Introduction
There has been an increasing interest regarding
natural orifice transluminal endoscopic surgery (NOTES) since
Kalloo et al reported transgastric peritoneoscopy in the
year 2004 (1). NOTES can be
performed with a flexible endoscope through the stomach, large
intestine, vagina or bladder into the abdominal cavity to diagnose
and treat intra-abdominal diseases (2). NOTES was initially developed as a way
to perform scarless surgery on the abdominal wall (3,4);
however, the procedure is technically challenging unless access
methods, retraction and closure methods, infection control and the
direction of endoscopy are improved (5).
To date, the field of minimally invasive surgery has
experienced enormous innovations, and future research is directed
toward decreasing abdominal pain, minimizing the risk of infection,
reducing the incidence of hernia formation and optimizing the
cosmetic effect associated with the surgery (6). Following the emergence of novel
technologies, including NOTES, transumbilical endoscopic surgery
(TUES) and single-port laparoscopy (SPL) (7,8), an
increasing level of attention has been paid to NOTES, representing
a major paradigm shift to scarless surgery (9,10).
However, NOTES is associated with a number of potential obstacles,
as instruments must be improved in order to obtain safe access to
peritoneal cavities, allow adequate exposure, ensure secure closure
methods, manage infection control and allow correct orientation
(11). The transumbilical approach
is favored, as it decreases the disadvantages associated with
NOTES; the transumbilical approach, using a flexible endoscope, is
a promising, single-incision approach (12).
Based on a number of laboratory animal studies, a
novel approach towards laparoscopic and endoscopic cooperative
surgery was performed in accordance with the protocol and criteria
formulated by the ASGE/SAGES Working Group (13). The present study reports a gastric
gastrointestinal stromal tumor (GIST) resection and cholecystectomy
that was successfully performed by adopting this novel
approach.
Case report
A 62-year-old man was admitted to the Provincial
Hospital Affiliated to Shandong Univeristy (Jinan, China) with a
complaint of acid regurgitation lasting for 8 months, and the
patient was diagnosed with cholecystic polypus by ultrasonography.
Gastroscopy revealed a submucosal tumor mass (~1.3×1.0 cm) with a
smooth surface located in the posterior wall of the lesser gastric
curvature of the lower gastric portion. Computed tomography and
