Introduction
Acute kidney injury (AKI) refers to a clinical
syndrome characterized by a rapid (hours to days) reduction in
renal excretory function, with the accumulation of creatinine and
urea nitrogen and other waste products that are not commonly tested
in clinical practice (1). AKI is
commonly observed in clinical practice, particularly following
major surgery and treatment in intensive care units (2). In addition, AKI mortality is high,
ranging between 24 and 62% (3).
Patients that survive AKI may have an increased long-term risk of
developing chronic kidney disease with poor prognosis (4). There is therefore an urgent requirement
for novel methods for the prevention and management of AKI.
In recent years, a promising and effective
therapeutic strategy for AKI involves the use of mesenchymal
stromal cells (MSCs) derived from various sources, such as bone
marrow or adipose (5,6). However, the mechanisms are not
understood well. It has been suggested that MSCs promote renal
injury repair, predominantly via paracrine/endocrine mechanisms as
opposed to direct transdifferentiation into kidney cells (7,8). In
previous studies, MSC-conditioned medium and MSCs released by
extracellular vesicles (Evs) were reported to exert renoprotective
effects against AKI (9–11). The researchers attributed these
effects to the favorable molecules, such as mRNA and miRNA
(11), in the Evs or secreted
soluble factors, such as hepatic growth factor. However,
contradictory findings have indicated that CM may not be able to
protect against kidney injury (12,13).
EVs, such as exosomes and shedding vesicles (also
known as microvesicles; Mvs) are membranous structures that deliver
bioactive molecular content, including proteins, mRNA and micro
(mi)RNA sequences (14). Evs is a
term suggested in recent years which describes a novel pathway of
cell-to-cell interaction, and it is also regarded as a crucial
point of endocrine (14). Since Evs
are released by cells and extracted from CM using differential
centrifugation, in the present study Evs were regarded as a type of
‘special CM’ or ‘improved CM’. On the basis of endocrine/paracrine
mechanism of MSCs, CM/Evs could provide a novel strategy of
cell-free therapy for tissue injuries (15).
Previous studies have produced inconsistent results
regarding the effects of CM/Evs therapy on AKI in rodent models
(8,12). This may be due to the variation of
injury models, treatment models, delivery route and cell type. In
present study, a meta-analysis was conducted to identify relevant
literature regarding CM/Evs therapy applied to AKI in rodent
models, using the serum creatinine (SCr) concentration, the classic
index of kidney function, as an analyzed parameter. In addition,
this study was intended to investigate the possible influential
factors for the therapeutic effects by sub-group and regression
analysis.
Materials and methods
Search strategy
PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Scopus
(http://www.scopus.com) were employed as searched
databases. The last search was updated on October 1st, 2014 using
the following key words and search terms: ([extracellular vesicles
or Evs or micro vesicles or micro-vesicles or microvesicles or Mvs
or exosome or shedding vesicles] or [conditioned medium or
conditioned culture media]) and (mesenchymal stromal cells or
mesenchymal stem cells or MSCs) and (acute kidney injury or acute
kidney disease or acute renal failure).
Eligibility criteria and data
extraction
AKI models in rats or mice were screened. The animal
experiments that investigated the effect of MSC-derived CM/Evs
therapy on impaired renal function as determined by the level of
SCr were analyzed. In addition, a sham- or placebo-operated control
group was a requirement for study inclusion. The exclusion criteria
for the studies were the following: i) Large animal/non-rodent
experiments; ii) renal function was not determined by SCr; iii) SCr
estimation was not included; iv) cell behavior was altered by
genetic modification. Comments, reviews, and editorials were
excluded. Only published English-language studies were considered
for inclusion.
The following data were extracted from the complete
manuscripts of the qualified studies: Basal characteristics of the
study, SCr concentration, CM or Evs therapy, time of the therapy
after injury and measurement time. If necessary, SCr data were
estimated using graphics, as previously described (16,17).
Accordingly, standard deviations were determined or recalculated
based on the standard error. SCr concentrations are expressed
herein as mg/dl (original data presented as µmol/l were changed
accordingly). All literature searching, screening and data
extraction were performed by two independent individuals, and
determined after discussion.
Data analysis
The outcome was presented in teh form of the
different in mean SCr between the control (AKI) and experimental
(CM/Evs therapy) animals. A random-effects model was applied
according to the results of heterogeneity tests. Continuous
variables are presented as weighted mean differences with 95%
confidence intervals (CIs) between the MSC-treated and control
groups. In the case of multiple experimental groups compared with
one control group within a single study, the number of animals in
the control group was divided equally by the number of experimental
groups. When there were multiple measurements, one study was
regarded as separate assessment (16,17).
