Introduction
Nociception is a sensory process that leads to pain
triggered by nociceptors, which are primary sensory neurons that
respond to thermal, mechanical or chemical stimuli (1). There are various major pain theories,
including the intensity theory of pain, which postulates that any
sensory stimulus with enough intensity can generate pain (2). The peripheral pattern theory suggests
that pain is produced by intense stimulation of all skin fiber
endings (3), which contradicts the
specificity theory, which proposes that there are numerous types of
sensory receptors, with each one responding to a specific type of
stimuli (4). All of these theories
propose that pain is induced by the hyperstimulation of sensory
receptors; however, research has suggested that pain may be
associated with other sensory stimuli, such as touch (5).
The Chinese Tuina massage is a traditional hand
massage, which has previously been associated with pain relief.
There are six main styles of physical Tuina therapy, including
wobbling, rubbing, vibrating, squeezing, knocking and articular
moving. Squeezing involves pressing, pinching, kneading, grasping
and rubbing, and is commonly used in Tuina pain treatments
(6). Constant softness and
penetration under consistent intensities, frequencies and
manipulation durations are applied to all styles of Tuina. Clinical
practice has demonstrated the therapeutic effects of Tuina for the
treatment of pain, depression, chronic inflammation and mechanical
injury (7–12). According to the theory of peripheral
sensory signaling, there exists an interacting network of sensory
nerves in the skin and beneath the muscles (13). Various nerve pathways of the
peripheral nervous system participate in distinct signal
transductions, and it has previously been demonstrated that
acupuncture may attenuate pain signaling by stimulating Aβ, Aδ and
C-type nerve fibers (14). Tuina,
which selectively stimulates specific nerves, may trigger similar
mechanisms, thus explaining its analgesic effects in clinical
practice. The present study established a rat model of pain in
order to analyze the effects of the Tuina massage on pain
threshold.
Materials and methods
Rats
Male Sprague-Dawley rats (age, 40–50 days; weight,
250–300 g) were purchased from Experimental Animal Center of Fudan
University (Shanghai, China) and housed in plastic cages at room
temperature, and natural diurnal cycles were applied. All
experiments were conducted in compliance with the Institutional
Animal Care and Use Committee (Shanghai Municipal Commission of
Health and Family Planning), and as few animals were used as
possible in order to achieve statistical significance. The present
study was approved by the Ethical Committee of the Yueyang Hospital
of Integrated Traditional Chinese and Western Medicine (Shanghai,
China).
Rat pain models
A total of 20 rats were randomly selected and
equally divided into either the 5.8% hypertonic saline solution
(HSS) injection group or the heat-induced pain group. In the HSS
group, 0.2 ml HSS was injected over 30 sec into the center of the
left gastrocnemius muscle of the hind limb at a depth of ~0.5 cm.
After HSS injection (0.2 ml), the rats in the heat-induced pain
group were placed into cages containing pre-heated transparent
glass bottoms with Plexiglass panels on all other sides. The rat
hind paws were directly heated using a thermal source (Model 336;
IITC Life Science Inc., Woodland Hills, CA, USA), the power of
which was adjusted according to the weight and size of each
individual rat, in order to avoid unnecessary injury. The longest
heating period applied was 20 sec, and the thermal pain threshold
was set between 8 and 12 sec for all rats. Hind paw mechanical
withdrawal and paw thermal withdrawal tests were conducted on the
bilateral feet, as outlined in previous studies (15,16).
Briefly, the rats were housed in a cage containing a metal grid
bottom (20×20×25 cm) and Plexiglass panels. The left paw was teased
with a brush pen (Electronic Von Frey; Stoelting Co., Wood Dale,
IL, USA), and the paw mechanical withdrawal response was recorded
upon contraction of the gastrocnemius muscles. The paw thermal pain
withdrawal test was conducted using the Model 33 instrument (IITC
Life Science Inc.). Pain thresholds were recorded at 40 and 20 min
prior to pain induction (B40 and B20), and 20, 40, 60, 80, 100,
120, 140 and 160 min, and at 1 and 2 days (A20, A40, A60, A80,
A100, A120, A140, A160, A1D and A2D, respectively) following pain
induction.
Tuina procedure
Hand manipulation maneuvers included clockwise
pressing and rubbing, with moderate to strong pressure on the skin.
Tuina massage was performed on the center of the gastrocnemius
muscle for the indicated frequencies and durations. Massage
intensity was 80% of the full potential (at this point, the rats
exhibited sensory stimuli feeling without signs of pain and paw
withdrawal). Prior to Tuina massage, rats were placed face down on
a frame platform to adapt to the experimental environment and to
minimize stress. Finger pressure recordings (units in Newton) were
monitored using a pressure recording instrument (FingerTPS™,
Pressure Profile Systems, Inc., Tokyo, Japan), as described in a
previous study (17).
Experimental design of Tuina
massages
Clinically, a Tuina massage lasts for 2–3 min and is
repeated 3–5 times with high hand pressure; however, no previous
experimental protocol exists for a rat model. In the present study,
Tuina massages were performed on the left and right hind
gastrocnemius muscles in three groups (n=10 each), as follows: i)
The 5 min group, in which the rats were massaged twice for 2 min
with a 1 min interval; ii) the 15 min group, in which the rats were
massaged for 2 min five times, with 1 min intervals; and iii) the
30 min group, in which the rats were massaged for 2 min ten times,
with 1 min intervals. In the control group, finger skin touch
without Tuina was applied. A frequency of 2 Hz was used for all
rats.
