Introduction
In normal human blood, every mm3 contains
10–30×104 platelets, the average lifespan of which is
8–10 days (1). If the platelet count
decreases to <10×104 mm3, the patient is
diagnosed with thrombocytopenia (2).
Thrombocytopenia, if severe, may cause symptoms, such as mucosal
bleeding from the nose, mouth and gastrointestinal tract (3). Tazobactam and piperacillin (TZP) are
antibiotics that are used to treat the majority of infections
caused by β-lactamase-producing bacteria (4,5). Common
adverse reactions associated with TZP treatment include
neutropenia, leukopenia and thrombocytopenia, urticaria, allergic
shock, exfoliative dermatitis, and adverse reactions of the nervous
system (6–8). These adverse reactions typically occur
simultaneously and thrombocytopenia rarely manifests independently
of other symptoms (9). The patients
with thrombocytopenia associated with TZP may report severe
bleeding at a number of locations, including gastrointestinal tract
bleeding, a cerebral hemorrhage or subcutaneous bleeding (10) The mechanism by which TZP causes
thrombocytopenia is unclear, however, drug-induced thrombocytopenia
is typically hypothesized to have three possible underlying
mechanisms; these are immune-mediated, direct platelet number
decreases and bone marrow suppression (11). The present study investigated the
occurrence of thrombocytopenia in a single patient treated with
TZP, as well as its clinical features, in order to investigate
potential underlying mechanisms.
Case report
A 76-year-old male patient with a 20-year history of
hypertension was admitted to the intensive care unit (ICU) of
Ningbo First Hospital (Ningbo, China) in February 2013 complaining
of dizziness, vomiting and slurred speech. Informed consent was
obtained from the patient, after which blood samples were taken and
a clinical evaluation was conducted. A computed tomography scan of
the brain and lungs suggested that the patient was suffering from a
cerebral infarction and pneumonia. The patient was treated with
aspirin, in order to reduce the levels of platelets, with
nifedipine, in order to control blood pressure and improve cerebral
circulation, and with TZP (dose, 4.5 g; administered every 8 h
intravenously), starting from the 3rd day following admission.
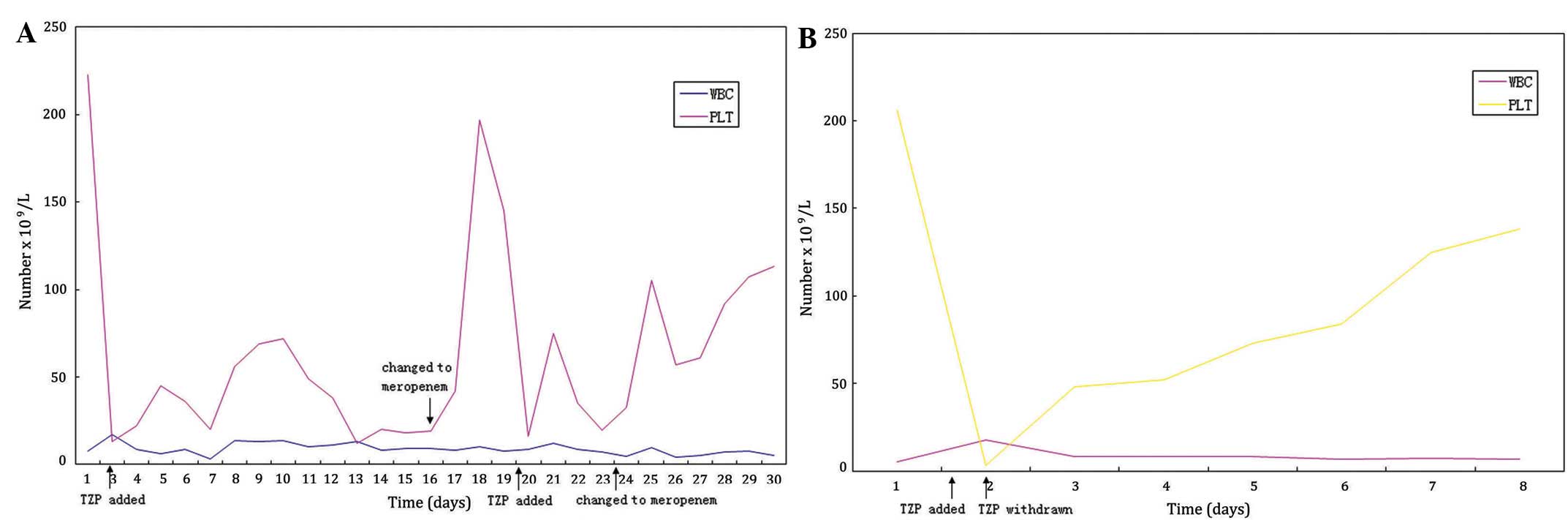
However, the platelet levels of the patient rapidly dropped to
13×109platelets/l, but the size of the liver and spleen
were deemed normal, determined using B-scan ultrasound examination.