P<0.05 was considered to indicate a statistically significant
difference in two-sided tests.
Sub-group analysis and multivariate meta-regression
were performed. The analyzed influential factors included: CM or
Evs; injury type, toxic or ischemia-reperfusion (IRI); cell type,
bone marrow MSC (BMSC) or non-BMSC; delivery route, intravenous or
others; time point of therapy after injury, <1, 1–24 and >24
h; and time point of measurement, ≤2, 3–4 and >4 days.
Furthermore, as publication bias is of concern for present
meta-analysis, publication bias was investigated by a funnel
plot.
All analysis was performed using Review Manager
(version 5.2.9; The Nordic Cochrane Centre, Cochrane Collaboration,
2012) and SPSS software, version 18.0 (SPSS, Inc., Chicago, IL,
USA). Meta-regression analysis was conducted using the ‘MetaReg’
macro written by David B. Wilson (http://mason.gmu.edu/~dwilsonb/ma.html).
Results
Literature characteristics
A total of 45 studies were retrieved from the PubMed
database and 254 from the Scopus database. After excluding
duplicate studies, a total of 274 remained. By excluding 96 review
articles, 17 books, 14 book chapters and 4 short surveys, a total
of 143 research articles remained. After screening for inclusion
eligibility based on reading titles and abstracts, there were 13
papers eligible for our review. Among the included animals, only
461 animals met the inclusion criteria and were analyzed.
Characteristics of the enrolled studies are described in Table I.
 | Table I.Characteristic of the included
studies. |
Table I.
Characteristic of the included
studies.
| Author, year | Injury type | n | CM or Evs | Cell type | Delivery route | Time point of therapy
after injury | Time point of SCr
measurement | Animal model | Ref. |
|---|
| Milwid et al,
2012 | Cisplatin | 14 | CM | BMSC | Intravenous | 3, 9, 24, 48 and 72
h | 3 and 5 days | Rat | 9 |
| Bruno et al,
2009 | Glycerol | 32 | Evs | BMSC | Intravenous | 3 days | 5 and 8 days | Mouse | 11 |
| Gheisari et