Recording of nerve impulses
Rats were anesthetized using 1% sodium pentobarbital
(40 mg/kg; Beijing Dingguo Changsheng Biotechnology, Co., Ltd.,
Beijing, China). Following loss of sensation, which was tested by a
foot pinch, a cannula was inserted into the trachea of the rats in
order to maintain an open airway and low resistance air flow. The
spine vertebrae of the rats were clipped for stability and the
right hind leg was tied in order to maintain steadiness during the
Tuina massage. The body temperature of all rats was maintained
between 36.5 and 37.5°C using a feedback controlled heating pad,
and blood flow in the bilateral hind legs was continuously
monitored. A 1.5 cm skin incision was made along the lower back and
over the left femur bone. In order to expose an enlargement of the
lumbar spinal cord and sciatic nerve, a T12-L1 laminectomy was
performed. A bipolar silver hook electrode was positioned beneath
the exposed sciatic nerve and the dorsal spinal cord for
physiological and electrophysiological recordings. In addition, the
exposed dorsal spinal cord area was filled with a medical wax
cotton ball and was stabilized using a warmed paraffin oil pool
situated in a skin flap. C-fiber-evoked field potentials were
initiated via stimulation of the sciatic nerve using rectangular
pulses (6–18 V; 500 µsec; interval, 60 sec), delivered by a silver
mercury membrane electrode (Beijing Xinhangxingye, Co., Ltd.,
Beijing, China). The sciatic nerves were stimulated using 1 mol/l
HSS injected through a micropipette, which was fabricated using
either a PC-5 N (Narishige Group, Tokyo, Japan) or a laser-based
micropipette puller (P-97; Sutter Instrument, Novato, CA, USA), in
order to induce a pain response. The T12-L1 nerve impulse signals
were displayed on an oscilloscope (VC-11; Nihon Kohden, Tokyo,
Japan), which was connected to a Pentium computer (Intel, Santa
Clara, CA, USA) via a CED 1401 interface for off-line analysis
using Spike2 software (Cambridge Electronic Design Ltd., Cambridge,
UK). Data are presented as C-fiber evoked field potentials and
nerve impulse thresholds at a defined baseline (%).
Immunohistochemical staining for
tissue damage
In order to assess the extent of tissue damage
following Tuina massage, treated rats were sacrificed via cervical
dislocation at the end of the experiments, and the gastrocnemius
muscle tissues of 12 rats from the 5, 15 and 30 min Tuina groups
were removed (n=4 each). Sections of gastrocnemius muscle tissue
(0.5×0.5 cm) were mounted on glass slides, fixed with acetone for
10 min and subsequently washed three times with 0.01 M
phosphate-buffered saline (PBS), after which the tissue sections
were blocked with 5% sheep serum (Experimental Animal Center of
Fudan University) at room temperature for 20 min. Mouse anti-desmin
monoclonal primary antibody (dilution, 1:5; cat. no. BM0036; Wuhan
Boster Biological Technology, Ltd., Wuhan, China) was added to the
slides and incubated at 37°C for 1 h, after which the slides were
washed three times with 0.01 M PBS (pH 7.2–7.6). The secondary
fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G
antibody (dilution, 1:100; cat. no. SA1062; Wuhan Boster Biological
Technology Co., Ltd.), was added to the slides, which were
subsequently incubated in a dark room for 2–3 h at room
temperature. The tissue sections were washed with PBS, and
4′,6-diamidino-2-phenylindole was added for 10 min in order to
stain cell nuclei. After washing three times with PBS, sections
were mounted in buffered glycerin and were visualized under a
fluorescence microscope (Nikon Eclipse E600; Nikon Corp., Tokyo,
Japan) within 24 h.
Hematoxylin and eosin (H&E)
staining
For H&E staining of muscle sections, slices were
incubated twice with xylene, for 10 min each time, followed by
rehydration twice in absolute alcohol (5 min each time) and then
twice in 95 and 75% alcohol (2 min each time). Next, the slices
were stained with Harris-modified hematoxylin solution (Bogu
Biological Science and Technology Co., Ltd., Shanghai, China) for 8
min, then rinsed with distilled water for 10 min, after which 1%
hydrochloric acid-alcohol solution was added for 30 sec followed by
washing with distilled water for 1 min. Subsequently, 0.2% ammonia
water saturated lithium carbonate (Yuanmu Biological Science and
Technology Co. Ltd., Shanghai, China) was added for 30–60 sec, and
the slides were washed again with distilled water for 5 min.
Finally, the samples were counterstained with eosin-phloxine
solution (Bogu Biological Science and Technology Co., Ltd.) for
30–60 sec subsequent to washing with 10 drops of 95% alcohol.
Following dehydration with absolute alcohol and 95% alcohol twice
for 5 min each time, the samples were cleared twice in xylene (5
min each time) and mounted with xylene-based mounting medium
(Yuanmu Biological Science and Technology Co. Ltd., Shanghai,
China).
Malondialdehyde (MDA) and superoxide
dismutase (SOD) assays
In order to measure the concentrations of MDA and
SOD in the gastrocnemius muscle tissue sections, 0.1 g muscle
samples were cut into small pieces. Subsequently, the tissues were
homogenized in 0.9 ml radioimmunoprecipitation assay cell lysis
buffer (Shanghai Changdao Biotechnology, Co., Ltd., Shanghai,
China) with an ultrasonic homogenizer (Qsonica, LLC, Newtown, CT,
USA) and were maintained on ice for 20 min. Following
centrifugation at 900 × g for 10 min, the supernatant was removed
for further experimentation.
MDA protein expression levels were measured using
the thiobarbituric acid reaction method. Briefly, samples were
heated at 95°C in a water bath for 40 min and were subsequently
cooled with tap water, after which 200 µl samples were added to a
96-well plate, followed by substrate reagents (Sigma-Aldrich,
Beijing, China) at 37°C, to allow color development. Optical
density (OD) was measured using an Epoch spectrometer (Biotek
Instruments, Inc., Winooski, VT, USA) at a wavelength of 532 nm.
The MDA concentration was calculated using the following equation:
MDA (nmol/mg protein) = [(Sample OD532-Control
OD532)/(Standard OD532-Control
OD532)] × standard concentration (10 nmol/ml)/sample
protein concentration (mg protein/ml).