In order to ameliorate the platelet deficit, aspirin treatment was
terminated, and the patient was administered 10 units infused
platelets, 40 mg methylprednisolone and 5 g gamma globulin once
daily, for 2 weeks. However, the platelet levels remained low and,
on the 16th day following admission, TZP treatment was substituted
with meropenem treatment (dose, 1.0 g; administered every 8 h
intravenously) in order to restore platelets to normal levels
(Table I and Fig. 1A). Over the next 2 days, the platelet
count of the patient increased from 19×109 to
19.7×1010 platelets/l. On the 20th day following
admission, the TZP treatment regimen was restored, inducing the
platelet count of the patient to decrease to 16.2×109
platelets/l; substitution of TZP with meropenem on the 23rd day
following admission again caused the platelet count to return to
normal (10.5×1010 platelets/l) over a period of 2 days.
The preliminary diagnosis of the patient was idiopathic
thrombocytopenic purpura.
 | Table I.WBC and PLT levels altered over time
during TZP and meropenem treatment. |
Table I.
WBC and PLT levels altered over time
during TZP and meropenem treatment.
| Day | WBC
(109/l) | PLT
(109/l) |
|---|
| 1 | 7.6 | 223 |
| 3 | 16.8 |
13 |
| 4 | 8.4 |
22 |
| 5 | 6.1 |
45 |
| 6 | 8.7 |
36 |
| 7 | 3.1 |
20 |
| 8 | 13.4 |
56 |
| 9 | 13.1 |
69 |
| 10 | 13.5 |
72 |
| 11 | 10 |
49 |
| 12 | 10.9 |
38 |
| 13 | 12.9 |
12 |
| 14 |
8 |
20 |
| 15 |
9 |
18 |
| 16 | 9.3 |
19 |
| 17 | 7.9 |
42 |
| 18 | 10.3 | 197 |
| 19 | 7.7 | 145 |
| 20 | 8.4 | 16.2 |
| 21 | 11.9 |
75 |
| 22 | 8.4 |
35 |
| 23 |
7 | 19.6 |
| 24 | 4.6 | 32.6 |
| 25 | 9.7 | 105 |
| 26 | 4.1 |
57 |
| 27 | 5.1 |
61 |
| 28 |
7 |
92 |
| 29 | 7.4 | 107 |
A laboratory examination demonstrated that the
erythrocyte sedimentation rate (ESR; 37 mm/h) and the levels of
immunoglobulin (Ig) G (2,260 mg/dl) were increased; however, IgA,
IgM, complement C3, complement C4, streptolysin and rheumatoid
factors, anti-cardiolipin (ACA), antinuclear, anti-Smith,
anti-U1-nuclear ribonucleoprotein,
anti-Ro/Sjögren's-syndrome-related antigen A, anti-Ro-52,
anti-La/Sjögren's-syndrome-related antigen B, anti-Scl-70,
anti-Jo-1, anti-dsDNA, anti-nucleosome, anti-proliferating cell
nuclear antigen, anti-mitochondrial-M2, anti-histone, anti-PM-Scl,
anti-ribosomal P-protein, anti-keratin and anti-neutrophil
cytoplasmic antibodies were normal (Table II), determined using
immunoturbidimetry, in accordance with a previous study (12). IgG levels were detected by
immunoturbidimetry with an IMMAGE 800 system (Beckman Coulter,
Inc., Brea, CA, USA). A bone marrow smear examination detected no
obvious abnormalities. Additional assays were conducted as follows:
ACA and anti-neutrophil antibodies were detected using western
blotting (data not shown); anti-hepatitis C and anti human
immunodeficiency virus using an enzyme-linked immunosorbent assay
(ELISA; Zhuhai Livzon Diagnostics, Inc., Zhuhai, China); and
platelet antibodies using a solid-phase antiglobulin test using an
immunity micro column incubator (Changchun Boyan Technology
Instrument Co., Ltd., Changchun, China). Assays for ACA,
anti-neutrophil, anti-hepatitis C virus, anti-human
immunodeficiency virus and plasma platelet antibodies were negative
(13). Furthermore, the thyroid
function of the patient was normal, and the liver and spleen sizes
were demonstrated to be normal via an abdominal B ultrasound. The
patient was discharged from the hospital after 41 days of
treatment.
 | Table II.Laboratory examination of the
patient. |
Table II.