ultrasonography inspection revealed a cholecystic polypus measuring
0.5×0.5 cm, which occupied the neck of the gallbladder. The medical
conditions which contraindicated a laparoscopic approach, such as
coagulopathy, severe dysrhythmias or chronic obstructive pulmonary
disease, were common exclusion criteria. This is because
pneumoperitoneum pressure has an impact on cardiopulmonary function
and the circulation system (14).
Therefore, an electrocardiogram, chest X-ray and laboratory test
are required for each inpatient, in order to identify whether a
patient can endure the operation. If the tests are abnormal, futher
examinations, such as heart doppler ultrasound, pulmonary function
tests and arterial blood gas analysis, are requested. At the same
time, consultations are held with cardiologists, respiratory
physicians or hematologists to assess the body function. Based on
these reviews, different treatment methods for patients are
adopted, for example drug therapy and aerosol inhalation to protect
and improve cardiopulmonary function. Regarding the current study,
the electrocardiogram, chest X-ray and other laboratory tests of
the patient presented no obvious abnormalities.
The protocol for the procedure was approved by the
ethical committee of the Provincial Hospital Affiliated to Shandong
University, and written informed consent was obtained from the
patient. Preoperative preparation and anesthesia were performed
using the same method adopted for conventional laparoscopic
cholecystectomy (15). A gastroscope
(EG2931; PENTAX Medical (Global), Tokyo, Japan), with a standard
hook knife (KD-620LR; Olympus Corporation, Tokyo, Japan) and a
trielcon, were used for endoscopic submucosal tumor resection.
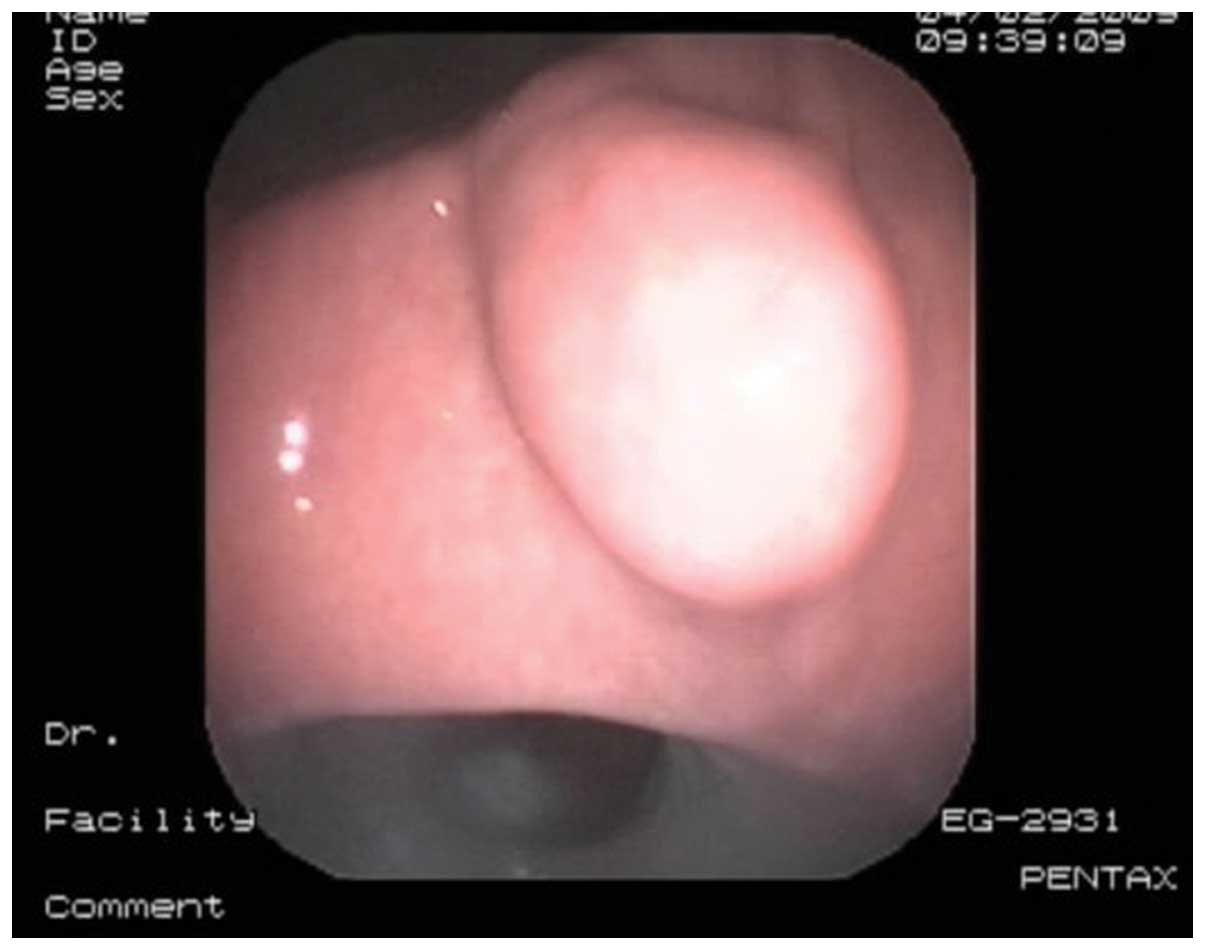
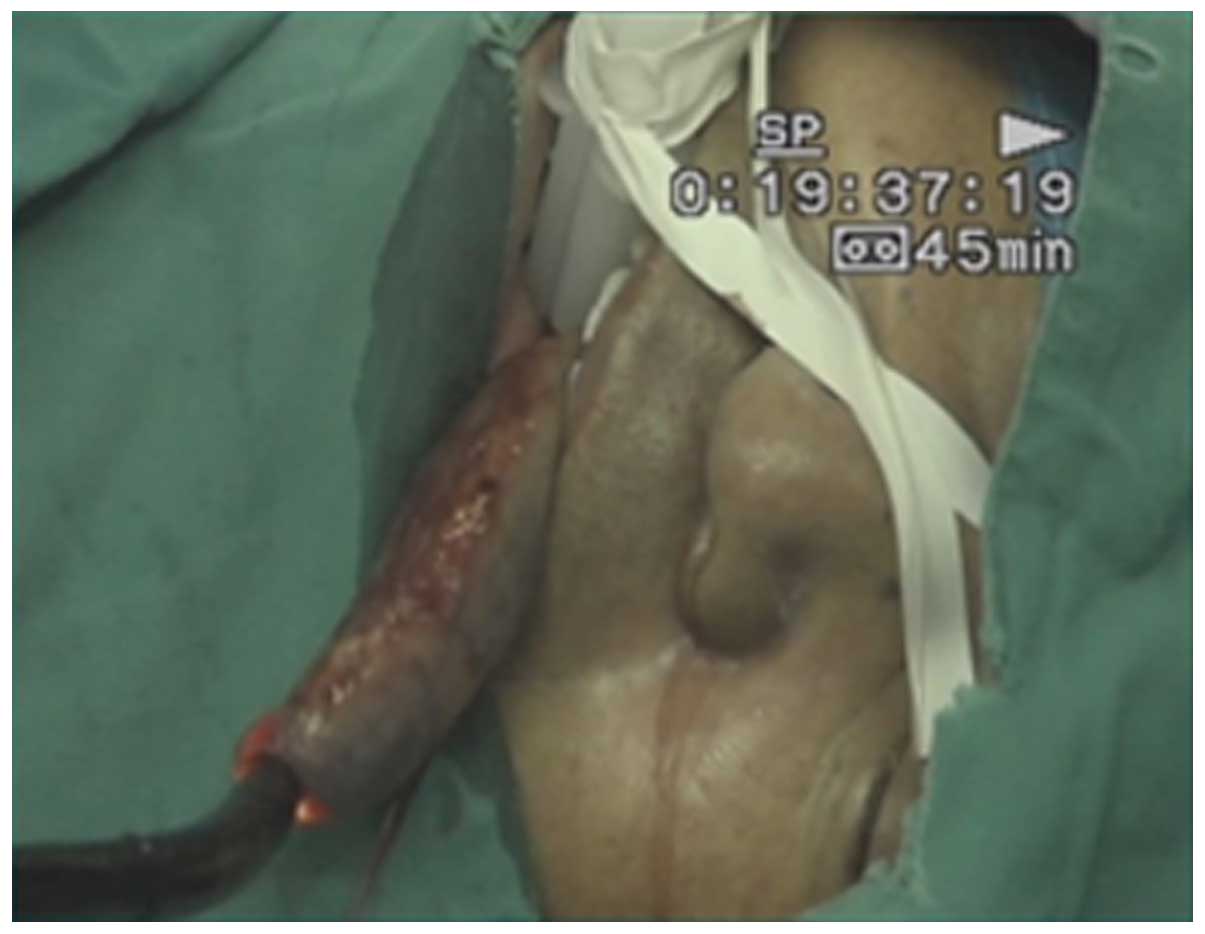
The tumor location was confirmed by gastroscopy
(Fig. 1) and ~2 cm around the
periphery of the tumor was marked (16). Adrenaline (dilution, 1:10,000 with
hypertonic saline; Shanghai Harvest Pharmaceutical Co., Ltd.,
Shanghai, China) was injected into the submucosal layer, and an
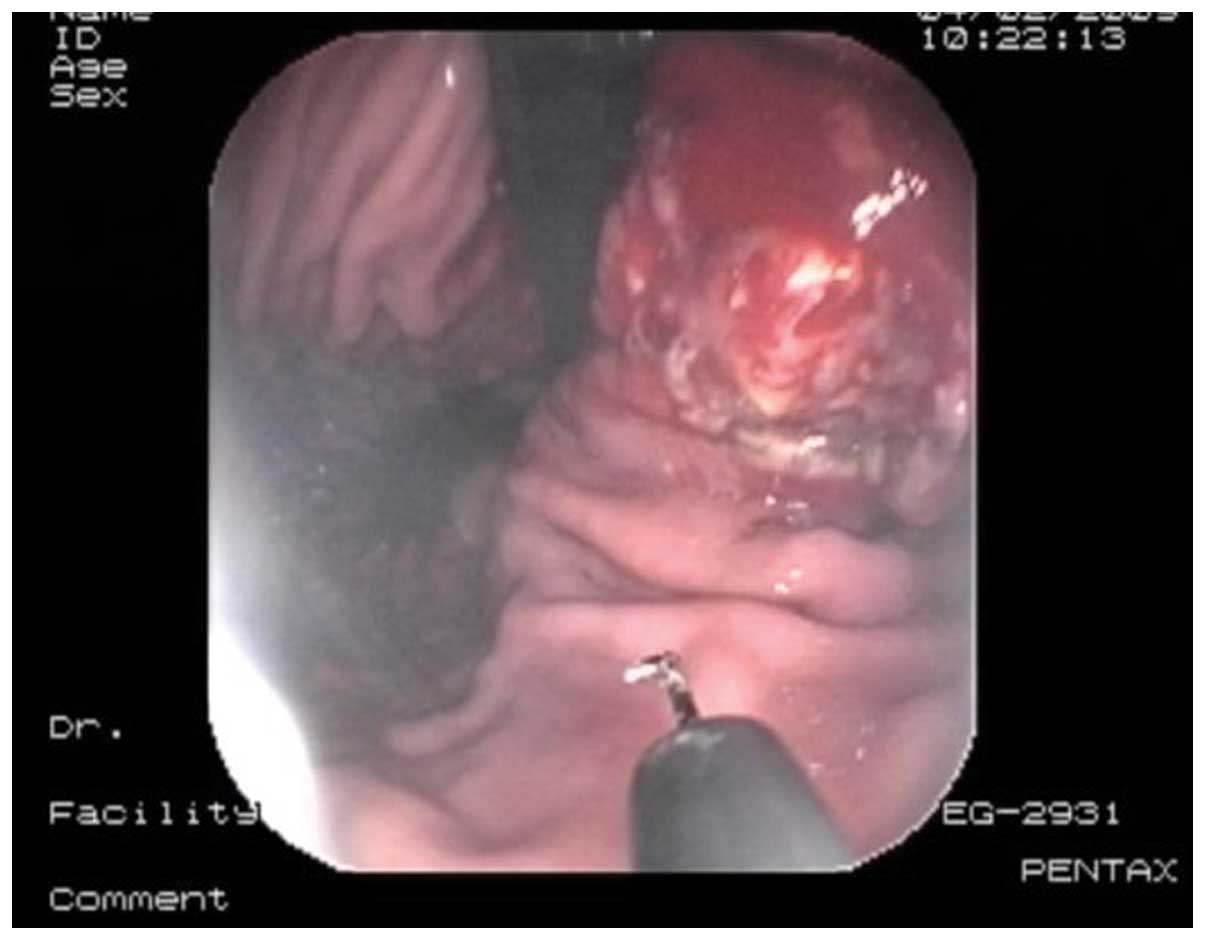
incision was made with a hook knife set at 100-W Endo-Cut mode. The
hook knife resected the tumor, and the marked area surrounded the
tumor was resected circumferentially (Fig. 2). Finally, the tumor was removed
using the trielcon through the peroral approach, and the endoscope
obtained access to abdominal cavity through the incision in the
stomach (Fig. 3).