SOD protein expression levels were measured using
the WST-1 method, according to the manufacturer's protocol (Dojindo
Laboratories, Kumamoto, Japan). Briefly, 200 µl protein samples
were added into a 96-well plate, after which substrate reagent was
added at 37°C for 20 min. OD was measured using a Epoch
spectrometer (Biotek Instruments, Inc.) at 450 nm. The SOD
concentration was calculated using the following equation: SOD
inhibitory rate = 100 ×
[(control-controlΔC)-(sample-sampleΔT)]/
[control-controlΔC], where C is the difference between
the control and the control blank, and T is the difference between
the sample and the sample blank. The SOD activity was calculated
using the following equation: SOD activity (U/mg protein) = SOD
inhibiting rate/50% × dilution/protein concentration.
Statistical analysis
Data analyses were performed using Sigmastat 3.5
softwate (Systat Software, Inc., San Jose, CA, USA). Mean
mechanical and thermal pain threshold values pre-Tuina were
calculated using the B40 and B20 groups, respectively. Post-Tuina
pain threshold percentages were calculated using the following
equation: Post-Tuina (%) = (post-Tuina mean value/pre-Tuina mean
value) × 100%. One-way repeated analysis of variance (ANOVA),
followed by a Student-Newman-Keuls test, was conducted in order to
compare the pain withdrawal threshold values between the pre- and
post-Tuina groups. In addition, the pain withdrawal threshold
values between each time point were compared using two-way repeated
ANOVA, followed by Student-Newman-Keuls test. Data are presented as
the mean pain withdrawal threshold value ± standard error. The
areas under the curves (AUC) of the C-fiber evoked field potentials
are presented as the mean ± standard error of four time points, and
were analyzed using two-way repeated ANOVA, followed by
Student-Newman-Keuls test. The SOD and MDA concentrations in the
gastrocnemius muscle are presented as the mean ± standard error of
four groups. Data were analyzed using one-way ANOVA, and Tamhane's
T2 polynomial test was used for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
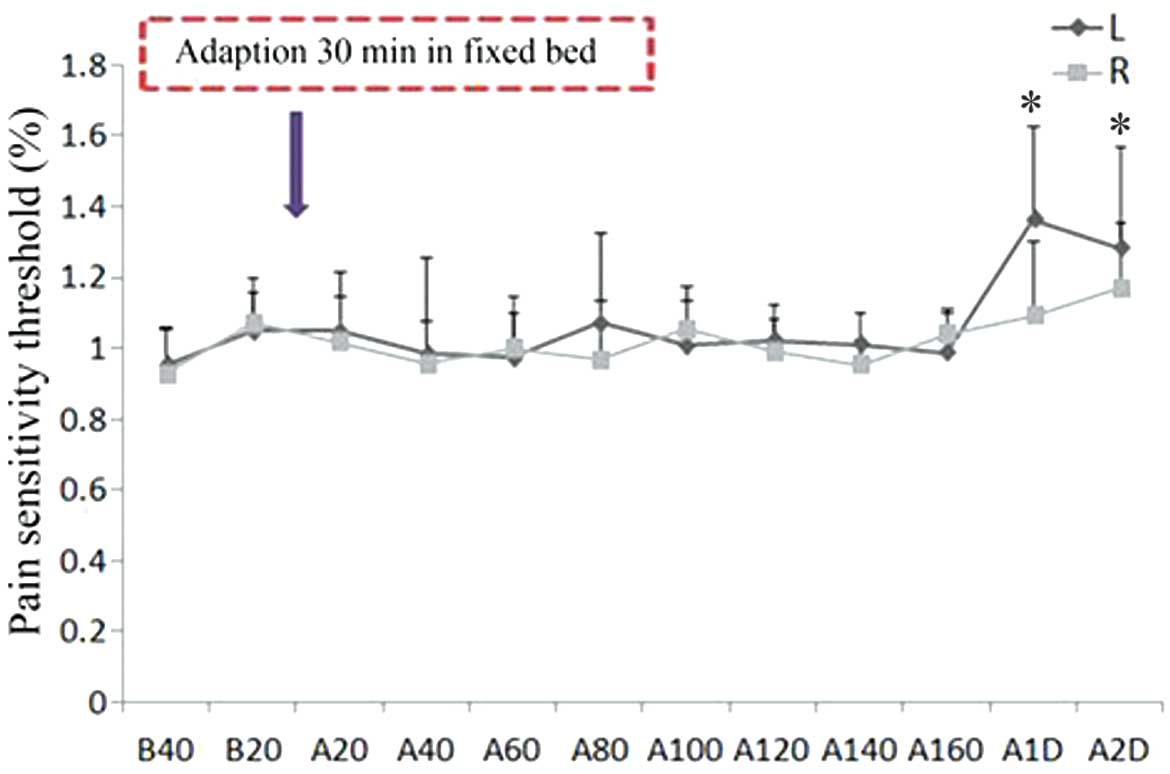
HSS (5.8%), but not thermal pain,
reduces the mechanical pain threshold
In order to establish a rat model of pain, 5.8% HSS
was injected into the left gastrocnemius muscle of the rats
(Fig. 1). Decreased mechanical pain
threshold values and hypersensitivity were detected in the injected
and contralateral gastrocnemius muscles of the A20, A40, A60, A80,
A100, A120, A140, A160, A1D and A2D group rats (n=10); thus
suggesting that 5.8% HSS was able to decrease the mechanical pain
threshold value and induce pain for 2 days post-injection (Fig. 1). Conversely, the thermal pain
threshold of the bilateral gastrocnemius muscles was not altered
until 160 min following 5.8% HSS injection; however, significantly
elevated thermal pain thresholds were detected at 1–2 days
post-injection in the left muscle (P<0.05; Fig. 2). Therefore, 5.8% HSS treatment, but
not thermal heat, may be considered a useful strategy for
developing a rat model of pain.
