Introduction
Breast cancer is a complex disease with a long-term
development and highly variable clinical prognosis, which are
strongly associated with the stromal microenvironment. Fibroblasts
are the predominant cells of tumor stroma (1), and play a major role in the initiation
and progression of cancer (2,3).
Cytokines are likely to have important effects on fibroblasts in
tumor stroma; they may be involved in the activation of effector
mechanisms that promote or limit certain functions of fibroblasts,
which could potentially influence the tumor microenvironment.
Interleukin (IL)-13 is a T helper 2 (Th2) cytokine
that participates in fibrosis, inflammation and the occurrence and
development of cancer (4). Results
of previous studies have confirmed the presence of IL-13 in breast
cancer (5–7). The microenvironment of human breast
tumor has been observed to display features of Th2 inflammation and
fibrosis, whose pathologies can promote tumor development (8). IL-13-mediated hyperactive functions of
breast stromal fibroblasts are associated with the malignant
transformation of breast epithelial cells (7,9,10).
The increased expression levels of autophagy-related
genes induced by IL-13 may be an abnormal event occurring in
fibroblasts of tumor stroma, which may modify or alter the tumor
stromal microenvironment and ultimately cause cells to become
malignant. The association between autophagy and tumor development
has been the subject of considerable attention from researchers
(11,12). Autophagy involves nuclear factor
(NF)κB, a transcription factor that is able to regulate the
expression levels of autophagy-related genes (13,14). A
study has shown that IL-13 induces inhibitor of κB kinase β (IKKβ)
phosphorylation, and subsequently activates the p65 subunit of NFκB
(NFκBp65) in bronchial smooth muscle cells in vitro
(15). However, the molecular and
cellular mechanisms contributing to the effect of IL-13 in breast
tumors remain unclear.
The aim of the present study was to investigate
whether the cytokine IL-13 signals to NFκBp65, and whether
autophagy-related genes are induced by IL-13 via the activation of
NFκBp65 as transcriptional targets in fibroblasts of breast tumor
stroma.
Materials and methods
Cell culture and co-culture
The human fibroblast line CCC-ESF-1 (ESF) was
obtained from the Cell Center of the Chinese Academy of Medical
Sciences (Beijing, China). The human breast cancer cell line BT474
was acquired from the Cell Resource Center of the Chinese Academy
of Sciences. ESF or BT474 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Waltham, MA, USA) with 10% fetal bovine serum (GE
Healthcare, Buckinghamshire, UK), 10 µg/ml streptomycin and 100
U/ml penicillin (both purchased form Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified 5% CO2
atmosphere. ESF and BT474 cells were co-cultured using
polycarbonate Transwell inserts (0.4 µm pore size; Corning Inc.,
Corning, NY, USA). ESF cells (2×105 cells/well) were
plated at the bottom of 6 well companion culture plates and then
allowed to adhere for at least 2 h without apical Transwell
inserts. ESF cells plated in the wells were exposed to BT474
cell-conditioned media by placing the Transwell inserts plated with
BT474 cells (1.5×105 cells/well) into these wells. This
method allowed the ESF and BT474 cells to grow in the same medium
without direct contact between them. Each experiment was repeated 3
times.
Treatment of cells
The quinoxaline derivative BMS345541 (Sigma-Aldrich,
Beijing, China) is an inhibitor of IKKβ/NFκBp65. When ESF cells
reached a confluence of 80–90%, they were cultured in serum-free
DMEM for 4 h. Subsequently, cultured of co-cultured ESF cells were
treated with BMS345541 (30 µmol/l) for 1 h, then treated with IL-13
(20 ng/ml; PeproTech, Rocky Hill, NJ, USA) for 30 min.