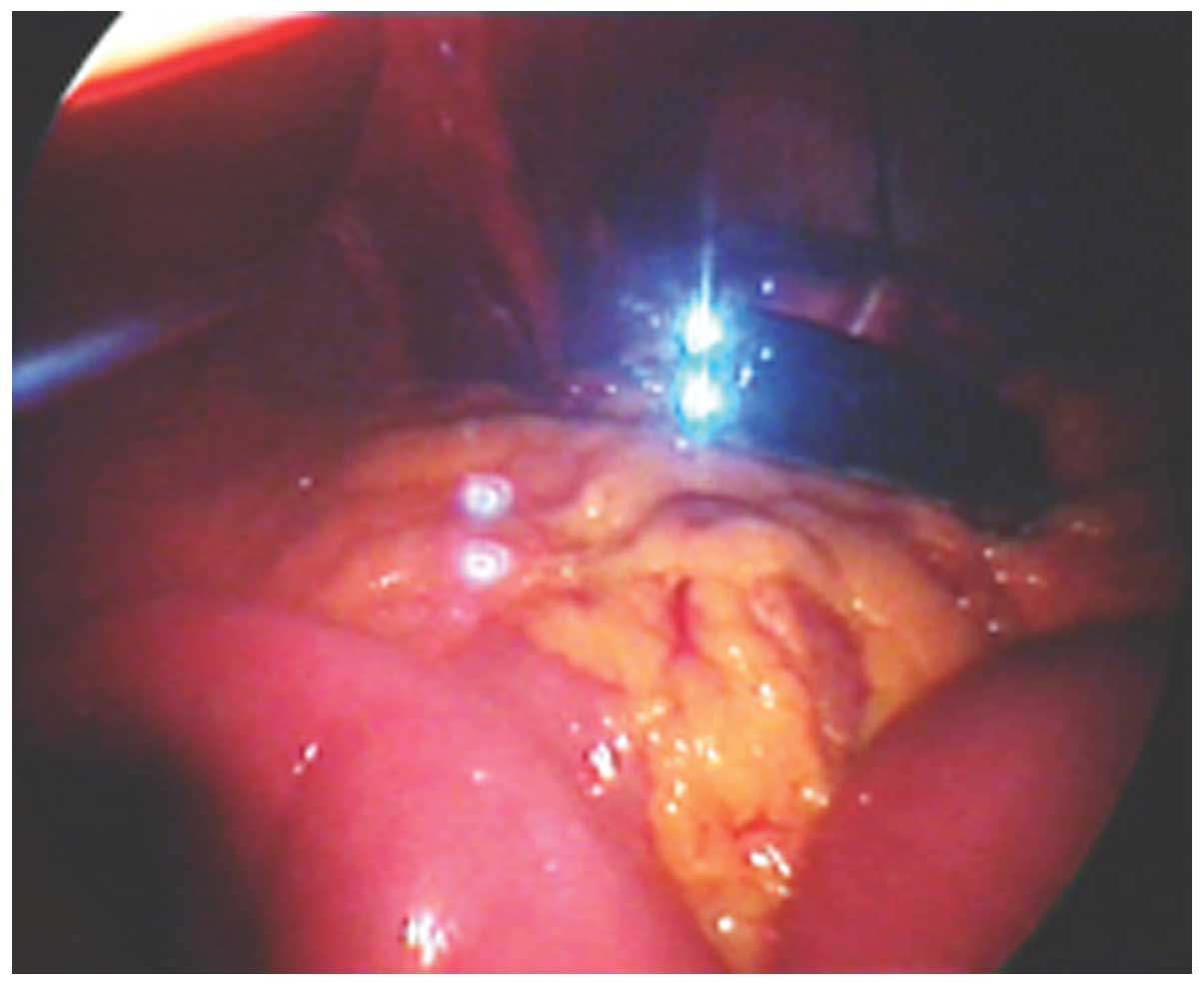
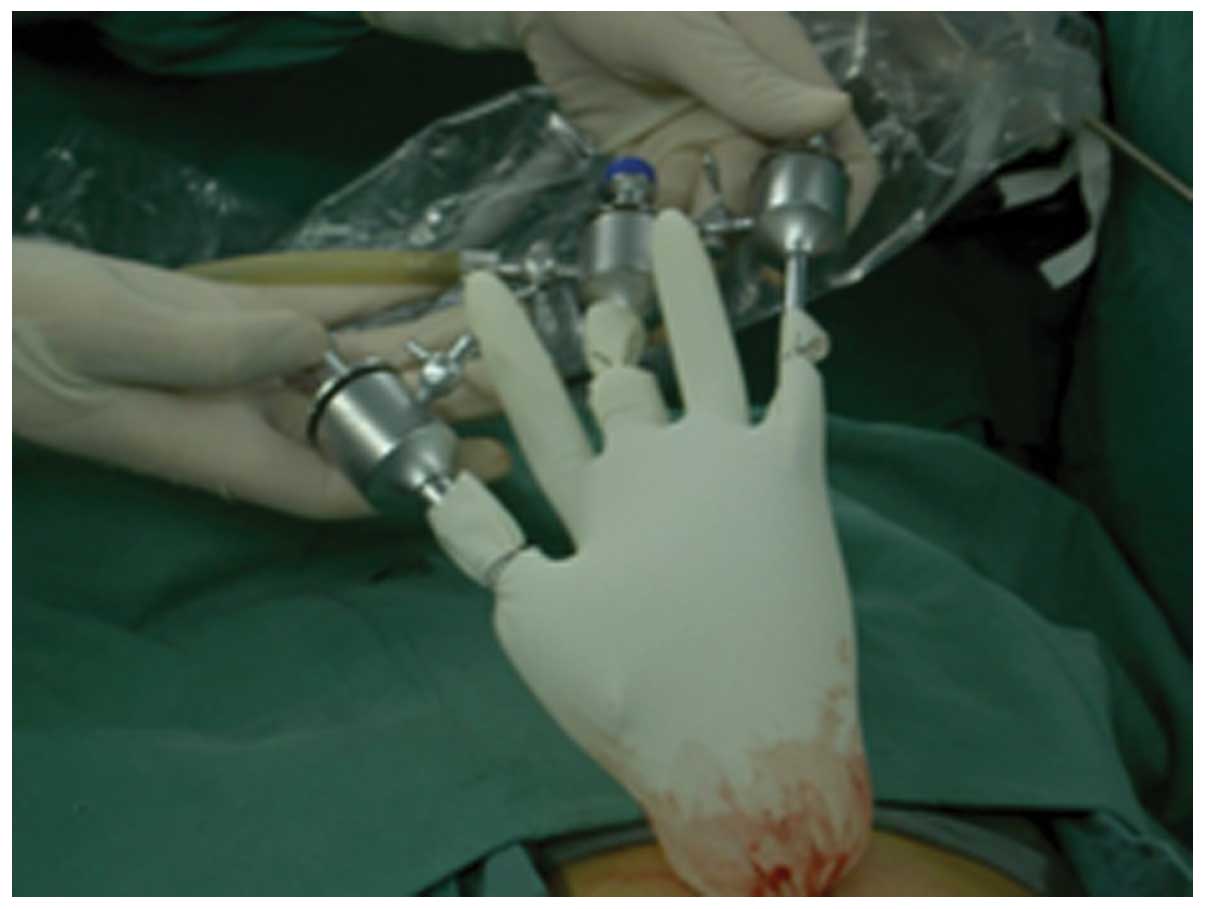
A simple method was used to establish the operation
channel for the laparoscopic cholecystectomy (16). A 15-mm trocar was required, on which
the wrist portion of a sterile glove was wrapped (Fig. 4). The fingertips of the glove were
opened and 5-mm trocars were inserted, allowing a 5-mm laparoscope
and two other instruments to be inserted through the channels
(Fig. 5). The 15-mm trocar was
placed through an arc-incision along the lower side of umbilicus.