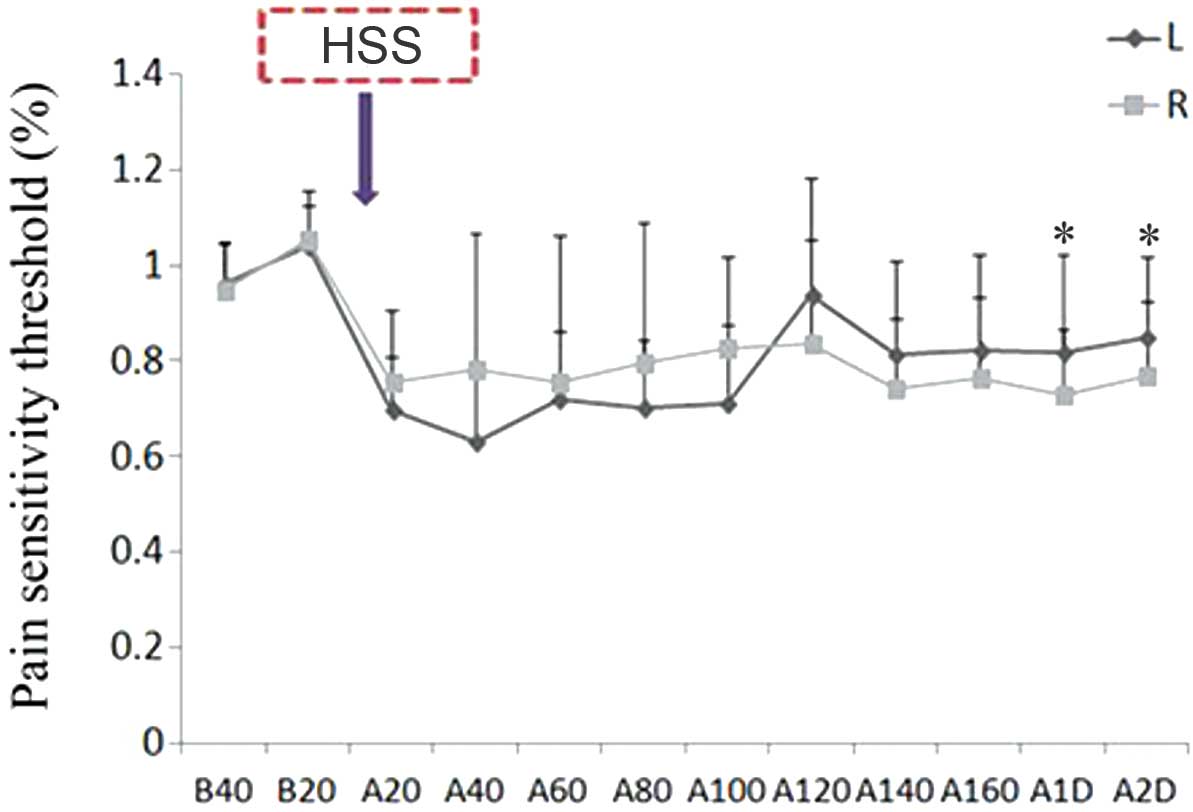 | Figure 1.HSS (5.8%) injection decreased the
mechanical pain threshold value of the bilateral hind legs of the
rats, and did not recover until 2 days post-injection (n=10). Left
leg: B20 vs. A20-A100, *P<0.01; B20 vs. A140-A2D, *P<0.05.
Right leg: B20 vs. A20-A80 and A14-A2D, *P<0.01; B20 vs. A100
and A120, *P<0.05. L, left leg; R, right leg; B, before HSS
injection; A, after HSS injection; HSS, hypertonic saline
solution. |
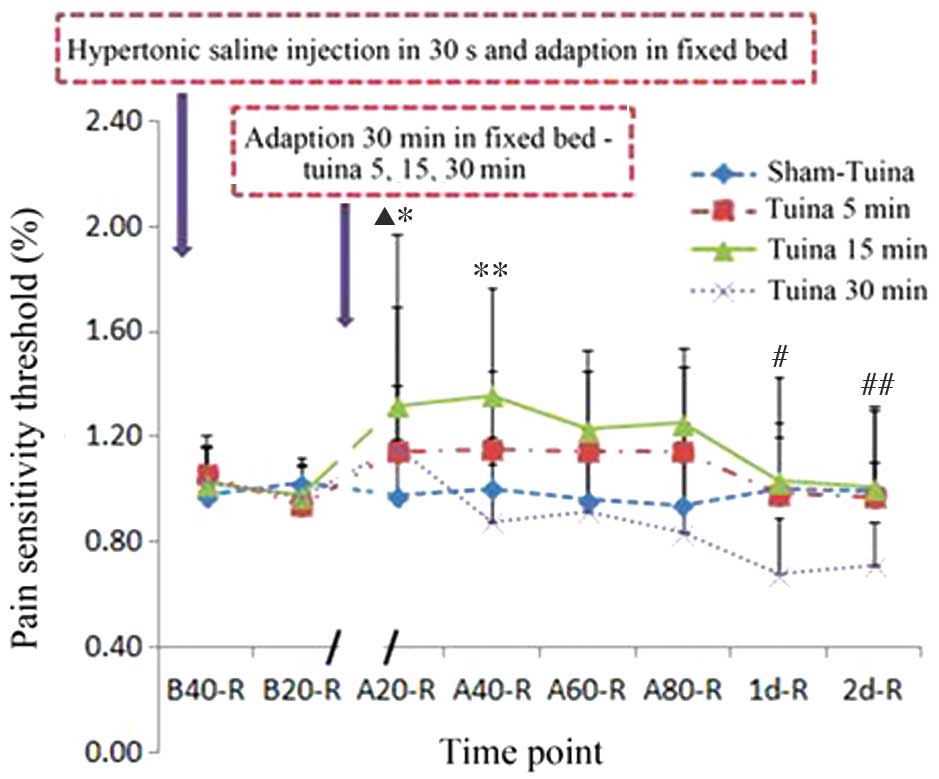
Tuina massage for 15 min increases
mechanical pain thresholds in the right gastrocnemius muscle
following injection of 5.8% HSS into the left gastrocnemius
muscle
In order to investigate whether Tuina affected the
mechanical pain sensitivity and threshold values of contralateral
muscles following HSS injection, a 15 min Tuina massage was
performed on the right gastrocnemius muscles of the treated rats.