Western blot assay
ESF cells were lysed in radioimmunoprecipitation
assay (RIPA) lysis buffer (Beyotime Institute of Biotechnology,
Shanghai, China). Nucleus or cytoplasm proteins were extracted
using a protein extraction kit according to the manufacturer's
instructions (Beyotime Institute of Biotechnology, Shanghai,
China). The protein content in each sample was determined using a
Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Equal amounts of protein were separated by electrophoresis on
an 8% or 10% sodium dodecyl sulfate-polyacrylamide gel (Beyotime
Institute of Biotechnology). The electrophoresed proteins were
transferred to a nitrocellulose membrane (Beyotime Institute of
Biotechnology); the 8% gel was run at 70–80 V and the 10% gel was
run at 110–120 V. After overnight incubation at 4°C in a blocking
buffer (5% non-fat dry milk and 0.05% Tween-20 in Tris-buffered
saline), the membranes were immunoblotted with antibodies against
IKKβ (mouse monoclonal; 1:200; sc-271782), pIKKβ (rabbit
polyclonal; 1:300; sc-21661), NFκBp65 (rabbit polyclonal; 1:500;
sc-109) and GAPDH (rabbit polyclonal; 1:500; sc-365062) (all
purchased from Santa Cruz Biotechnology, Dallas, TX, USA), beclin 1
(rabbit polyclonal; 1:500; AP1818b) and microtubule-associated
protein 1 light chain 3β (MAP1LC3B or LC3B; rabbit polyclonal;
1:500; AP1802a) (both purchased from Abgent, Inc., San Diego, CA,
USA) for 2 h at room temperature (RT). After washing, the membranes
were incubated with horseradish peroxidase-conjugated secondary
antibody (Santa Cruz Biotechnology) at RT for 1 h. Finally, the
membranes were developed using ECL plus (Beyotime Institute of
Biotechnology). The electrophoresis results of the western blot
were analyzed using ImageJ software (version 1.45; National
Institutes of Health, Bethesda, MD, USA).
Reverse transcription- quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the ESF cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific) and measured
using an SMA400 spectrophotometer (Merinton Instrument, Ltd.,
Beijing, China) at wavelengths of 260 and 280 nm. The extracted RNA
was reverse-transcribed into cDNA using a cDNA reverse
transcription kit (Takara Bio, Inc., Kyoto, Japan). The first cDNA
strand was synthesized at 25°C for 5 min, 37°C for 60 min and 70°C
for 5 min in a 20 µl reaction mixture containing 11 µl (0.6- 0.8
µg) of total RNA, 4 µl 5X RT buffer, 2 µl dNTP mixture (10 mM of
each dNTP), 1 µl RNAse inhibitor (20 U/µl), 1 µl reverse
transcriptase (200 U/µl) and 1 µl random primers (50 µM). Following
the first- strand cDNA synthesis, the cDNA was stored at - 20°C.
qPCR analysis of the cDNA was performed in triplicate using IQ SYBR
Green Supermix (Bio-Rad Laboratories, Inc.) and SuperReal PreMix
Plus (Tiangen Biotech Co., Ltd., Beijing, China) on a CFX connect
Real-Time PCR detection system (Bio-Rad Laboratories, Inc.).
Relative changes were calculated using the ΔΔCt method (16). The primer sequences used (Takara
Biotechnology Co., Ltd., Dalian, China) were as follows: Beclin 1,
forward: 5′AACCAACGTCTTTAATGCAACCTTC3′ and reverse:
5′AGCAGCATTAATCTCATTCCATTCC3′ (GenBank accession number
NM_003766.3); LC3B, forward: 5′AACATGAGCGAGTTGGTCAAG3′ and reverse:
5′GCTCGTAGATGTCCGCGAT3′; GAPDH forward: 5′AGAAGGCTGGGGCTCATTTG3′
and reverse: 5′AGGGGCCATCCACAGTCTTC3′ (GenBank accession number
NM_022818.4). PCR products were analyzed using Bio-Rad CFX Manager
software (version 1.6; Bio-Rad, Laboratories, Inc.).
Monodansylcadaverin (MDC) staining for
the detection of autophagosomes
Glass slides were put into culture plates. When ESF
cells cultured on the slides reached 80–90% confluence, the cells
were pre-treated with BMS345541 (30 µmol/l), then treated with
IL-13 (20 ng/ml). After washing, autophagosomes in the ESF cells
were stained using MDC (Sigma-Aldrich) at RT for 20 min. The
stained autophagosomes were observed using a laser confocal
microscope (Zeiss LSM510-Meta confocal; Zeiss AG, Oberkochen,
Gemany), and fluorescence intensity was analyzed using Image-Pro
Plus software (Media Cybernetics, Inc., Bethesda, MD, USA).