al, 2011 | Cisplatin | 113 | CM | BMSC | Intravenous | 1 d | 4 days | Mouse | 12 |
| Xing et al
2014 | IRI | 104 | CM | BMSC | Intravenous | 1 day | 2 and 3 days | Mouse | 13 |
| Bruno et al,
2012 | Cisplatin | 24 | Evs | BMSC | Intravenous | 8 h and 8 h, 2
days | 4 days | Mouse | 18 |
| Gatti et al,
2011 | IRI | 12 | Evs | BMSC | Intravenous | <1 h | 2 days | Rat | 19 |
| Kilpinen et
al, 2013 | IRI | 26 | Evs | hu-UCBMSC | Intra-arterial | <1 h | 1 and 2 days | Rat | 20 |
| Kim et al,
2012 | Cisplatin | 15 | CM | ADMSC |
Intraperitoneal | 1–2 days | 3 days | Rat | 21 |
| Reis et al,
2012 | Gentamicin | 20 | CM | BMSC | Intravenous | 1 day | 5 days | Rat | 22 |
| Zarjou et
al, 2011 | Cisplatin | 23 | CM | BMSC |
Intraperitoneal | 6 h | 3 days | Mouse | 23 |
| Zhang et al,
2014 | IRI | 12 | Evs | hu-WJMSC | Intravenous | <1 h | 14 days | Rat | 24 |
| Zhou et al,
2014 | cisplatin | 54 | CM and Evs | hu-UCMSC | Renal capsule
injection | 1 day | 3, 4 and 5
days | Rat | 25 |
| Zou et al,
2014 | IRI | 12 | Evs | hu-WJMSC | Intravenous | <1 h | 14 days | Rat | 26 |
Meta-analysis
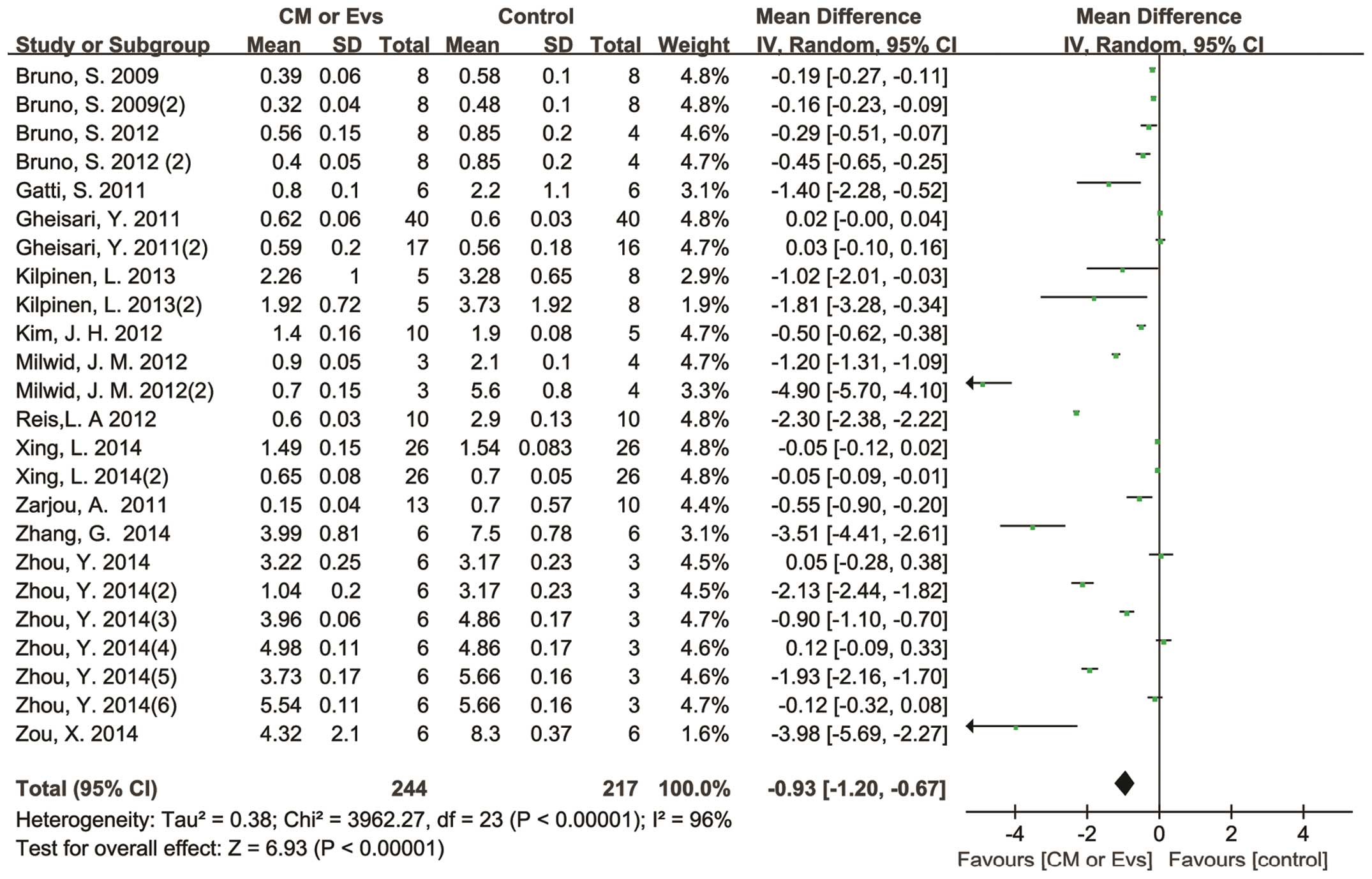
SCr data were continuous, as shown by the mean and
standard deviation. Pooled analysis showed a SCr reduction of 0.93
mg/dl (95% CI, 0.67–1.20 mg/dl) in the CM/Evs therapy groups, as
compared with the control groups with significant heterogeneity
(P<0.00001; I2=96%; Fig. 1).
Overall, no significant difference in SCr at baseline between the
control and therapy groups was detected (P=0.83). In addition,
several subgroup analyses were performed in order to determine
whether CM or Evs have comparable therapeutic effects, and the
optimum choice in CM/Evs therapy time after injury, time point
measurement, delivery route, cell type and animal species.
The multivariable meta-regression analysis showed
that measurement time point (P=0.041), therapeutic time point
(P=0.03), Evs or CM (P=0.0003) and cell type (P<0.0001) were
independent influential factors of SCr reduction.