Tuina treatment for 15 min significantly increased the mechanical
pain thresholds in the right muscle at 20 and 40 min following
Tuina massage, as compared with the non-massaged control group
rats; however, the elevated thresholds reached a plateau at 1 day
post-Tuina massage. In the 30 min Tuina massage group with high
hand pressure, the mechanical pain threshold of the right muscle
increased within 20 min of treatment; however, it had decreased at
1–2 days post-Tuina massage, as compared with the A20 group
(Table I and Fig. 3).
 | Table I.Mechanical pain threshold in the
ipsilateral leg following Tuina massage (mean% ± SE). |
Table I.
Mechanical pain threshold in the
ipsilateral leg following Tuina massage (mean% ± SE).
| Group | Non-Tuina | Tuina 5 min | Tuina 15 min | Tuina 30 min |
|---|
| N | 10 | 10 | 10 | 10 |
| B40 | 0.98±0.06 | 1.06±0.14 | 1.02±0.14 | 1.03±0.13 |
| B20 | 1.02±0.06 | 0.94±0.14 | 0.98±0.14 | 0.97±0.13 |
| A20 | 0.98±0.21 | 1.14±0.25 |
1.32±0.38a |
1.16±0.82b |
| A40 | 1.00±0.19 | 1.15±0.30 |
1.36±0.41c | 0.88±0.22 |
| A60 | 0.96±0.22 | 1.14±0.31 | 1.23±0.30 | 0.92±0.21 |
| A80 | 0.94±0.23 | 1.14±0.32 | 1.25±0.29 | 0.83±0.38 |
| A1D | 1.00±0.20 | 0.98±0.27 |
1.03±0.39d | 0.68±0.22 |
| A2D | 1.00±0.30 | 0.97±0.13 |
1.01±0.31e | 0.71±0.17 |
Tuina massage of the right
gastrocnemius muscle for 15 min increases the mechanical pain
threshold of the left gastrocnemius muscle in HSS-injected
rats
In order to investigate whether Tuina massage of the
right gastrocnemius muscle was able to affect the pain sensitivity
and threshold of the left contralateral muscle, 15 min Tuina was
performed on the right gastrocnemius muscles of HSS-treated rats
and the pain sensitivity of the left gastrocnemius muscles were
measured. An increased pain threshold was detected for the left
muscle at 80 min post-Tuina, and was shown to plateau at 1 day
post-Tuina, as compared with the non-Tuina control groups.
Similarly, 30 min Tuina massage was associated with an increased
pain threshold at 20 min post-Tuina (Table II and Fig. 4).
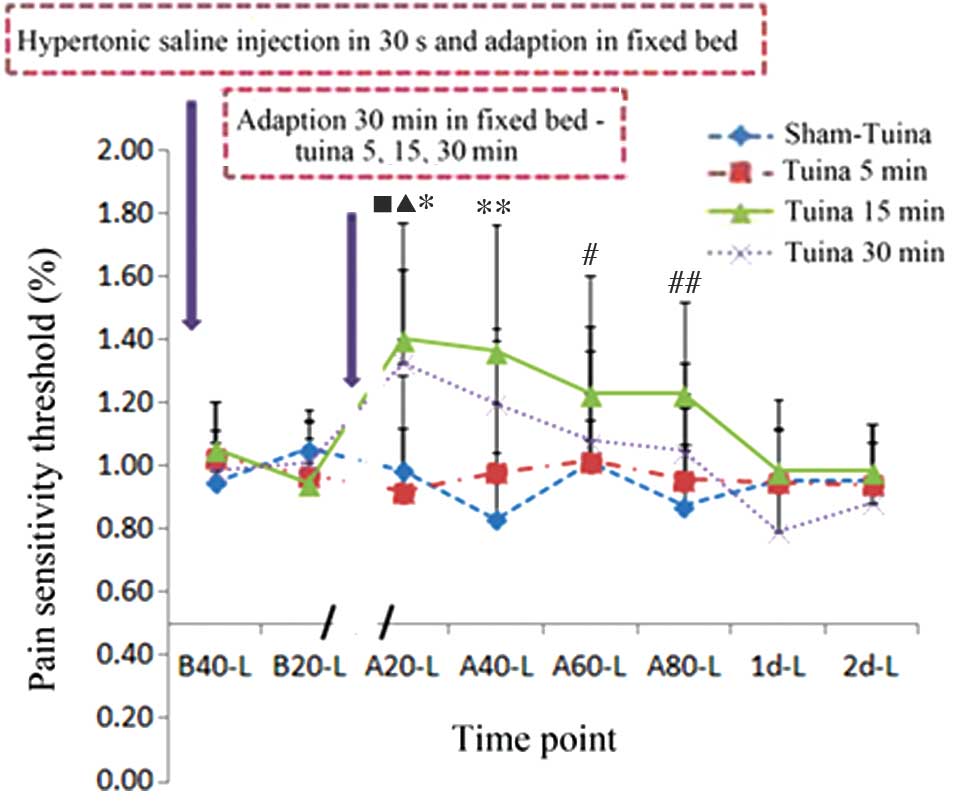 | Figure 4.Tuina massage affects the mechanical
pain threshold of the contralateral leg. Hypertonic saline solution
(5.8%) injection decreased the left leg pain threshold, which was
reversed following adaptation in a fixed bed and Tuina massage
using high hand pressure. Tuina 15 min group: *P<0.001, B20 vs.
A20; **P<0.001, B20 vs. A40; #P<0.05, B20 vs. A60;
##P<0.05, B20 vs. A80. Tuina 30 min group:
▲P<0.05, B20 vs. A20; nP<0.05, B40 vs.
A20. B, before-Tuina; A, after-Tuina; L, left leg. |
 | Table II.Mechanical pain threshold in the
contralateral leg following Tuina massage (mean% ± SE). |
Table II.