Statistical analysis
Experimental data were analyzed using SPSS
Statistics software, version 17.0 (SPSS Inc., Chicago, IL, USA).
Data are presented as mean ± standard deviation. The differences
between two groups were analyzed by the Student's t-test. A
probability value (P) of <0.05 was considered to indicate a
statistically significant difference.
Results
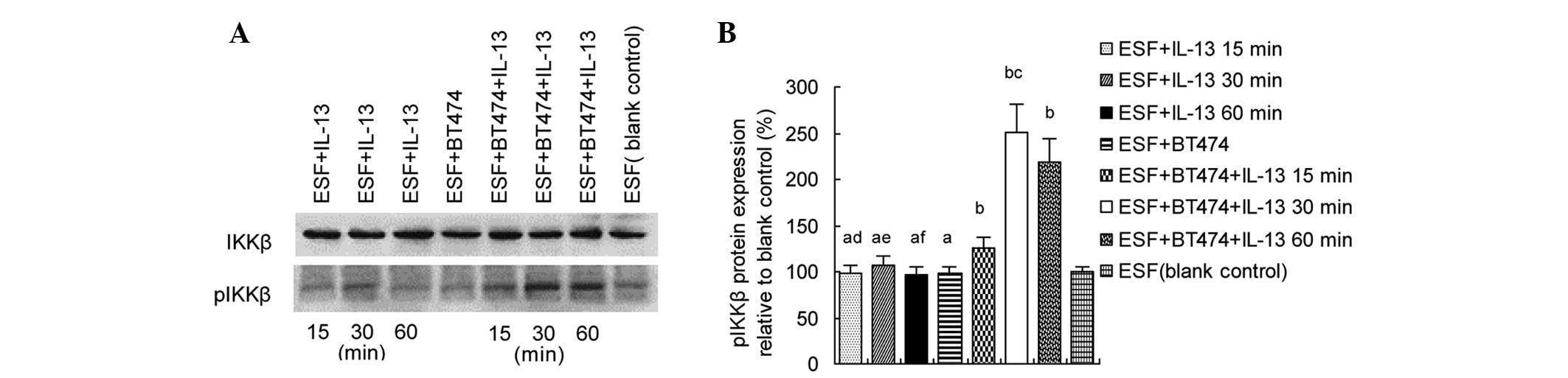
IL-13 induces IKKβ phosphorylation in
co-cultured ESF cells
In order to determine whether IL-13 induces IKK
phosphorylation in fibroblasts co-cultured with breast cancer
cells, ESF cells were treated with IL-13 (20 ng/ml) for 15, 30 or
60 min. Western blot analyses were performed to detect pIKKβ
protein in the ESF cells. The results showed that pIKKβ levels were
significantly increased in the ESF + BT474 co-culture group treated
by IL-13 compared with the blank ESF control (P<0.05), and were
markedly higher in the ESF + BT474 + IL-13 30 min co-culture group
than in the ESF + BT474 + IL-13 15 min or ESF + BT474 + IL-13 60
min co-culture groups (P<0.05; Fig.
1).