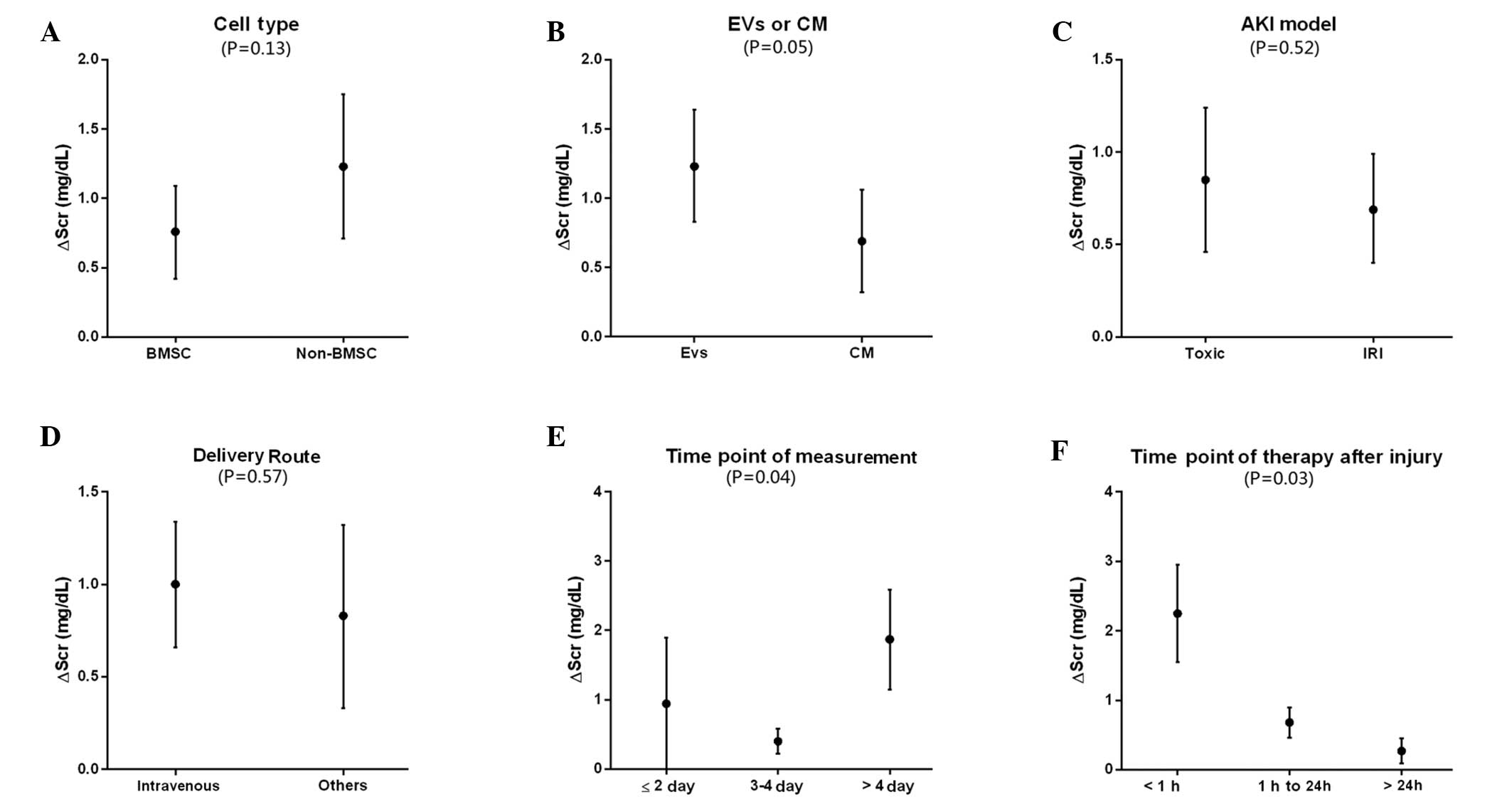
In the sub-group analysis, no difference in SCr
reduction was detected between BMSC and non-BMSC therapy groups
(0.76 mg/dl [1.09,0.42] vs. 1.23 mg/dl [1.75,0.71]; P=0.13).
(Fig. 2A). These results were
inconsistent with the result of meta-regression, which indicated
that cell type was an influential factor for SCr reduction. The
sub-group analysis also detected significant differences between
Evs and CM (P=0.05), which showed the SCr reduction (0.7 mg/dl,
[0.32,1.07] vs. 1.23 mg/dl, [0.84,1.63], for Evs and CM
respectively; Fig. 2B). Furthermore,
the SCr reduction induced by Evs (1.23 mg/dl, [0.84,1.63]) was
increased compared with CM (0.7 mg/dl,[0.32,1.07]). In the present
study, AKI model or CM/Evs delivery route were not identified to be
associated with SCr reduction (Fig. 2C
and D). As shown in Fig. 2E, SCr
measurement at >4 days after therapy was associated with more
favorable effects (1.87 mg/dl, [1.14,2.59]), while measurement at
≤2 days showed no beneficial effects (−0.94 mg/dl, [-1.89,-0.00]).
