tumors, malignant tumors and
precancerous lesions (13). Cystic
tumors of the pancreas are subsequently divided into serous
cystadenoma, mucinous cystic neoplasms, solid pseudopapilloma and
intraductal papillary mucinous tumors (13–16). The
correct preoperative diagnosis of cystic pancreatic tumors
determines their treatment and surgical management (17,18).
Mucinous cystadenoma, also known as large
cystadenoma, is the most common type of cystic pancreatic tumor,
accounting for ~40% of cystic pancreatic tumors and 2–3% of primary
pancreatic tumors (19). Mucinous
cystadenoma occurs most frequently in middle-aged women, with 75%
of these tumors occurring in the 40–60 age range, and anatomically,
it occurs both in the body and tail of the pancreas (19). As a precancerous lesion, mucinous
cystadenoma often requires surgery. In the present study, 17 cases
of mucinous cystic neoplasms were investigated, of which 8 cases
were benign and 9 cases were malignant. The CT scans of the
majority of the cases demonstrated large, round cystic lesions with
a single vesicle diameter >2 cm. In certain cases, mural nodules
were visible in the cavity; eggshell calcification in the wall was
visible in 10–25% cases, which is a characteristic change of the
disease (20). When subjected to
enhanced examination, the walls, mural nodules and intracapsular
intervals were moderately enhanced. In general, the cysts were
unevenly spaced, the capsules were thick with mural nodules, and
the CT signals of the tissues were significantly enhanced.
Pancreatic duct obstruction may be associated with
localized mucinous cystadenocarcinoma (4); however, the results of the present
study suggested that the expansion of the pancreatic duct and the
lesion do not appear to be associated. In the present study, 2
cases of mucinous cystadenoma were misdiagnosed as cystic
adenocarcinoma due to the presence of mural nodules and moderate
enhancement on the CT. Furthermore, 2 cases of bladder cancer were
misdiagnosed as mucinous cystadenoma due to slight enhancement of
the wall. The CT scans of mucinous cystadenoma and
cystadenocarcinoma were similar, indicating that there may be
potential difficulties in differential diagnosis between these
tumor types.
Serous cystadenoma, also known as microcapsules or
small cystic adenoma, is the second most common type of cystic
pancreatic tumor, accounting for ~30% of cystic pancreatic tumors
and 1–2% of primary pancreatic tumors (4). Serous cystadenoma tumors originate in
the central acinar and tubular cells and are typically located in
the pancreatic head (14). They are
most frequent in women aged ≥60 years (male:female ratio, 1:4)
(14). As serous cystadenomas are
benign tumors which rarely undergo malignant transformation,
surgery is only indicated for large tumors (14). Among the 6 cases of serous
cystadenoma evaluated in the present study, 4 cases occurred in
women aged 60–70 years and were located in the pancreatic head,
whereas 2 cases occurred in the pancreatic tail. The majority of
cases exhibited multiple cystic lesions with a mean diameter of 5
cm (range, 1.4–27 cm) and pale lobulated edges, and multiple
(>6) vesicles (diameter, 0.2–2 cm). Furthermore, ~33.3% of cases
exhibited a honeycomb-like appearance; 20% of cases exhibited a
single cystic lesion or cystic lesions >2 cm in diameter; and
30% of cases exhibited a central fibrous scar, with or without
astral calcification, with slight enhancement of the wall and
capsule and no association with dilatation of the pancreatic
duct.
Comparison of the aforementioned observations for
serous and mucinous cystadenoma indicates that mucinous cystadenoma
typically occurs in younger patients (40–60 years old) and is
located in the body and tail of the pancreas, with large vesicles
(diameter, >2 cm), and thick-walled and unevenly spaced cysts
with a density higher than that of water, whereas serous
cystadenoma typically occurs in patients ≥60 years old and is
located in the head of pancreas, with a small capsule (diameter,
<2 cm), thin walls, and cysts with a density similar to that of
water. Astral calcification is a typical characteristic of serous
cystadenoma.
As compared with serous and mucinous cystadenoma,
solid pseudopapilloma has a lower morbidity and accounts for 5% of
cystic pancreatic tumors and 0.9–2.7% of primary pancreatic tumors
(21). Notably, 91% of these tumors
occur during adolescence or in young women (average age, 28 years;
male:female ratio, 1:9), and are often located in the head and tail
of the pancreas (21). As a benign
or low-grade malignant tumor, solid pseudopapilloma requires
surgical treatment (21). CT
examination showed large cystic, solid lesions (diameter, 2.5–25
cm; general diameter, >5 cm), which were often associated with
bleeding within the tumor and enlarged reactive lymph nodes; 66.7%
of cases demonstrated pleomorphic calcifications. On enhanced CT
examination, the enhancement of the portal venous phase was
elevated, as compared with the arterial phase.