Laboratory examination of the
patient.
| Variable | Result | Change | Normal range | Unit |
|---|
| ESR | 37 | + | <15 | mm/h |
| IgG | 2,260 | + | 726–1,685 | mg/dl |
| IgA | 158 | n | 69–382 | mg/dl |
| IgM | 67 | n | 63–277 | mg/dl |
| C3 | 70.4 | − | 85–193 | mg/dl |
| C4 | 12.1 | n | 12–36 | mg/dl |
| ASO | 70.9 | n | 0–200 | IU/ml |
| RF |
<20 | n | 0–30 | IU/ml |
| ACA | N | n | N | N/A |
| ANCA | N | n | N | N/A |
| ANA | N | n | N | N/A |
| Sm-Ab | N | n | N | N/A |
| U1-Nrnp-Ab | N | n | N |
N/A |
| Anti-SSA | N | n | N |
N/A |
| Ro-52-Ab | N | n | N |
N/A |
| Anti-SSB | N | n | N |
N/A |
| Scl-70-Ab | N | n | N |
N/A |
| Jo-1-Ab | N | n | N |
N/A |
| dsDNA-Ab | N | n | N |
N/A |
| AnuA-Ab | N | n | N |
N/A |
| PCNA-Ab | N | n | N |
N/A |
| AMA-M2 | N | n | N |
N/A |
| AHA-Ab | N | n | N |
N/A |
| PM-Scl-Ab | N | n | N |
N/A |
| r-Prot-Ab | N | n | N |
N/A |
| AKA | N | n | N |
N/A |
| anti-PLT | N | n | N |
N/A |
In December 2013, the same patient was
re-hospitalized complaining of an inability to swallow, pulmonary
aspiration and a high temperature. The patient was diagnosed with
aspiration pneumonia and was treated with 4.5 g TZP every 8 h, in
order to eliminate the infection; however, on the same day, the
levels of platelets were markedly reduced to 3×109
platelets/l, as determined via manual counting. Conversely,
substitution of TZP treatment with cefoperazone (dose, 2.0 g;
administered every 8 h intravenously) resulted in the platelet
levels returning to normal after 5 days, suggesting that there was
an association between TZP and the occurrence of thrombocytopenia.
Therefore, the initial diagnosis of idiopathic thrombocytopenic
purpura was revised to drug-induced thrombocytopenia (Table III and Fig. 1B).
 | Table III.WBC and PLT levels altered over time
following the second TZP treatment. |
Table III.
WBC and PLT levels altered over time
following the second TZP treatment.
| Day | WBC
(x109/l) | PLT
(x109/l) |
|---|
| 1 |
5.4 |
206 |
| 4 | 17.6 |
3 |
| 5 |
8.2 | 48 |
| 6 |
8 | 52 |
| 7 |
8 | 73 |
| 8 |
6.7 | 84 |
| 9 |
7.1 |
125 |
| 10 |
6.5 |
138 |
Discussion
TZP treatment is commonly used in the ICU; however,
TZP-induced thrombocytopenia is not commonly reported. In the
present study, an elderly patient admitted to the ICU presented
with thrombocytopenia, which was associated with TZP treatment.
Clinical laboratory results demonstrated that the TZP-induced
thrombocytopenia was abrupt (12 h later it had markedly decreased)
and reversible, as platelet numbers were normal within 3–5 days
following withdrawal of TZP. Although the platelet count decreased
following treatment with methylprednisolone and gamma globulin, the
platelet number remained particularly low for 1 month. However,
subsequent to withdrawal of TZP treatment, the platelet level
increased, confirming the hypothesis that thrombocytopenia was
induced by TZP.
The main reason that doctors may ignore the
association between TZP treatment and platelet reduction is that it
has only rarely been reported in the literature (10,14,15). The
present study suggested that doctors should be aware of the risks
of TZP-induced thrombocytopenia, and that TZP treatment should be
discontinued following detection of drug-induced
thrombocytopenia.
The mechanism underlying TZP-induced
thrombocytopenia is currently unclear. Previous studies have
suggested that drug-induced thrombocytopenia may occur due to
drug-induced suppression of the bone marrow (16,17).
Conversely, other studies have suggested that TZP, which is a
500–1,000 Da drug, may associate with the platelet membrane
antigen, stimulating the body to produce antibodies against the
TZP-platelet complex; this, in turn, may activate the complement
system in order to promote platelet destruction (18–20). In
the present study, the adverse effects of TZP were predominantly
associated with immune-mediated thrombocytopenia; however, the
molecular pathogenesis underlying TZP-induced thrombocytopenia has
yet to be elucidated and requires additional study.
Acknowledgements
The present study was supported by grants from the
Ningbo Natural Science Foundation of China (grant no. 2013A610234),
the Zhejiang Natural Science Foundation of China (grant no.