Two 5-mm laparoscopic instruments were introduced through the
trocars fixed on the glove tips. Guided by the endoscope, the
laparoscopic cholecystectomy procedure was performed, and the
gallbladder was extracted from the mouth using a trielcon (Fig. 6).
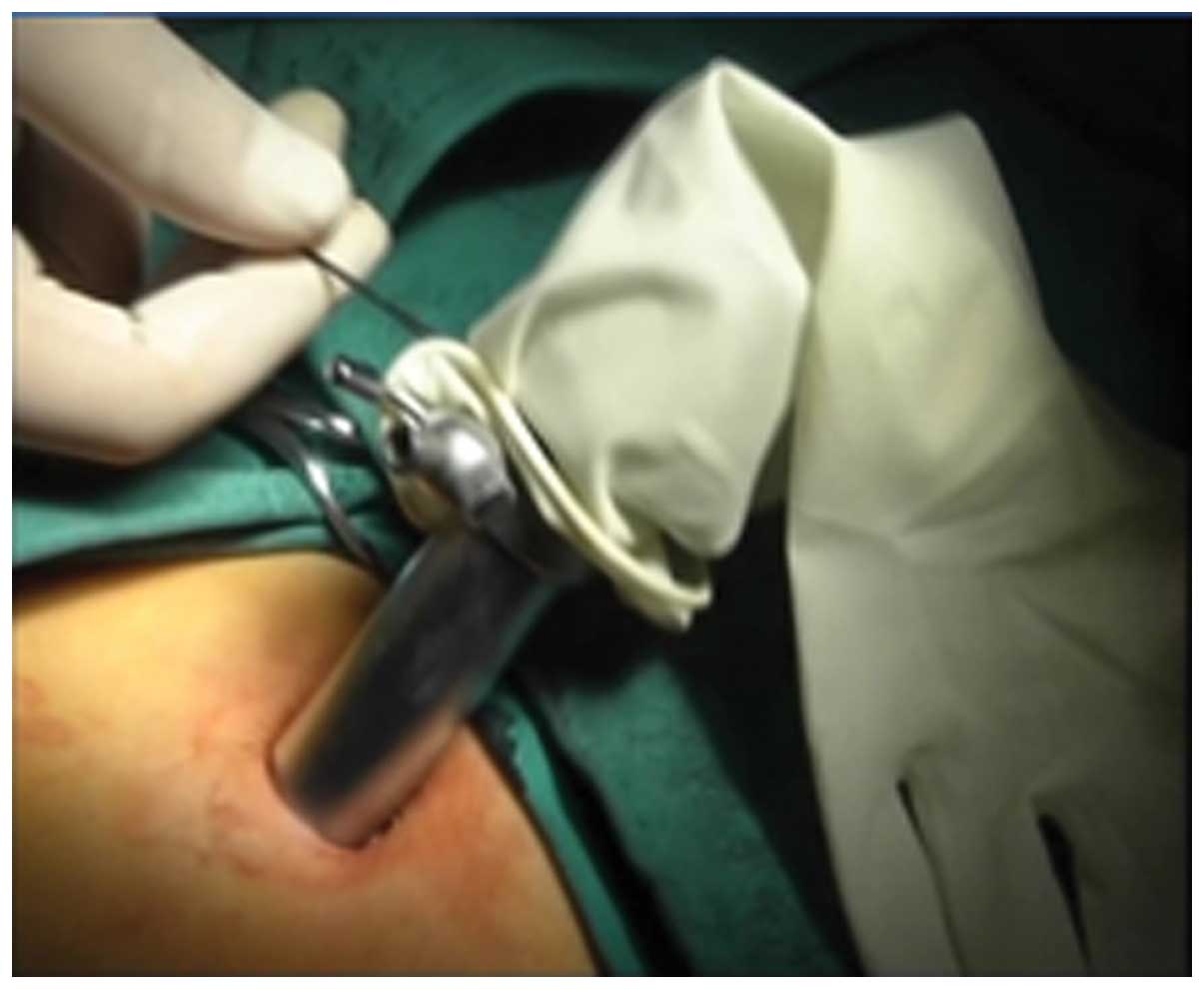
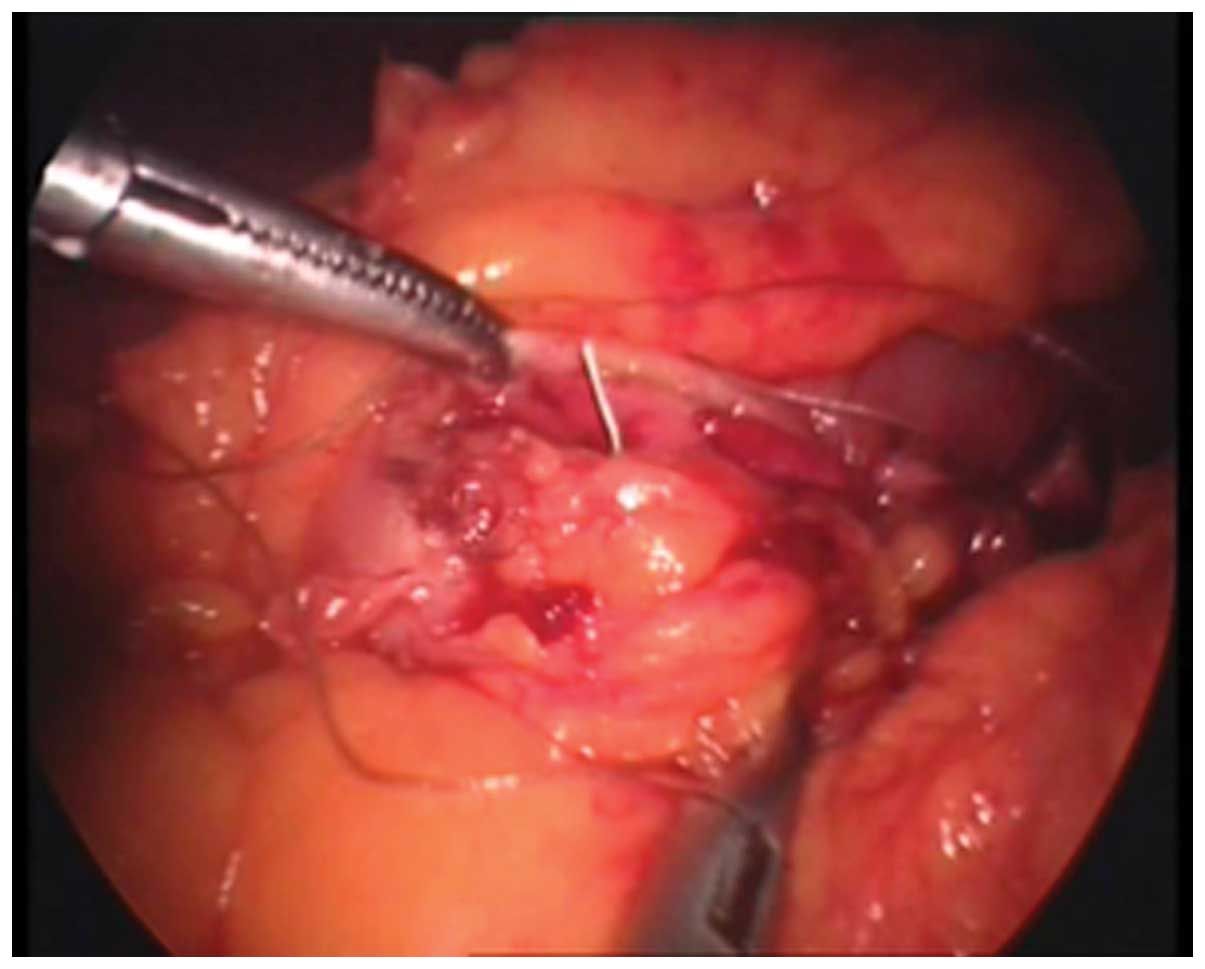
In accordance with the criteria established by the
NOSCAR Consortium (13), the
endoscope was removed and a 5-mm laparoscope was inserted through
the trocar fixed on the glove tips. The incision then was
hand-sutured laparoscopically using a 2/0 absorbable suture
(Fig. 7).
The procedure was successfully performed in 178 min,
with ≤20 ml blood loss. No postoperative complications, such as