Significant differences were also detected in the sub-group
analysis (P=0.041). In addition, the therapeutic time point was an
influential factor for SCr reduction (Fig. 2F). It was observed that CM/Evs
injected within 1 h after injury were associated with a favorable
outcome (2.25 mg/dl, [2.95,1.55]), while at 1 day after injury the
therapeutic effects were reduced (0.27 mg/dl, [0.09,0.45]). The
subgroup analysis demonstrated the significant differences
associated with treatment time point (P=0.03). In present study,
two species of animal were investigated, namely rats and mice. The
sub-group analysis showed no significant difference between these
animals (P=0.72), which was consistent with the meta-regression
analysis.
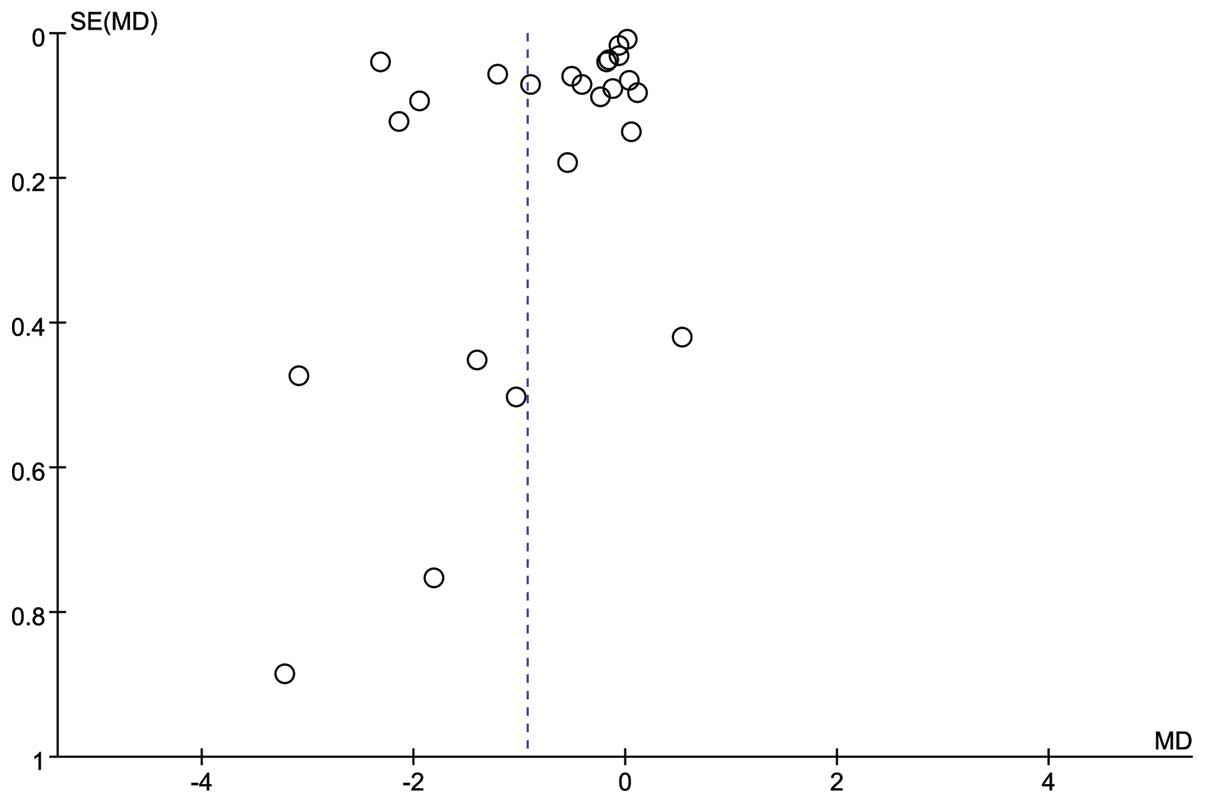
As shown in Fig. 3,
the funnel plot for SCr indicated no publication bias.
Sensitivity analysis
Sub-group and multivariable meta-analyses were
performed to investigate the source of significant heterogeneity
among the involved studies.
Analyzed factors included CM or Evs, injury type,
animal type, cell type, delivery route, therapeutic time point
(after injury) and measurement time point. Meta-regression showed
that measurement time point (P=0.041), therapeutic time point
(P=0.03), Evs or CM (P=0.0003) and cell type (P<0.0001) were
independent influential factors of SCr reduction. No trend in SCr
reduction was observed regarding animal model (P=0.72).