Mechanical pain threshold in the
contralateral leg following Tuina massage (mean% ± SE).
| Group | Non-Tuina | Tuina 5 min | Tuina 15 min | Tuina 30 min |
|---|
| N | 10 | 10 | 10 | 10 |
| B40 | 0.95±0.13 | 1.03±0.18 | 1.06±0.15 | 0.99±0.12 |
| B20 | 1.05±0.13 | 0.97±0.18 | 0.94±0.15 | 1.01±0.12 |
| A20 | 0.99±0.14 | 0.92±0.37 |
1.40±0.37a |
1.33±0.30b,c |
| A40 | 0.83±0.21 | 0.98±0.42 |
1.36±0.40d | 1.20±0.24 |
| A60 | 1.01±0.13 | 1.01±0.42 |
1.23±0.38e | 1.08±0.29 |
| A80 | 0.87±0.19 | 0.96±0.37 |
1.23±0.29f | 1.05±0.14 |
| A1D | 0.96±0.16 | 0.95±0.17 | 0.98±0.22 | 0.79±0.16 |
| A2D | 0.96±0.12 | 0.94±0.19 | 0.98±0.15 | 0.89±0.25 |
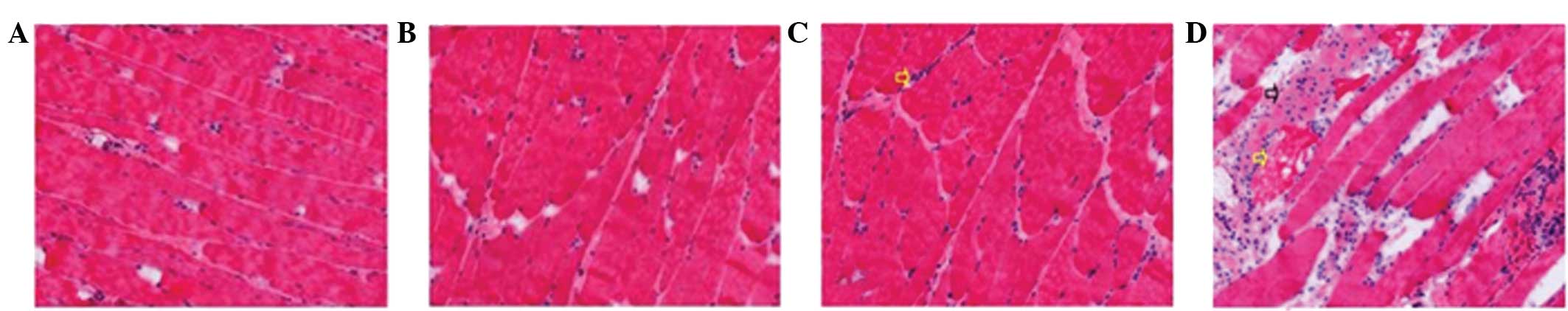
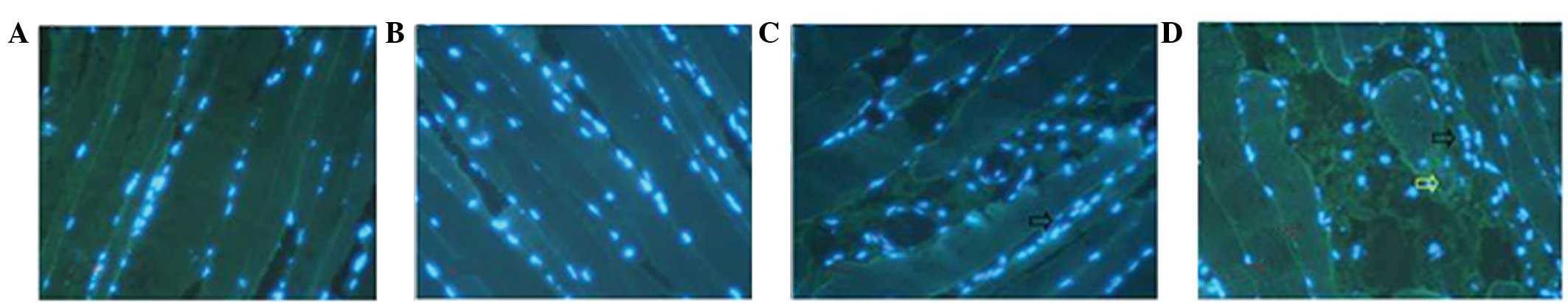
Muscle damage was not detected following Tuina
massage for 5 and 15 min; however, Tuina massage performed for 30
min was shown to induce moderate damage to the treated muscles. In
order to investigate whether Tuina massage caused damage to the
gastrocnemius muscles, the muscles were removed immediately
following Tuina massage treatments for histological and
immunohistochemical analyses. There were no detectable histological
alterations in the Tuina 15 and 20 min groups, as compared with the
non-Tuina controls (Figs. 5A–C and
6A–C). However, in the 30 min Tuina
group, abnormal muscle fibers, minor swelling and necrotic areas
were observed.
Tuina increases SOD content, but not
MDA content, in the bilateral gastrocnemius muscles
In order to investigate whether damaged muscle
tissues post-Tuina were associated with altered SOD and MDA protein
expression levels, SOD and MDA concentrations were measured in the
bilateral gastrocnemius muscles. Tuina massage for 15 and 30 min
significantly increased the levels of SOD, but not MDA, in the
bilateral gastrocnemius muscles, as compared with the ipsilateral
muscle tissue in the non-Tuina group; thus suggesting that Tuina
massage may induce moderate biochemical alterations (Table III).
 | Table III.SOD and MDA content in bilateral
gastrocnemius muscles. |
Table III.