bleeding, leakage or infection occurred. The maximum postoperative
temperature was ≤37.4°C. The postoperative defecation time was
three days, and the stomach tube was withdrawn after defacation.
The patient resumed free oral intake five days after the procedure
and was discharged on day six with no laboratory abnormalities,
such as white blood cell, neutrophil and red blood cell count,
hemoglobin levels, albumin levels, and levels of K+ and
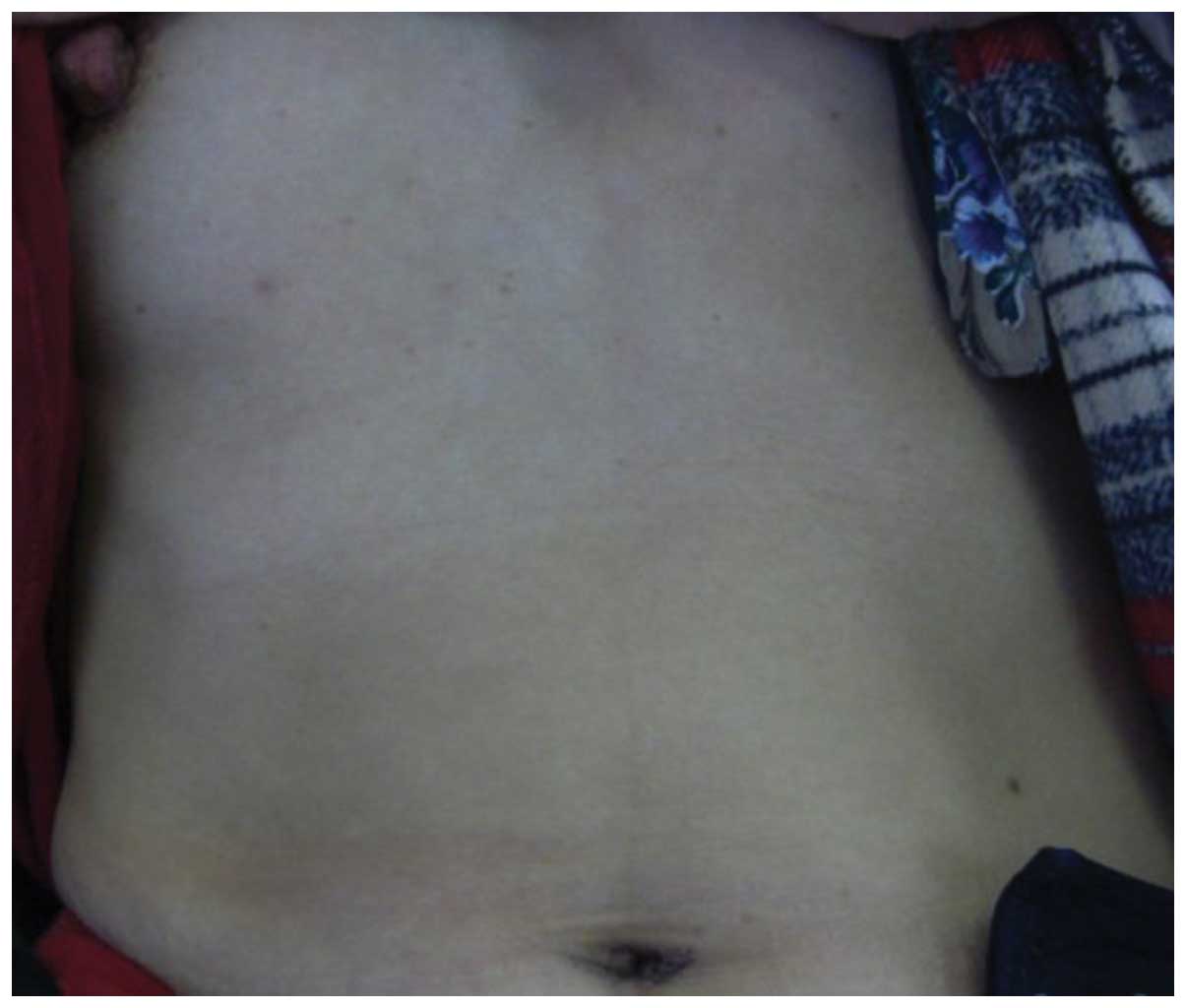
Na+. To data, the patient has not developed any wound
infections or hernias, no discomfort has been experienced and the
patient was satisfied with the cosmetic result at a follow-up three
months after the operation (Fig.