LQ15H150001) and the Chinese Medicine Research Program of Zhejiang
Province, China (grant no. 2015ZA185). The authors of the present
study would like to thank Dr Xiang Hou (Ningbo University, Ningbo,
China) for assistance in platelet quantification.
Glossary
Abbreviations
Abbreviations:
|
ESR
|
erythrocyte sedimentation rate
|
|
TZP
|
tazobactam and piperacillin
|
References
|
1
|
Bautista AP, Buckler PW, Towler HM, Dawson
AA and Bennett B: Measurement of platelet life-span in normal
subjects and patients with myeloproliferative disease with indium
oxine labeled platelets. Br J Haematol. 58:679–687. 1985.
View Article : Google Scholar
|
|
2
|
Smock KJ and Perkins SL: Thrombocytopenia:
An update. Int J Lab Hematol. 36:269–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Provan D, Stasi R, Newland AC, Blanchette
VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB,
Godeau B, et al: International consensus report on the
investigation and management of primary immune thrombocytopenia.
Blood. 115:168–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gonçalves-Pereira J and Póvoa P:
Antibiotics in critically ill patients: A systematic review of the
pharmacokinetics of β-lactams. Crit Care. 15:R2062011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bourget P, Lesne-Hulin A, Le Reveillé R,
Le Bever H and Carsin H: Clinical pharmacokinetics of
piperacillin-tazobactam combination in patients with major burns
and signs of infection. Antimicrob Agents Chemother. 40:139–145.
1996.PubMed/NCBI
|
|
6
|
Finsterer J and Kotzailias N:
Thrombocytosis under ciprofloxacin and tazobactam/piperacillin.
Platelets. 14:329–331. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reichardt P, Handrick W, Linke A, Schille
R and Kiess W: Leukocytopenia, thrombocytopenia and fever related
to piperacillin/tazobactam treatment - a retrospective analysis in
38 children with cystic fibrosis. Infection. 27:355–356. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anand A and Chauhan HK: Piperacillin and
vancomycin induced severe thrombocytopenia in a hospitalized
patient. Platelets. 22:294–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Macwilliam JL, Mistry R, Floyd MS Jr and
Baird AD: Piperacillin/tazobactam induced thrombocytopaenia - a
delayed response. BMJ Case Rep. 2012:bcr03201259812012.PubMed/NCBI
|
|
10
|
Uzun G, Onem Y, Hatipoglu M, Turhan V,
Mutluoglu M and Ay H: Piperacillin/tazobactam-induced neutropenia,
thrombocytopenia, and fever during treatment of a diabetic foot
infection. Scand J Infect Dis. 45:73–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramot Y and Nyska A: Drug-Induced
Thrombosis - Experimental, clinical, and mechanistic
considerations. Toxicol Pathol. 35:208–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cines DB, Bussel JB, Liebman HA and Prak
Luning ET: The ITP syndrome: Pathogenic and clinical diversity.
Blood. 113:6511–6521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Li H, Zhao H, Ji L and Yang R:
Hepatitis C virus-related adult chronic idiopathic thrombocytopenic
purpura: Experience from a single Chinese centre. Eur J Haematol.
70:196–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Macwilliam JL, Mistry R, Floyd MS Jr and
Baird AD: Piperacillin/tazobactam-induced thrombocytopenia - a
delayed response. BMJ Case Rep. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shaik S, Kazi HA and Ender PT: Rapid-onset
piperacillin-tazobactam induced thrombocytopenia. J Pharm Pract.
28:204–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruiz-Irastorza G, Barreiro G and Aguirre
C: Reversible bone marrow depression by high-dose
piperacillin/tazobactam. Br J Haematol. 95:611–612. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar A, Choudhuri G and Aggarwal R:
Piperacillin induced bone marrow suppression: A case report. BMC
Clin Pharmacol. 3:22003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kelton JG, Meltzer D, Moore J, Giles AR,
Wilson WE, Barr R, Hirsh J, Neame PB, Powers PJ, Walker I, et al:
Drug-induced thrombocytopenia is associated with increased binding
of IgG to platelets both in vivo and in vitro. Blood. 58:524–529.
1981.PubMed/NCBI
|
|
19
|
Pérez-Vázquez A, Pastor JM and Riancho JA:
Immune thrombocytopenia caused by piperacillin/tazobactam. Clin
Infect Dis. 27:650–651. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grégoire C, Brumpt C, Loirat D, Lau N,
Bruel C, Philippart F, Couzigou C, Garrouste-Orgeas M and Misset B:
A case of daptomycin-induced immune thrombocytopenia. Antimicrob
Agents Chemother. 56:6430–6431. 2012. View Article : Google Scholar : PubMed/NCBI
|