SOD and MDA content in bilateral
gastrocnemius muscles.
| Group | Non-Tuina | Tuina 5 min | Tuina 15 min | Tuina 30 min |
|---|
| N |
|
|
|
|
|
Left | 8 | 8 | 7 | 8 |
|
Right | 8 | 8 | 8 | 8 |
| SOD (U/mg
prot) |
|
|
|
|
|
Left | 0.68±0.08 | 1.03±0.19 |
2.13±0.22a |
1.81±0.43b |
|
Right | 0.59±0.11 | 0.79±0.13 | 1.48±0.22 |
2.11±0.53c |
| MDA (nmol/mg
prot) |
|
|
|
|
|
Left | 0.64±0.08 | 0.57±0.07 | 1.18±0.32 | 0.83±0.13 |
|
Right | 0.79±0.06 | 0.61±0.07 | 0.68±0.05 | 0.70±0.04 |
Tuina inhibits harmful spinal dorsal
horn C-fiber activity
C-fiber evoked field potentials in the superficial
spinal dorsal horn were recorded, and the results of the Tuina 15
min and non-Tuina control groups were compared. Local 5.8% HSS
injection into the left gastrocnemius muscle was found to be
associated with a decreased peak amplitude and AUC of the sciatic
nerves. However, subsequent to Tuina massage for 15 min, the peak
amplitude of the C-fiber discharge and the AUC of the ipsilateral
and contralateral muscles were significantly decreased, when
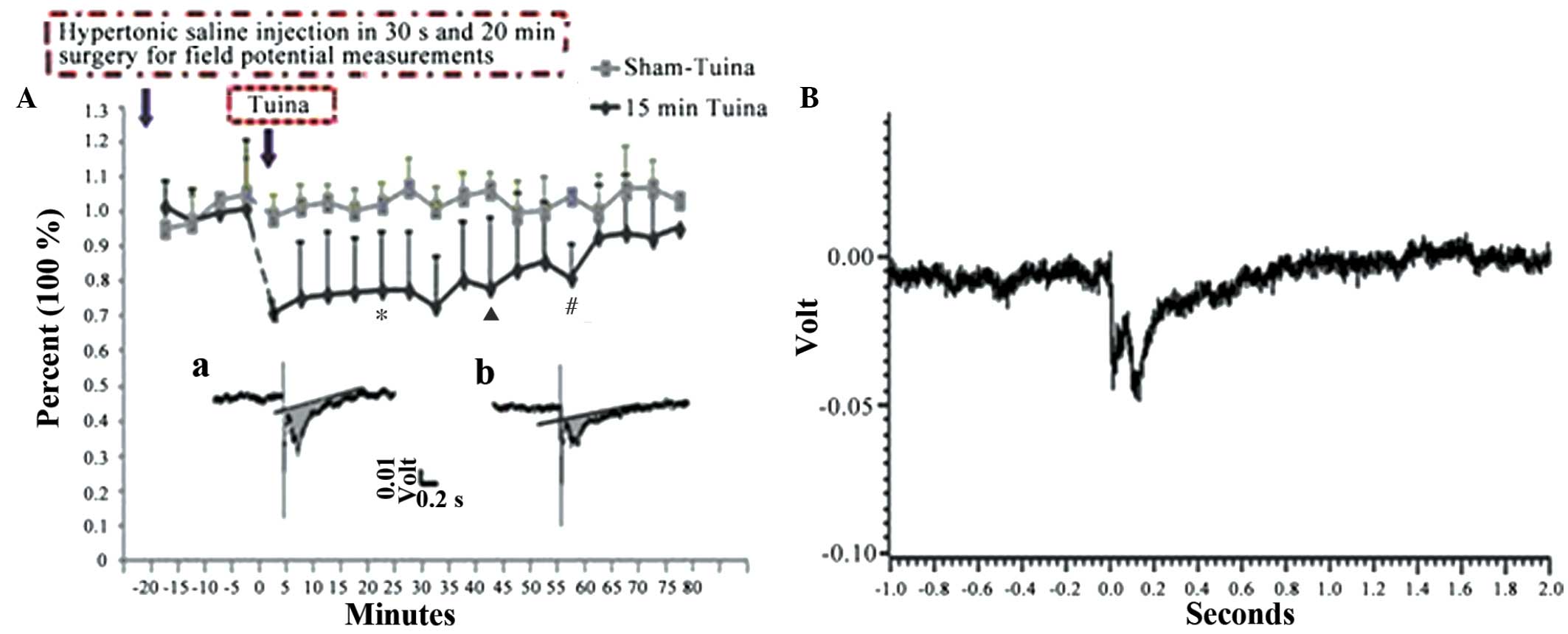
compared with those in the control group (P<0.001; Table IV and Fig. 7). The aforementioned results
suggested that Tuina is able to decrease the sensitivity of the
C-fibers in the sciatic nerves of the bilateral gastrocnemius
muscles. Similar results were detected for the other Tuina groups,
including the A20, A40, A60 and A80 groups.
 | Table IV.Spinal dorsal horn C-fiber-evoked
field potential in sciatic nerves following 5.8% HSS injection
(mean% ± standard error). |
Table IV.
Spinal dorsal horn C-fiber-evoked
field potential in sciatic nerves following 5.8% HSS injection
(mean% ± standard error).
| Group | Non-Tuina | Tuina 15 min |
|---|
| N | 3 | 8 |
| B20 | 0.95±0.06 | 1.02±0.10 |
| A20 | 1.00±0.05 |
0.77±0.18a |
| A40 | 1.04±0.06 |
0.80±0.15b |
| A60 | 1.05±0.10 |
0.81±0.18c |
| A80 | 1.03±0.08 | 0.95±0.17 |
Discussion
Tuina massage, which has been used in China for
clinical purposes for >1,000 years, is an effective therapeutic
physical massage that provides pain relief and helps to alleviate
the symptoms of chronic inflammatory diseases (18). However, the underlying mechanism
remains obscure, due to the lack of experimental animal models. The
present study established a rat model of pain via intramuscular
injection of 5.8% HSS, in order to reduce mechanical pain
thresholds. This effect was reversed by 15 min Tuina massage
treatment of the ipsilateral and contralateral gastrocnemius
muscles. The results of the present study suggested that the spinal
dorsal horn C-fiber responses of the sciatic nerve were reduced
following Tuina massage, as demonstrated by a modified protocol for
recording C-fiber evoked field potentials (15,16). The
underlying physiological and biochemical mechanisms underlying
Tuina massage-induced analgesic effects are currently unknown.