8).
Discussion
Following the rapid development of laparoscopy, the
field of minimally invasive surgery requires minimal skin
incisions, reduced pain and complications, faster postoperative
recovery periods, shorter lengths of hospitalization, improved
cosmetic effects and faster recovery times (17). NOTES receives the attention and
interest of surgeons and gastroenterologists as it is scarless and
is relatively painless (18).
However, NOTES faces a number of obstacles as a result of
inadequate surgical instruments that do not allow safe access to
the peritoneal cavity, and do not allow adequate exposure, secure
closure methods, effective infection control and correct
orientation (19,20).
In comparison with NOTES, TUES may be an ideal
choice when attempting invisible abdominal scar surgery. A number
of SPL procedures have been reported in recent years and have
produced satisfactory results (21,22). It
has also been reported that multi-visceral resection operations
have been successfully performed by TUES (23,24).
However, endoscopic cholecystectomy combined with gastric GIST
resection has not previously been reported. Designed using a number
of porcine model studies (25,26), the
present report describes a novel, successful procedure of a
cholecystectomy combined with gastric GIST resection using
laparoscopy and endoscopy.
GISTs are rare, non-epithelial mesenchymal tumors
with a potential for malignant transformation, accounting for
<3% of all gastrointestinal neoplasms (27), and the most common sites of
occurrence is the stomach (40–60%) (28). Despite the development of a new
chemotherapeutic agent, imatinib mesylate (29), surgical resection remains the primary
alternative treatment for primary GISTs (30). A number of studies have demonstrated
that survival is dependent on tumor size and histological features,
rather than the extent of resection (31,32).
Therefore, major anatomical resections are not required for a
number of GISTs (33) in which case
minimally invasive surgery is performed, particularly for gastric
GISTs (34). The first report of a
laparoscopically excised gastric GIST was published in 1992
(35), and there is evidence that
the technique is effective with minimal morbidity and mortality
(36). Endoscopic dissection is
gaining acceptance as a new procedure for submucosal GIST and
attains minimal morbidity and mortality (37).
A number of key factors limit the performance of
endoscopic resection of GIST. With regards to multi-visceral
resection, the procedure is dependent on the location of the GIST.
Tumors that are located in the fundus or in the body of the stomach
and along the greater gastric curvature are accessible by
endoscopic resection, as there is sufficient space and mobility for
extensive resection and manual sewing of the incision of the
stomach (38). Endoscopic resection
is also applicable for anterior lesions of the stomach as the
endoscope can gain access to the peritoneal cavity via an incision
in the stomach with clear broad vision (39,40).
However, the endoscopic GIST dissection procedure is restricted to
submucosal dissection due to unfavorable bleeding of the muscle
layer (41). The final, most
controversial limitation is the size of the tumor. It has been
reported that a large submuscosal tumor (diameter, ≥5 cm) was
successfully resected (42). It is
also reported that tumors <2 cm in diameter have a low risk of
metastasis. However, using current surgical principles and
instruments, it is recommended that only tumors <2 cm in
diameter are suitable for endoscopic surgery (43,44).
Due to the rare occurance of lymph node metastases
of GIST, the resection of a tumor using a 2 cm periphery is
sufficient for surgery (45,46). As conventional laparoscopic surgery,
benign disease of gallbladder is confined to the cooperative
surgery such as cholecystolithiasis and gallbladder polyps
(47).
In contrast to NOTES, GIST is technically easier and
provides more satisfactory cosmetic results, requiring only one
small incision in the abdomen that can be concealed when the
umbilicus is reconstructed (48). In
addition, TUES results in reduced blood loss, a quicker flatus
time, shorter periods of postoperative hospitalization and fewer
complication in comparison with conventional laparoscopic surgery
(49,50).
It has been previously reported that endoscopic
submucosal dissection is prone to result in a large quantity of
bleeding, given the rich blood supply of the stomach and the
unfavorable control of bleeding endoscopically (51). However, studies have demonstrated
that a standard hook knife in 100-W Endo-Cut mode does not result
in excessive bleeding that could coagulate in small vessels and
gain access to the abdominal cavity (52).
Secure closure of the visceral incision is a
critical step in avoiding intra-abdominal infection (53,54). A
number of devices have been developed and used in the clinic, such
as flexible endo-stitch, endoscopic clips and G-Pros (55,56).
However, the incision in the stomach may be closed by
hand-suturing, which has been proved to be a safe, easy and low
cost method in comparison with other methods (57). To confirm the tightness of the
stomach incision closure, a combination of intra-abdominal
perfusion of saline and filling the stomach with air may be a good
method to evaluate the closure of the incision by examining if
there are bubbles out of the water.
Infection control is an increasing concern. A recent
laboratory study from Case Western Reserve University demonstrated
that NOTES may result in less impairment of the peritoneal immune
system and thus improve the infectious outcome (58). The temperature of the patient in the
present study reached no higher than 37.4°C, and the white blood
cell and neutrophil (%) count were recorded as being within their
normal ranges, indicating that there was no increased risk of
infection during the operation.
In conclusion, the present study demonstrated that
the novel method described in the present report is technically
simple, feasible and safe with a reasonable operation time, reduced
bleeding and rapid recovery period. The technique provides an
alternative for minimally invasive surgery, in particular with
regards to multi-visceral resection. However, a large sample is
required in order to confirm its safety, and a longer follow-up
period must be recorded in order to observe the long-term effects
of the operation. In addition, patients must be carefully selected
for the operation using strict criteria. Furthermore, more suitable
instruments, greater experience of the surgeon and more refined
techniques for the operation will facilitate this novel approach
towards gastric GIST resection. It can be speculated that the novel
method will become a popular use for laproscopy and endoscopy.
Acknowledgements
The present study was supported by the Shandong
Provincial Science and Technology Development Project Foundation of
China (grant nos. 2012GSF11820 and 2013GSF11827).
References
|
1
|
Kalloo AN, Singh VK, Jagannath SB, Niiyama
H, Hill SL, Vaughn CA, Magee CA and Kantsevoy SV: Flexible
transgastric peritoneoscopy: A novel approach to diagnostic and
therapeutic interventions in the peritoneal cavity. Gastrointest
Endosc. 60:114–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Auyang ED, Santos BF, Enter DH, Hungness
ES and Soper NJ: Natural orifice translumenal endoscopic surgery
(NOTES): A technical review. Surg Endosc. 25:3135–3148. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Voermans RP, Van Berge Henegouwen MI and
Fockens P: Natural orifice transluminal endoscopic surgery (NOTES).