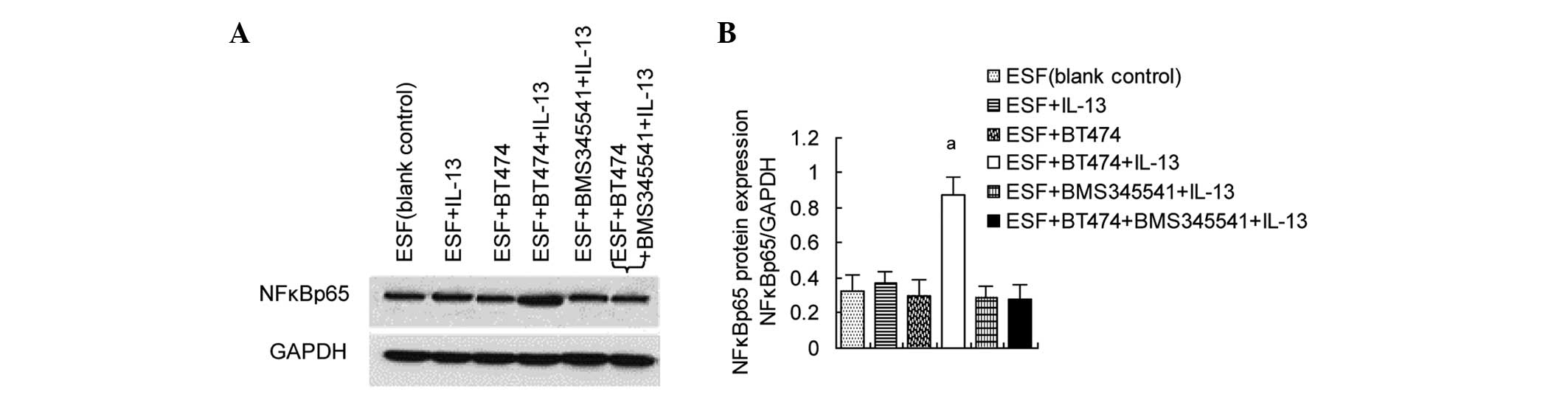
Upregulation of NFκBp65 levels in
co-cultured ESF cells by IL-13/pIKKβ
The previous experiment showed that IL-13 can induce
IKKβ phosphorylation in co-cultured ESF cells. In unstimulated
cells, the activation of NFκB complexes is inhibited by inhibitor
of NFκB (IκB) which binds to NFκB. pIKKβ can induce the
phosphorylation and degradation of IκB, subsequently activating
NFκB (17). Since stimulation with
IL-13 can induce the phosphorylation of IKKβ, whether NFκBp65 in
ESF cells is activated when stimulated by IL-13 was investigated.
The results of western blot analysis showed that NFκBp65 levels
were significantly increased in the ESF + BT474 co-culture group
treated with IL-13 compared with those in untreated co-cultured
cells. BMS345541, an inhibitor of IKKβ/NFκBp65, significantly
inhibited the IL-13/pIKKβ-induced upregulation of NFκBp65 in
co-cultured ESF cells (Fig. 2).
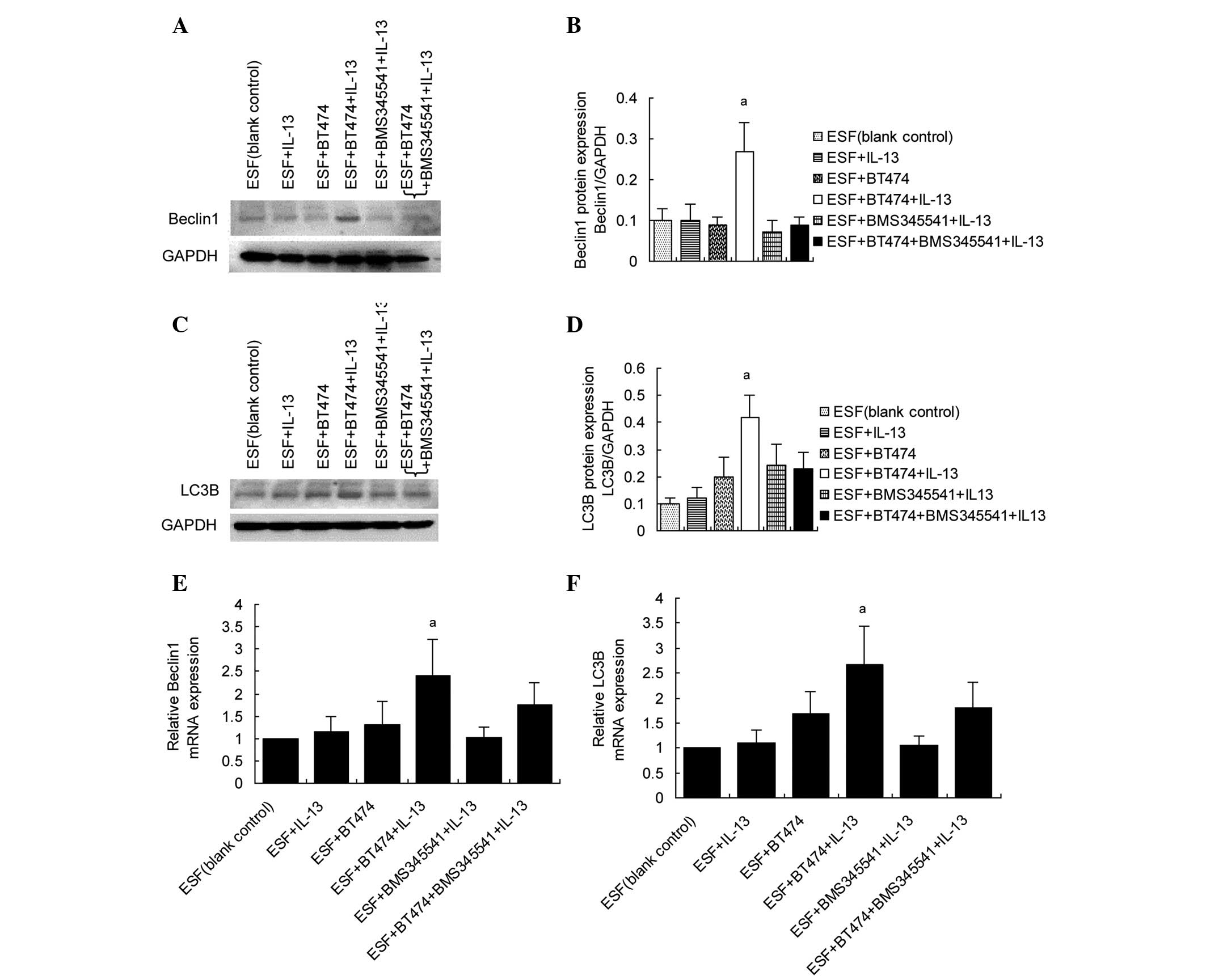
Upregulation of beclin 1 and LC3B in
co-cultured ESF cells by IL-13/pIKKβ-induced NFκBp65
activation
Activated NFκB can translocate into the nucleus and
activate target genes. In the present study, the expression levels
of autophagy-related genes were examined to determine whether they
are targeted by NFκBp65 when stimulated by IL-13. The results of
western blot analysis showed that beclin 1 and LC3B expression