Discussion
At present, adult stem cells have been extensively
investigated with regard to their potential implications in
regenerative medicine (27). MSCs
from various tissues have been applied to the therapy for kidney
injury, ischemia myocardial infarction and other diseases in
clinical trials, a number of which produced favorable results
(28). However, there remain a
number of limitations associated with MSC transplantation,
including immune-mediated rejection, senescence-induced genetic
instability or loss of function and limited cell survival (29). Besides these issues, the primary
problem related to the use of MSCs in clinical applications is the
possibility of malignant transformation (30). On the basis of the
endocrine/paracrine mechanism that may be involved in MSC therapy,
CM/Evs may offer a strategy which avoids a number of the risks and
limitations mentioned above (15).
The present meta-analysis comprised 13 published
studies concerning on CM/Evs for AKI, and the pooled analysis
showed a more marked SCr reduction (0.93 mg/dl [0.67,1.20]) in
CM/Evs therapy groups compared with control groups, suggesting that
CM/Evs were able to protect rodent model animals against AKI. The
sub-group analysis showed that CM and Evs administration could lead
to SCr reduction (0.7 mg/dl [0.32,1.07] and 1.23 mg/dl [0.84,1.63],
respectively). Furthermore, SCr reduction in Evs sub-group was
significantly elevated compared with the CM sub-group (P=0.05).
Thus, Evs may offer more substantial therapeutic effects compared
with CM. After the long-time concerning on growth factors and
cytokines which is an important part of the cellular secretome, it
now appears that the cells secreted Evs instead of soluble factors,
which has previously been regarded as the main cellular secretome
with a more important function. Evs, including exosomes and
shedding vesicles, have been shown to deliver genetic information
and functional proteins as well as bioactive membrane. Previous
studies have attributed the therapeutic effects of Evs to their
role in cell-to-cell communication (14) or the capability to reprogram injured
cells (31). Evs can be extracted
from CM in vitro using differential centrifugation, although
the protocol may vary between studies. Thus, we hypothesize that
the more marked protective role of Evs may be attributed to higher
concentration of effective ingredient, such as functional protein,
mRNA, miRNA and DNA, in Evs compared with CM.
The sub-group analysis showed that the rapid
delivery of CM/Evs (1 h after injury) may lead to greater SCr
reduction (Fig. 2F), and the
therapeutic effects may emerge after 4 days (Fig. 2E), while there was no significant SCr
reduction after 2 days. Furthermore, the review data suggested that
the delivery route and kidney injury type might not affect SCr
reduction. Notably, in a previous meta-analysis concerning MSCs
therapy for impaired renal function in small animal models
(16), increased SCr reduction was
observed using an arterial delivery route compared with an
intravenous route. For MSCs transplantation, intravenously
delivered cells were retained in the lung capillaries (32), while intra-arterial delivery may lead
to more efficient infusion. This may explain why arterial injection
therapy is able to produce improved treatment effects. By contrast,
no retained cells were detected in the lung capillaries after
intravenous injection in CM/Evs therapy (11). In addition, Evs were able to migrate
toward injured tissue, thus functioning in a similar manner to MSCs
(15). Therefore, the results
mentioned above indicate that delivery route may not affect the
therapeutic efficacy of an Evs-based treatment for AKI.
Thus far, cell-free therapy using CM/Evs for AKI
experiments have been performed only in small animals. Therefore,
further animal experiments involving different species are
necessary in order to assess the safety and efficiency of CM/Evs
therapy, prior to human clinical trials. Meta-analysis of animal
studies was not common, yet they were recommended in several
settings (33–35), and could often guide research
(36), even clinical endeavors.
Based on the present meta-analysis, our recommendations for MSCs
cell-free MSCs therapy (CM/Evs) for AKI are as follows: i) Compared
with CM, Evs have the priority as they possess greater therapeutic
potential; ii) the time point of treatment should be as early as
possible after injury; iii) the therapeutic effects may emerge at a
later time; and iv) the delivery route could not affect the
therapeutic effects.
However, there were still limitations of present
study. The limitation of meta-analysis is well known (37), our analysis was based on study
outcomes, and we did not have access to individual data. Another
limitation is that some data were estimated using graphics during
data extraction. Besides, there was significant heterogeneity,
which might be due to other unknown influential factors varied in
the included studies. Nevertheless, by using the random-effect
analysis, the risk of finding erroneous estimates was
minimized.