Intraductal papillary mucinous tumors account for
20% of cystic pancreatic tumors and 0.5–9.8% of primary pancreatic
tumors (22). These tumors are more
frequent in elderly men with 87% occurring in the 60–70 year age
range (4). They originate in the
ductal epithelium and are divided into three types, namely main
duct, branch duct and mixed. Branch duct intraductal papillary
mucinous tumors most commonly occur in the uncinate process of the
pancreas, and exhibit similarities with the main pancreatic duct
type in terms of grape-like cysts, cystic masses and a dilated
branch duct. CT scans of the main pancreatic duct type typically
demonstrate diffuse main pancreatic duct or localized expansion,
with surrounding pancreatic atrophy and papillary nodules within
the pancreatic duct (4).
Furthermore, multiple mural nodules, which are the solid components
of tumors, expand into the main pancreatic duct and thus likely
contribute to tumor malignancy (23). Mixed lesions are associated with the
main pancreatic duct and branches. In order to confirm the relative
locations of cystic pancreatic tumors in the present study, MinIP
and CPR techniques were applied, which may aid the differential
diagnosis of these tumors. In particular, it is important that
branch-type intraductal papillary mucinous and serous tumors are
identified (24,25), as branch-type intraductal papillary
mucinous tumors occur in older men and are characterized by a
dilated pancreatic duct that communicates with the tumor. By
contrast, serous cystadenoma often occurs in older women, without
dilatation or communication with the pancreatic duct. Therefore,
the differential diagnosis of cystic pancreatic tumors should
consider gender, age, anatomical location, cavity size, number of
cysts, wall thickness, wall nodules and duct morphology data.
The present study demonstrated that, as a
non-invasive, convenient and widely applied imaging method,
multi-slice CT can provide detailed information on pancreatic
cystic lesions. Although cystic tumors demonstrate similar CT
changes, this imaging technique facilitates a more accurate
diagnosis of cystic pancreatic tumors and provides important
information for their surgical management. As the sample size of
the present study was small, future studies should be conducted
with larger patient samples.
References
|
1
|
Wilentz RE, Albores-Saavedra J and Hruban
RH: Mucinous cystic neoplasms of the pancreas. Semin Diagn Pathol.
17:31–42. 2000.PubMed/NCBI
|
|
2
|
Cubilla AL and Fitzgerald PJ:
Classification of pancreatic cancer (nonendocrine). Mayo Clin Proc.
54:449–458. 1979.PubMed/NCBI
|
|
3
|
Sener SF, Fremgen A, Imperato JP,
Sylvester J and Chmiel JS: Pancreatic cancer in Illinois. A report
by 88 hospitals on 2,401 patients diagnosed 1978–84. Am Surg.
57:490–495. 1991.PubMed/NCBI
|
|
4
|
Limaiem F, Khalfallah T, Farhat LB,
Bouraoui S, Lahmar A and Mzabi S: Pancreatic cystic neoplasms. N Am
J Med Sci. 6:413–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hara T, Kato H, Akiyama M and Murata K:
Basic examination of in-plane spatial resolution in multi-slice CT.
Nihon Hoshasen Gijutsu Gakkai Zasshi. 58:473–478. 2002.(In
Japanese). PubMed/NCBI
|
|
6
|
Tan YLYG, Mo JC, Zheng RB, Ye DK, Wu D,
Luo DL and Peng S: Comparison of diagnostic value between DR and
MSCT in fracture and dislocation of foot and ankle. Zhongguo Gu
Shang. 26:553–556. 2013.(In Chinese). PubMed/NCBI
|
|
7
|
Devireddy SK, Kumar RV, Gali R, Kanubaddy
SR, Rao DM and Siddhartha M: Three-dimensional assessment of
unilateral subcondylar fracture using computed tomography after
open reduction. Indian J Plast Surg. 47:203–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai CL, Yang ZG, Xue LP and Li YM:
Application value of multi-slice spiral computed tomography for
imaging determination of metastatic lymph nodes of gastric cancer.