levels were increased in co-cultured ESF cells following treatment
with IL-13. BMS345541 significantly inhibited the expression of
beclin 1 and LC3B targeted by IL-13/pIKKβ-induced NFκBp65
activation (Fig. 3A–D). The mRNA
levels of beclin 1 and LC3B were also examined in co-cultured ESF
cells using RT-qPCR. The results of RT-qPCR (Fig. 3E and F) were consistent with those of
the western blot assay.
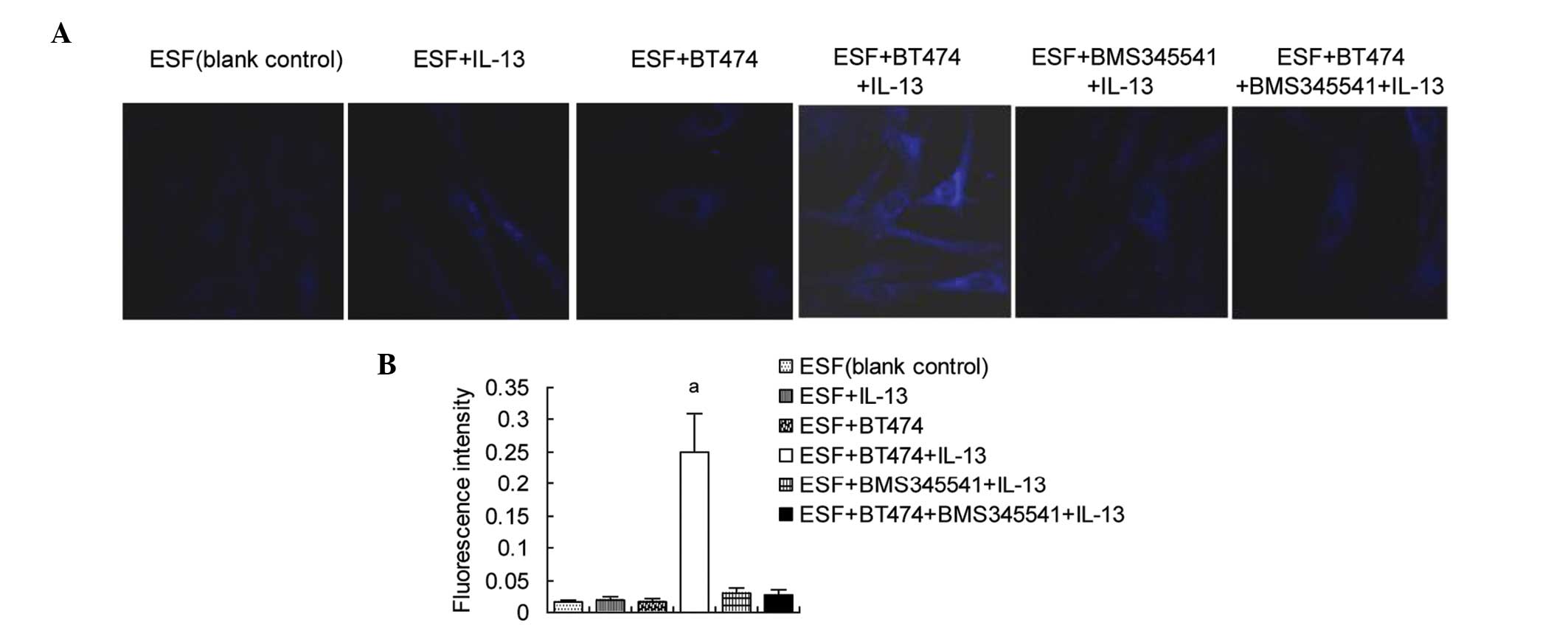
Autophagosomes are increased in
co-cultured ESF cells by the IL-13-induced activation of
NFκBp65/beclin 1 and LC3B
Finally, the formation of autophagosomes was
examined. ESF cells were treated with BMS345541 and/or IL-13.
Autophagosomes were stained using MDC, and observed by laser
confocal microscopy. The results (Fig.
4) showed that the fluorescence intensity was significantly
higher in the co- cultured ESF cells treated with IL-13 than in the
blank control, untreated co- cultured ESF cells and IL-13-treated
ESF cells, indicating that autophagosomes were increased in the ESF
+ BT474 + IL-13 group. Adding BMS345541 prior to IL-13 stimulation
significantly inhibited the formation of autophagosomes in the co-
culture, which was proportional to the expression levels of
NFκBp65-targeted beclin 1 and LC3B.
Discussion
IL-13, a Th2 cytokine, is involved in allergic
reactions, fibrosis, inflammation and tumor development (18). Studies of the IL-13 signaling pathway
have focused on the signal transducer and activator of
transcription 6 (STAT6) pathway (15,19).
However, IL-13 is able to activate the IKK/NFκB signaling pathway,
and autophagy can be activated by multiple conditions and signaling
agents including cytokines (15,20). In
the present study, the associations between IL-13, IKKβ/NFκBp65 and
autophagy genes in fibroblasts co-cultured with breast cancer cells
were investigated.
Fibroblasts are the predominant cells of the stromal
microenvironment (1); they
participate in the formation of stroma, and play an important role
in stromal rebuilding or remodeling. Hyperactivity of fibroblasts
can cause abnormalities of the components in stroma (21). Disorders of the immune system with
abnormally high levels of IL-13 could lead to the abnormal
activation of fibroblasts, which may be a significant cause of
pathological changes of the stroma in the development of
inflammation, autoimmune diseases and tumors. Breast diseases such
as hyperplasia and tumors are closely associated with the
IL-13-mediated abnormal active functions of fibroblasts in breast
stroma. In the present study, a co-culture model of breast cancer
cells with fibroblasts was established. This co-culture model
allowed fibroblasts and breast cancer cells to grow in the same
medium without direct contact between the two types of cells.