However, a cross-talk between the skin and the underlying muscles
is provided by peripheral sensory systems. Impulses are transmitted
from skin sensors to the central nervous system via the spinal cord
and various nerve impulses are transmitted by distinct nerve
sensors and fibers. According to the gate-control theory, small
nerve fibers transmit pain signals and large nerve fibers transmit
normal signals to the brain (19).
However, both types of nerve fiber interact with projection cells,
which extend through the spinothalamic tract to the brain and to
inhibitory interneurons within the dorsal horn (19). Pain signals from the projection
neurons are transmitted to the brain upon the inactivation of
inhibitory neurons, which is thought to occur when small-fiber
stimulation is dominant. In the case of predominant long nerve
fiber signaling, activated inhibitory neurons prevent the
projection neurons from sending signals to the brain (19).
The present study hypothesized that Tuina massage
may induce long nerve fiber signaling and thereby inhibit pain
signaling to the central nervous system by the activation of
inhibitory neurons. Low frequency electroacupuncture has previously
been shown to reduce C-fiber-evoked nerve volleys in the dorsal
horn following sciatic nerve stimulation. However, this effect was
inhibited by the opioid receptor antagonist naloxone; thus
suggesting that endogenous opioids may also have a role in
modifying pain signals (20). Future
studies should investigate the underlying mechanisms of Tuina
massage modified pain signaling.
In conclusion, the present study demonstrated an
analgesic effect for middle point gastrocnemius muscle Tuina
massage in a rat model of pain. The optimal application mode was 2
Hz for 15 min, consisting of 2 min cycles and 1 min intervals
between cycles. No alterations were detected in the gastrocnemius
muscle morphology following a 15 min Tuina massage. The pain relief
effects of Tuina were associated with elevated pain thresholds and
reduced AUC of C-fiber-evoked field potentials of the ipsilateral
and contralateral nerves.
Acknowledgements
The present study was supported by grants from the
National Science Foundation for Distinguished Young Scholars (grant
nos. 81025022 and 81503673), the budgetary funds of the Project of
Shanghai University of Traditional Chinese Medicine (grant no.
2013JW30), the ‘Sailing program’ of the Shanghai Science and
Technology Committee (grant no. 14YF1411700), Research Projects in
the Industry of Traditional Chinese Medicine (grant no.
2015468003-1) and Research Projects from Shanghai Municipal
Commission of Health and Family Planning (grant no.
20154Y0175).
References
|
1
|
Dubin AE and Patapoutian A: Nociceptors:
The sensors of the pain pathway. J Clin Invest. 120:3760–3772.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Norrsell U, Finger S and Lajonchere C:
Cutaneous sensory spots and the ‘law of specific nerve energies’:
History and development of ideas. Brain Res Bull. 48:457–465. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sinclair DC: Cutaneous sensation and the
doctrine of specific energy. Brain. 78:584–614. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moayedi M and Davis KD: Theories of pain:
From specificity to gate control. J Neurophysiol. 109:5–12. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Melzack R and Wall PD: Pain mechanisms: A
new theory. Science. 150:971–979. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
YU TY: Science of Chinese massage. Chinese
Medicine Press. 88–95. 2013.
|
|
7
|
Cherkin DC, Sherman KJ, Kahn J, Wellman R,
Cook AJ, Johnson E, Erro J, Delaney K and Deyo RA: A comparison of
the effects of 2 types of massage and usual care on chronic low
back pain: A randomized, controlled trial. Ann Intern Med. 155:1–9.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Astin JA and Ernst E: The effectiveness of
spinal manipulation for the treatment of headache disorders: A
systematic review of randomized clinical trials. Cephalalgia.
22:617–623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Degirmen N, Ozerdogan N, Sayiner D,
Kosgeroglu N and Ayranci U: Effectiveness of foot and hand massage
in postcesarean pain control in a group of Turkish pregnant women.
Appl Nurs Res. 23:153–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitchinson AR, Kim HM, Rosenberg JM,
Geisser M, Kirsh M, Cikrit D and Hinshaw DB: Acute postoperative
pain management using massage as an adjuvant therapy: A randomized
trial. Arch Surg. 142:1158–1167; discussion 1167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piotrowski MM, Paterson C, Mitchinson A,
Kim HM, Kirsh M and Hinshaw DB: Massage as adjuvant therapy in the
management of acute postoperative pain: A preliminary study in men.
J Am Coll Surg. 197:1037–1046. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sherman KJ, Cherkin DC, Hawkes RJ,
Miglioretti DL and Deyo RA: Randomized trial of therapeutic massage
for chronic neck pain. Clin J Pain. 25:233–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katz B: Depolarization of sensory
terminals and the initiation of impulses in the muscle spindle. J
Physiol. 111:261–282. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao ZQ: Neural mechanism underlying
acupuncture analgesia. Prog Neurobiol. 85:355–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schouenborg J and Weng HR: Sensorimotor
transformation in a spinal motor system. Exp Brain Res.
100:170–174. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferreira SH, Lorenzetti BB and Corrêa FM:
Central and peripheral antialgesic action of aspirin-like drugs.
Eur J Pharmacol. 53:39–48. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Randall LO and Selitto JJ: A method for
measurement of analgesic activity on inflamed tissue. Arch Int
Pharmacodyn Ther. 111:409–419. 1957.PubMed/NCBI
|
|
18
|
Ilic D, Djurovic A, Brdareski Z,
Vukomanovic A, Pejovic V and Grajic M: The position of chinese
massage (Tuina) in clinical medicine. Vojnosanit Pregl.
69:999–1004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melzack R and Wall PD: Pain mechanisms: A
new theory. Science. 150:971–979. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xing GG, Liu FY, Qu XX, Han JS and Wan Y:
Long-term synaptic plasticity in the spinal dorsal horn and its
modulation by electroacupuncture in rats with neuropathic pain. Exp
Neurol. 208:323–332. 2007. View Article : Google Scholar : PubMed/NCBI
|