Acknowledgements
This study was supported by grants from the Research
Program of Science and Technology Commission of Shanghai
Municipality (grant no. 10411967200), Shanghai å Health Bureau
(grant no. 2011PD06), National Natural Science Foundation of China
(grant nos. 81170642 and 81470919) and a Shanghai Shen Kang
Platform Grant (grant no. SHDC12007206). The authors thank Dr
Changxin Song of Qinghai Normal University for consultation of
statistical analysis.
References
|
1
|
Bellomo R, Kellum JA and Ronco C: Acute
kidney injury. Lancet. 380:756–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bagshaw SM, George C and Bellomo R: ANZICS
Database Management Committee: Early acute kidney injury and
sepsis: A multicentre evaluation. Crit Care. 12:R472008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waikar SS, Liu KD and Chertow GM:
Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J
Am Soc Nephrol. 3:844–861. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wald R, Quinn RR, Luo J, Li P, Scales DC,
Mamdani MM and Ray JG: University of Toronto Acute Kidney Injury
Research Group: Chronic dialysis and death among survivors of acute
kidney injury requiring dialysis. JAMA. 302:1179–1185. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian H, Yang H, Xu W, Yan Y, Chen Q, Zhu
W, Cao H, Yin Q, Zhou H, Mao F and Chen Y: Bone marrow mesenchymal
stem cells ameliorate rat acute renal failure by differentiation
into renal tubular epithelial-like cells. Int J Mol Med.
22:325–332. 2008.PubMed/NCBI
|
|
6
|
Gao J, Liu R, Wu J, Liu Z, Li J, Zhou J,
Hao T, Wang Y, Du Z, Duan C and Wang C: The use of chitosan based
hydrogel for enhancing the therapeutic benefits of adipose-derived
MSCs for acute kidney injury. Biomaterials. 33:3673–3681. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duffield JS, Park KM, Hsiao LL, Kelley VR,
Scadden DT, Ichimura T and Bonventre JV: Restoration of tubular
epithelial cells during repair of the postischemic kidney occurs
independently of bone marrow-derived stem cells. J Clin Invest.
115:1743–1755. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tögel F, Hu Z, Weiss K, Isaac J, Lange C
and Westenfelder C: Administered mesenchymal stem cells protect
against ischemic acute renal failure through
differentiation-independent mechanisms. Am J Physiol Renal Physiol.
289:F31–F42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Milwid JM, Ichimura T, Li M, Jiao Y, Lee
J, Yarmush JS, Parekkadan B, Tilles AW, Bonventre JV and Yarmush
ML: Secreted factors from bone marrow stromal cells upregulate
IL-10 and reverse acute kidney injury. Stem Cells Int.
2012:3920502012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bi B, Schmitt R, Israilova M, Nishio H and
Cantley LG: Stromal cells protect against acute tubular injury via
an endocrine effect. J Am Soc Nephrol. 18:2486–2496. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bruno S, Grange C, Deregibus MC, Calogero
RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati
B, et al: Mesenchymal stem cell-derived microvesicles protect
against acute tubular injury. J Am Soc Nephrol. 20:1053–1067. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gheisari Y, Ahmadbeigi N, Naderi M,
Nassiri SM, Nadri S and Soleimani M: Stem cell-conditioned medium
does not protect against kidney failure. Cell Biol Int. 35:209–213.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xing L, Cui R, Peng L, Ma J, Chen X, Xie
RJ and Li B: Mesenchymal stem cells, not conditioned medium,
contribute to kidney repair after ischemia-reperfusion injury. Stem
Cell Res Ther. 5:1012014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Camussi G, Deregibus MC, Bruno S,
Cantaluppi V and Biancone L: Exosomes/microvesicles as a mechanism
of cell-to-cell communication. Kidney Int. 78:838–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baglio SR, Pegtel DM and Baldini N:
Mesenchymal stem cell secreted vesicles provide novel opportunities
in (stem) cell-free therapy. Front Physiol. 3:3592012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, He J, Pei X and Zhao W: Systematic
review and meta-analysis of mesenchymal stem/stromal cells therapy
for impaired renal function in small animal models. Nephrology
(Carlton). 18:201–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Spoel TI, Jansenof Lorkeers SJ,
Agostoni P, van Belle E, Gyöngyösi M, Sluijter JP, Cramer MJ,
Doevendans PA and Chamuleau SA: Human relevance of pre-clinical
studies in stem cell therapy: Systematic review and meta-analysis
of large animal models of ischaemic heart disease. Cardiovasc Res.