NFκB complexes are retained in a latent cytoplasmic
form in unstimulated cells through binding to inhibitor of NFκB
(IκB). Activation of IKK (the IκB kinase) complex induces the
phosphorylation and subsequent degradation of IκB. Subsequently,
free NFκB dimers translocate to the nucleus and activate target
genes. NFκB, a pleiotropic transcription factor, can be activated
by a diverse spectrum of modulating stimuli, linking a number of
genetic targets (22). In the
present study, ESF cells exhibited basic activation of IKKβ and
NFκBp65, which may be a response to the essential growth or
metabolism of cells, but the addition of IL-13 increased pIKKβ
protein expression in fibroblasts co-cultured with breast cancer
cells, indicating that the phosphorylation of IKKβ was induced by
IL-13. Subsequently, the expression levels of NFκBp65 were
upregulated, suggesting that NFκBp65 was activated by IL-13-induced
pIKKβ. Evidence of mechanism was provided by the use of BMS345541,
an inhibitor of the IKKβ/NFκBp65 signaling pathway, which inhibited
the IKKβ/NFκBp65 response to IL-13 stimulation, suggesting that
IL-13 plays the role of an upstream activator in the IKKβ/NFκBp65
signaling pathway.
NFκB is frequently activated by cytokines or stress
and has either pro- or anti-autophagic functions, according to the
cellular content (23). Direct
molecular interactions exist between the canonical NFκBp65
activation pathway and the autophagic core machinery (24). The present study examined the
consequences of NFκBp65 activation in fibroblasts co-cultured with
breast cancer cells following stimulation with IL-13. The results
showed that autophagy genes beclin 1 and LC3B were NFκBp65
transcriptional targets, which may be responsible for the increased
quantity of autophagosomes in the fibroblasts co-cultured with
breast cancer cells. The level of LC3B is proportional to the
amount of autophagosomes (25). On
the basis of these properties, western blots to quantify LC3B
levels can be used to assess autophagosomal accumulation. Beclin 1,
the mammalian ortholog of yeast Atg6, has a central role in
autophagy. Beclin 1 can intervene at every major step in autophagic
pathways, from autophagosome formation to autophagosome/endosome
maturation (26). NFκBp65 can
directly bind the beclin 1 promoter and upregulate its mRNA and
protein levels (27).
Notably, the co-culture of fibroblasts with breast
cancer cells fundamentally influenced the effects of IL-13 on the
fibroblasts. The addition of IL-13 to the co-culture caused the
occurrence of activation or regulation events, including the
phosphorylation of IKKβ, the activation of NFκBp65, the
upregulation of beclin 1 and LC3B, and the increase of
autophagosomes in fibroblasts co-cultured with breast cancer cells,
suggesting that modifications in tumor stroma can play a
significant role in functional changes to, or oncogenic
transformation of, fibroblasts.
In conclusion, this study highlights that IL-13
induced the activation of IKKβ/NFκBp65 and their regulatory events,
including the upregulation of beclin 1 and LC3B expression, and the
increase of autophagosomes in fibroblasts co-cultured with breast
cancer cells. These results provide new evidence concerning the
associations between IL-13, IKKβ/NFκBp65 and autophagy, indicating
that IL-13 regulates the autophagy genes beclin 1 and LC3B through
IKKβ/NFκBp65 in fibroblasts of breast tumor stroma, and that IL-13
may play role in the activation of IKKβ/NFκBp65.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (Grant No. 91229118).
References
|
1
|
Cirri P and Chiarugi P: Cancer associated
fibroblasts: The dark side of the coin. Am J Cancer Res. 1:482–497.
2011.PubMed/NCBI
|
|
2
|
Beacham DA and Cukierman E: Stromagenesis:
The changing face of fibroblastic microenvironments during tumor
progression. Semin Cancer Biol. 15:329–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gabitass RF, Annels NE, Stocken DD, Pandha
HA and Middleton GW: Elevated myeloid-derived suppressor cells in
pancreatic, esophageal and gastric cancer are an independent
prognostic factor and are associated with significant elevation of
the Th2 cytokine interleukin-13. Cancer Immunol Immunother.