Endoscopy. 39:1013–1017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rattner D and Kalloo A: ASGE/SAGES Working
Group: ASGE/SAGES Working Group on natural orifice translumenal
endoscopic surgery. Surg Endosc. 20:329–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adelsdorfer C, Taura P, Ibarzabal A,
Vendrell M, Delitala A, Deulofeu R, Adelsdorfer W, Delgado S and
Lacy AM: Effect of transgastric natural orifice transluminal
endoscopic surgery peritoneoscopy on abdominal organ
microcirculation: An experimental controlled study. Gastrointest
Endosc. 83:427–433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fuchs KH: Comments on the current status
and future development of natural orifice transluminal endoscopic
surgery. ANZ J Surg. 85:201–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee SW and Lee JY: Laparoendoscopic
single-site urological surgery using a homemade single port device:
The first 70 cases performed at a single center by one surgeon. J
Endourol. 25:257–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fransen SA, Broeders E, Stassen L and
Bouvy N: The voice of Holland: Dutch public and patient's opinion
favours single-port laparoscopy. J Minim Access Surg. 10:119–125.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Magdeburg R and Kaehler G: Natural orifice
transluminal endoscopic surgery in humans: Feasibility and safety
of transgastric closure using the OTSC system. Surg Endosc.
30:73–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee GC and Sylla P: Shifting paradigms in
minimally invasive surgery: Applications of transanal natural
orifice transluminal endoscopic surgery in colorectal surgery. Clin
Colon Rectal Surg. 28:181–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rattner DW: Looking back and forward at
natural orifice translumenal endoscopic surgery. Cir Esp.
93:421–422. 2015.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noguera JF, Cuadrado A, Dolz C, Olea JM
and García JC: Prospective randomized clinical trial comparing
laparoscopic cholecystectomy and hybrid natural orifice
transluminal endoscopic surgery (NOTES) (NCT00835250). Surg Endosc.
26:3435–3441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
ASGE; SAGES: ASGE/SAGES working group on
natural orifice translumenal endoscopic surgery white paper October
2005. Gastrointest Endosc. 63:199–203. 2006.PubMed/NCBI
|
|
14
|
Frasson M, Braga M, Vignali A, Zuliani W
and Di Carlo V: Benefits of laparoscopic colorectal resection are
more pronounced in elderly patients. Dis Colon Rectum. 51:296–300.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolthuis AM, Fieuws S, Van Den Bosch A, de
Buck van Overstraeten A and D'Hoore A: Randomized clinical trial of
laparoscopic colectomy with or without natural-orifice specimen
extraction. Br J Surg. 102:630–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsujimoto H, Yaguchi Y, Kumano I, Takahata
R, Ono S and Hase K: Successful gastric submucosal tumor resection
using laparoscopic and endoscopic cooperative surgery. World J
Surg. 36:327–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gaillard M, Tranchart H, Lainas P and
Dagher I: New minimally invasive approaches for cholecystectomy:
Review of literature. World J Gastrointest Surg. 7:243–248. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antoniou SA, Antoniou GA, Antoniou AI and
Granderath FA: Past, present, and future of minimally invasive
Abdominal surgery. JSLS. 19:e2015000522015. View Article : Google Scholar
|
|
19
|
Olweny EO, Best SL, Tracy CR and Cadeddu
JA: New technology and applied research: What the future holds for
LESS and NOTES. Arch Esp Urol. 65:434–443. 2012.PubMed/NCBI
|
|
20
|
Morgan M, Olweny EO and Cadeddu JA: LESS
and NOTES instrumentation: Future. Curr Opin Urol. 24:58–65. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shussman N, Kedar A, Elazary R, Abu Gazala
M, Rivkind AI and Mintz Y: Reusable single-port access device
shortens operative time and reduces operative costs. Surg Endosc.
28:1902–1907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quaranta D, Lambaudie E, Heinnemann M,
Houvenaeghel G and Chéreau E: Evaluation of single-port laparoscopy
for peritoneal carcinomatosis assessment in advanced ovarian
cancer. Eur J Obstet Gynecol Reprod Biol. 181:60–65. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pitiakoudis M, Zezos P, Kouklakis G,
Tsalikidis C, Romanidis K, Vradelis S, Tsaroucha AK, Kakolyris S
and Simopoulos C: Endoscopically assisted transumbilical
single-incision laparoscopic gastric resection for GIST treatment.
J Invest Surg. 2:1–8. 2015.
|
|
24
|
Wang Y, Liu R, Zhang Z, Xue Q, Yan J, Yu
J, Liu H, Zhao L, Mou T, Deng H and Li G: A safety study of
transumbilical single incision versus conventional laparoscopic
surgery for colorectal cancer: Study protocol for a randomized
controlled trial. Trials. 16:5392015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dhumane P, Donatelli G, Chung H,
Dallemagne B and Marescaux J: Feasibility of transumbilical
flexible endoscopic preperitoneoscopy (FLEPP) and its utility for
inguinal hernia repair: Experimental animal study. Surg Innov.
20:5–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang QY, Zhang GY, Wang L, Wang ZG, Li F,
Li YQ, Ding XJ and Hu SY: Infection during transgastric and
transvaginal natural orifice transluminal endoscopic surgery in a
live porcine model. Chin Med J (Engl). 124:556–561. 2011.PubMed/NCBI
|
|
27
|
Serrano C and George S: Recent advances in
the treatment of gastrointestinal stromal tumors. Ther Adv Med
Oncol. 6:115–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rammohan A, Sathyanesan J, Rajendran K,
Pitchaimuthu A, Perumal SK, Srinivasan U, Ramasamy R, Palaniappan R
and Govindan M: A gist of gastrointestinal stromal tumors: A
review. World J Gastrointest Oncol. 5:102–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Künstlinger H, Binot E, Merkelbach-Bruse
S, Huss S, Wardelmann E, Buettner R and Schildhaus HU:
High-resolution melting analysis is a sensitive diagnostic tool to
detect imatinib-resistant andimatinib-sensitive PDGFRA exon 18
mutations in gastrointestinal stromal tumors. Hum Pathol.
45:573–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel S: Navigating risk stratification
systems for the management of patients with GIST. Ann Surg Oncol.
18:1698–1704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Kalaawy M, El-Zohairy MA, Mostafa A,
Al-Kalaawy A and El-Sebae H: Gastrointestinal stromal tumors
(GISTs), 10-year experience: Patterns of failure and prognostic
factors for survival of 127 patients. J Egypt Natl Canc Inst.
24:31–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng F, Liu Z, Zhang X, Guo M, Xu G, Ren
G, Hong L, Sun L, Yang J and Zhang H: Comparison of endoscopic and
open resection for small gastric gastrointestinal stromal tumor.
Transl Oncol. 8:504–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Al-Thani H, El-Menyar A, Rasul KI,
Al-Sulaiti M, El-Mabrok J, Hajaji K, Elgohary H and Tabeb A:
Clinical presentation, management and outcomes of gastrointestinal
stromal tumors. Int J Surg. 12:1127–1133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirahara N, Matsubara T, Kidani A,
Hyakudomi R, Fujii Y and Tajima Y: A novel technique to minimize
deformation of the stomach in laparoscopic partial gastrectomy for
intraluminal gastric GISTs. J Laparoendosc Adv Surg Tech A.