World J Gastroenterol. 19:5732–5737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rebibo L, Chivot C, Fuks D, Sabbagh C,
Yzet T and Regimbeau JM: Three-dimensional computed tomography
analysis of the left gastric vein in a pancreatectomy. HPB
(Oxford). 14:414–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng ZZ, Yang NJ, Xi XQ, Zhao K, Hu SB,
Xu GH, Ren J and Zhou P: Diagnostic and application value of
64-slice spiral CT scanning in preoperative staging of esophageal
cancer. Zhonghua Zhong Liu Za Zhi. 33:929–932. 2011.(In Chinese).
PubMed/NCBI
|
|
11
|
Yu Y, Guo M and Han X: Comparison of
multi-slice computed tomographic angiography and dual-source
computed tomographic angiography in resectability evaluation of
pancreatic carcinoma. Cell Biochem Biophys. 70:1351–1356. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoon SE, Byun JH, Kim KA, Kim HJ, Lee SS,
Jang SJ, Jang YJ and Lee MG: Pancreatic ductal adenocarcinoma with
intratumoral cystic lesions on MRI: Correlation with
histopathological findings. Br J Radiol. 83:318–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hruban R, Klöppel G, Boffetta P, Maitra A,
Hiraoka N and Offerhaus GJA: Tumours of the pancreas. WHO
Classification of Tumours of the Digestive System. Bosman T,
Carneiro F, Hruban R and Theise ND: 3:(4th). (Lyon). IARC Press.
280–330. 2010.
|
|
14
|
Kosmahl M, Pauser U, Peters K, Sipos B,
Lüttges J, Kremer B and Klöppel G: Cystic neoplasms of the pancreas
and tumour-like lesions with cystic features: A review of 418 cases
and a classification proposal. Virchows Arch. 445:168–178. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoon WJ, Lee JK, Lee KH, Ryu JK, Kim YT
and Yoon YB: Cystic neoplasms of the exocrine pancreas: An update
of a nationwide survey in Korea. Pancreas. 37:254–258. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Basturk O, Coban I and Adsay NV:
Pancreatic cysts: Pathologic classification, differential diagnosis
and clinical implications. Arch Pathol Lab Med. 133:423–438.
2009.PubMed/NCBI
|
|
17
|
Klöppel G and Heitz PU: Pancreatic
endocrine tumors. Pathol Res Pract. 183:155–168. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krechler T, Ulrych J, Dvořák M, Hoskovec
D, Macášek J, Švestka T and Hořejš J: Cystic tumors of the pancreas
- our experience with diagnostics. Vnitr Lek. 59:572–577. 2013.(In
Czech). PubMed/NCBI
|
|
19
|
Hruban RH, Pitman MB and Klimstra DS: AFIP
Atlas of Tumor Pathology. Tumors of the Pancreas. Fascicle. 6:4th
series. (6th). (Washington, DC). Armed Forces Institute of
Pathology. 2007.
|
|
20
|
Klimstra DS: Cystic, mucin-producing
neoplasms of the pancreas: The distinguishing features of mucinous
cystic neoplasms and intraductal papillary mucinous neoplasms.
Semin Diagn Pathol. 22:318–329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren Z, Zhang P, Zhang X and Liu B: Solid
pseudopapillary neoplasms of the pancreas: Clinicopathologic
features and surgical treatment of cases. Int J Clin Exp Pathol.
15:6889–6897. 2014.
|
|
22
|
Acar M and Tatli S: Cystic tumors of the
pancreas: A radiological perspective. Diagn Interv Radiol.
17:143–149. 2011.PubMed/NCBI
|
|
23
|
Khouri J and Saif MW: Intraductal
papillary mucinous neoplasms of the pancreas (IPMNs): New insights
on clinical outcomes and malignant progression. JOP. 15:310–312.
2014.PubMed/NCBI
|
|
24
|
Yokoyama S, Sasaki Y, Hashimoto K, Takeda
M, Toshiyama R, Fukuda S, Naito A, Matsumoto S, Tokuoka M, Ide Y,
et al: A case of invasive ductal carcinoma of the pancreas
originating from an intraductal papillary mucinous tumor that was
initially misdiagnosed as a mucinous cystic tumor. Gan To Kagaku
Ryoho. 39:2149–2151. 2012.(In Japanese). PubMed/NCBI
|
|
25
|
Palmucci S, Trombatore C, Foti PV, Mauro
LA, Milone P, Milazzotto R, Latino R, Bonanno G, Petrillo G and Di
Cataldo A: The utilization of imaging features in the management of
intraductal papillary mucinous neoplasms. Gastroenterol Res Pract.
2014:7654512014. View Article : Google Scholar : PubMed/NCBI
|