60:1419–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aspord C, Pedroza-Gonzalez A, Gallegos M,
Tindle S, Burton EC, Su D, Marches F, Banchereau J and Palucka AK:
Breast cancer instructs dendritic cells to prime interleukin
13-secreting CD4+ T cells that facilitate tumor development. J Exp
Med. 204:1037–1047. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chawey C, Bibeau F, Gourgou-Bourgade S,
Burlinchon S, Boissiere F, Laune D, Roques S and Lazennec G:
Oestrogen receptor negative breast cancers exhibit high cytokine
content. Breast Cancer Res. 9:R152007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Srabovici N, Mujagic Z,
Mujanovic-Mustedanagic J, Muminovic Z, Softic A and Begic L:
Interleukin 13 expression in the primary breast cancer tumour
tissue. Biochem Med (Zagreb). 21:131–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C,
Tindle S, Marches F, Gallegos M, Burton EC, Savino D, Hori T, et
al: Thymic stromal lymphopoietin fosters human breast tumor growth
by promoting type 2 inflammation. J Exp Med. 208:479–490. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pula B, Jethon A, Piotrowska A,
Gomulkiewicz A, Owczarek T, Calik J, Wojnar A, Witkiewicz W, Rys J,
Ugorski M, et al: Podoplanin expression by cancer-associated
fibroblasts predicts poor outcome in invasive ductal breast
carcinoma. Histopathology. 59:1249–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aboussekhra A: Role of cancer-associated
fibroblasts in breast cancer development and prognosis. Int J Dev
Biol. 55:841–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esteve JM and Knecht E: Mechanisms of
autophagy and apoptosis: Recent developments in breast cancer
cells. World J Biol Chem. 2:232–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martinez-Outschoorn UE, Whitaker-Menezes
D, Pavlides S, Chiavarina B, Bonuccelli G, Casey T, Tsirigos A,
Migneco G, Witkiewicz A, Balliet R, et al: The autophagic tumor
stroma model of cancer or ‘battery-operated tumor growth’: A simple
solution to the autophagy paradox. Cell Cycle. 9:4297–4306. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nivon M, Richet E, Codogno P, Arrigo AP
and Kretz-Remy C: Autophagy activation by NFkappaB is essential for
cell survival after heat shock. Autophagy. 5:766–783. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cianfanelli V and Cecconi F:
Autophagy-dependent NFκB regulation. Cell Cycle. 11:436–437. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goto K, Chiba Y and Misawa M: IL-13
induces translocation of NF-kappa B in cultured human bronchial
smooth muscle cells. Cytokine. 46:96–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real- time quantitative PCR and
the 2− ΔΔCt method. Methods. 25:402–408.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayden MS and Ghosh S: Signaling to NF-
kappaB. Genes Dev. 18:2195–2224. 2006. View Article : Google Scholar
|
|
18
|
Wynn TA: Type 2 cytokines: Mechanisms and
therapeutic strategies. Nat Rev Immunol. 15:271–282. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goenka S and Kaplan MH: Transcriptional
regulation by STAT6. Immunol Res. 50:87–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salabei JK and Hill BG: Autophagic
regulation of smooth muscle cell biology. Redox Biol. 4:97–103.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barker HE, Bird D, Lang G and Erler JT:
Tumor-secreted LOXL2 activates fibroblasts through FAK signaling.
Mol Cancer Res. 11:1425–1436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan F and Lenardo MJ: The nuclear
signaling of NF-κB: Current knowledge, new insights and future
perspectives. Cell Res. 20:24–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Criollo A, Senovilla L, Authier H, Maiuri
MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S,
et al: IKK connects autophagy to major stress pathways. Autophagy.
6:189–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niso-Santano M, Criollo A, Malik SA,
Michaud M, Morselli E, Mariño G, Lachkar S, Galluzzi L, Maiuri MC
and Kroemer G: Direct molecular interactions between Beclin1 and
the canonical NFκB activation pathway. Autophagy. 8:268–270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang JH: Teaching the basics of autophagy
and mitophagy to redox biologists-mechanisms and experimental
approaches. Redox Biol. 4:242–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Copetti T, Bertoli C, Dalla E, Demarchi F
and Schneider C: p65/RelA modulates BECN1 transcription and
autophagy. Mol Cell Biol. 29:2594–2608. 2009. View Article : Google Scholar : PubMed/NCBI
|