24:707–711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lukaszczyk JJ and Preletz RJ Jr:
Laparoscopic resection of benign stromal tumor of the stomach. J
Laparoendosc Surg. 2:331–334. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Vogelaere K, Van De Winkel N, Aerts M,
Haentjens P, Spitali C, Van Loo I and Delvaux G: Surgical
management of gastrointestinal stromal tumours: A single centre
experience during the past 17 years. Acta Chir Belg. 114:167–173.
2014.PubMed/NCBI
|
|
37
|
Honda M, Hiki N, Nunobe S, Ohashi M,
Kiyokawa T, Sano T and Yamaguchi T: Long-term and surgical outcomes
of laparoscopic surgery for gastric gastrointestinal stromal
tumors. Surg Endosc. 28:2317–2322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahn JY, Park HJ, Park YS, Lee JH, Choi KS,
Jeong KW, Kim DH, Choi KD, Song HJ, Lee GH and Jung HY: Endoscopic
resection for undifferentiated-type early gastric cancer: Immediate
endoscopic outcomes and long-term survivals. Dig Dis Sc.
29–Dec;2015.(Epub ahead of print).
|
|
39
|
Berelavichus SV, Kriger AG, Kaldarov AR
and Kalinin DV: Minimally invasive surgical treatment of
gastrointestinal stromal tumor. Khirurgiia (Mosk). 38–41. 2015.(In
Russian). PubMed/NCBI
|
|
40
|
Hirano Y, Watanabe T, Uchida T, Yoshida S,
Kato H and Hosokawa O: Laparoendoscopic single site partial
resection of the stomach for gastrointestinal stromal tumor. Surg
Laparosc Endosc Percutan Tech. 20:262–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee IL, Lin PY, Tung SY, Shen CH, Wei KL
and Wu CS: Endoscopic submucosal dissection for the treatment of
intraluminal gastric subepithelial tumors originating from the
muscularis propria layer. Endoscopy. 38:1024–1028. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin J, Huang C, Zheng C, Li P, Xie J, Wang
J and Lu J: Laparoscopic versus open gastric resection for larger
than 5 cm primary gastric gastrointestinal stromal tumors (GIST): A
size-matched comparison. Surg Endosc. 28:2577–2583. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Blay JY, Bonvalot S, Casali P, Choi H,
Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC,
Joensuu H, et al: Consensus meeting for the management of
gastrointestinal stromal tumors. Report of the GIST consensus
conference of 20–21 March 2004, under the auspices of ESMO. Ann
Oncol. 16:566–578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Demetri GD, Benjamin RS, Blanke CD, Blay
JY, Casali P, Choi H, Corless CL, Debiec-Rychter M, DeMatteo RP,
Ettinger DS, et al: NCCN task force report: Management of patients
with gastrointestinal stromal tumor (GIST)-update of the NCCN
clinical practice guidelines. J Natl Compr Canc Netw. 5(Suppl 2):
S1–S29; quiz S30. 2007.PubMed/NCBI
|
|
45
|
Tokunaga M, Ohyama S, Hiki N, Fukunaga T,
Yamamoto N and Yamaguchi T: Incidence and prognostic value of lymph
node metastasis on c-Kit-positive gastrointestinal stromal tumors
of the stomach. Hepatogastroenterology. 58:1224–1228. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gong N, Wong CS and Chu YC: Is lymph node
metastasis a common feature of gastrointestinal stromal tumor?
PET/CT correlation. Clin Nucl Med. 36:678–682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Egawa T, Kenmochi T, Irino T, Mihara K,
Okamura A, Eto E, Inaba Y, Murakawa M, Segami K, Ito Y and
Nagashima A: Laparoscopic partial gastrectomy for gastric
submucosal tumor-indications and limitations of single-incision
laparoscopic surgery. Gan To Kagaku Ryoho. 38:1960–1962. 2011.In
Japanese. PubMed/NCBI
|
|
48
|
Takata A, Nakajima K, Kurokawa Y,
Takahashi T, Yamasaki M, Miyata H, Takiguchi S, Mori M and Doki Y:
Single-incision laparoscopic partial gastrectomy for gastric
submucosal tumors without compromising transumbilical stapling.
Asian J Endosc Surg. 7:25–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sasaki K, Fujiwara Y, Kishi K, Miyashiro
I, Sugimura K, Miyoshi N, Akita H, Motoori M, Gotoh K, Takahashi H,
et al: Usefulness of laparoscopy endoscopy cooperative surgery for
gastric gastrointestinal stromal tumor. Gan To Kagaku Ryoho.
41:2223–2225. 2014.(In Japanese). PubMed/NCBI
|
|
50
|
Zhou H, Ming S, Ma L, Wang C, Liu X, Zhou
X, Xie H, Tao T, Ma S and Cheng W: Transumbilical single-incision
laparoscopic versus conventional laparoscopic upper pole
heminephroureterectomy for children with duplex kidney: A
retrospective comparative study. Urology. 84:1199–1204. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jang YS, Lee BE, Kim GH, Park Do Y, Jeon
HK, Baek DH, Kim DU and Song GA: Factors associated with outcomes
in endoscopic submucosal dissection of gastric cardia tumors: A
retrospective observational study. Medicine (Baltimore).
94:e12012015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Suk KT, Ham YL, Baik GH, Sung HT, Sohn KM,
Kim DY and Hong SH: Efficacy of partial endoscopic submucosal
dissection with polypectomy of gastric neoplasm during a learning
period. Hepatogastroenterology. 60:2107–2112. 2013.PubMed/NCBI
|
|
53
|
Gonzalez JM, Saito K, Kang C, Gromski M,
Sawhney M, Chuttani R and Matthes K: Prospective randomized
comparison of gastrotomy closure associating tunnel access and
over-the-scope clip (OTSC) with two other methods in an
experimental ex vivo setting. Endosc Int Open. 3:E83–E89.
2015.PubMed/NCBI
|
|
54
|
Rausei S, Dionigi G, Boni L, Rovera F,
Minoja G, Cuffari S and Dionigi R: Open abdomen management of
intra-abdominal infections: Analysis of a twenty-year experience.
Surg Infect (Larchmt). 15:200–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hart S: The benefits of automated suturing
devices in gynecologic endoscopic surgeries: The Endo stitchand
SILS stitch. Surg Technol Int. 22:159–164. 2012.PubMed/NCBI
|
|
56
|
Lin S, Laeeq K, Ishii M, Kim J, Lane A,
Reh D and Bhatti N: Development and pilot testing of a feasible,
reliable, and valid operative competency assessment tool for the
endoscopic sinus surgery. Am J Rhinol Allergy. 23:354–359. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nemecek E, Negrin L, Beran C, Nemecek R
and Hollinsky C: The application of the V-Loc closure device for
gastrointestinal sutures: A preliminary study. Surg Endosc.
27:3830–3834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
McGee MF, Marks JM, Onders RP, Chak A,
Rosen MJ, Williams CP, Jin J, Schomisch SJ and Ponsky JL: Case
Advanced Surgical Endoscopy Team [CASE-T]: Infectious implications
in the porcine model of natural orifice transluminal endoscopic
surgery (NOTES) with PEG-tube closure: A quantitative bacteriologic
study. Gastrointest Endosc. 68:310–318. 2008. View Article : Google Scholar : PubMed/NCBI
|