91:649–658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bruno S, Grange C, Collino F, Deregibus
MC, Cantaluppi V, Biancone L, Tetta C and Camussi G: Microvesicles
derived from mesenchymal stem cells enhance survival in a lethal
model of acute kidney injury. PLoS One. 7:e331152012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gatti S, Bruno S, Deregibus MC, Sordi A,
Cantaluppi V, Tetta C and Camussi G: Microvesicles derived from
human adult mesenchymal stem cells protect against
ischaemia-reperfusion-induced acute and chronic kidney injury.
Nephrol Dial Transplant. 26:1474–1483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kilpinen L, Impola U, Sankkila L, Ritamo
I, Aatonen M, Kilpinen S, Tuimala J, Valmu L, Levijoki J and
Finckenberg P: Extracellular membrane vesicles from umbilical cord
blood-derived MSC protect against ischemic acute kidney injury, a
feature that is lost after inflammatory conditioning. J Extracell
Vesicles. 2:2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JH, Park DJ, Yun JC, Jung MH, Yeo HD,
Kim HJ, Kim DW, Yang JI, Lee GW, Jeong SH, et al: Human adipose
tissue-derived mesenchymal stem cells protect kidneys from
cisplatin nephrotoxicity in rats. Am J Physiol Renal Physiol.
302:F1141–F1150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reis LA, Borges FT, Simões MJ, Borges AA,
Sinigaglia-Coimbra R and Schor N: Bone marrow-derived mesenchymal
stem cells repaired but did not prevent gentamicin-induced acute
kidney injury through paracrine effects in rats. PLoS One.
7:e440922012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zarjou A, Kim J, Traylor AM, Sanders PW,
Balla J, Agarwal A and Curtis LM: Paracrine effects of mesenchymal
stem cells in cisplatin-induced renal injury require heme
oxygenase-1. Am J Physiol Renal Physiol. 300:F254–F262. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang G, Zou X, Miao S, Chen J, Du T,
Zhong L, Ju G, Liu G and Zhu Y: The anti-oxidative role of
micro-vesicles derived from human wharton-jelly mesenchymal stromal
cells through NOX2/gp91(phox) suppression in alleviating renal
ischemia-reperfusion injury in rats. PLoS One. 9:e921292014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y,
Zhang B, Wang M, Mao F and Yan Y: Exosomes released by human
umbilical cord mesenchymal stem cells protect against
cisplatin-induced renal oxidative stress and apoptosis in vivo and
in vitro. Stem Cell Res Ther. 4:342013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju
G, Miao S, Liu G, Lu M and Zhu Y: Microvesicles derived from human
Wharton's Jelly mesenchymal stromal cells ameliorate renal
ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem
Cell Res Ther. 5:402014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fisher MB and Mauck RL: Tissue engineering
and regenerative medicine: recent innovations and the transition to
translation. Tissue Engineering Part B: Reviews. 19:1–13. 2013.
View Article : Google Scholar
|
|
28
|
Wei X, Yang X, Han ZP, Qu FF, Shao L and
Shi YF: Mesenchymal stem cells: A new trend for cell therapy. Acta
Pharmacol Sin. 34:747–754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim PK, Bliss SA, Patel SA, Taborga M,
Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS and Rameshwar P:
Gap junction-mediated import of microRNA from bone marrow stromal
cells can elicit cell cycle quiescence in breast cancer cells.
Cancer Res. 71:1550–1560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rubio D, Garcia S, Paz MF, De la Cueva T,
Lopez-Fernandez LA, Lloyd AC, Garcia-Castro J and Bernad A:
Molecular characterization of spontaneous mesenchymal stem cell
transformation. PLoS One. 3:e13982008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Camussi G, Deregibus MC and Cantaluppi V:
Role of stem-cell-derived microvesicles in the paracrine action of
stem cells. Biochem Soc Trans. 41:283–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du T, Cheng J, Zhong L, Zhao XF, Zhu J,
Zhu YJ and Liu GH: The alleviation of acute and chronic kidney
injury by human Wharton's jelly-derived mesenchymal stromal cells
triggered by ischemia-reperfusion injury via an endocrine
mechanism. Cytotherapy. 14:1215–1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sandercock P and Roberts I: Systematic
reviews of animal experiments. Lancet. 360:5862002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Horn J, De Haan R, Vermeulen M, Luiten P
and Limburg M: Nimodipine in animal model experiments of focal
cerebral ischemia: A Systematic Review. Stroke. 32:2433–2438. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roberts I, Kwan I, Evans P and Haig S:
Does animal experimentation inform human healthcare? Observations
from a systematic review of international animal experiments on
fluid resuscitation. BMJ. 324:474–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Biondi-Zoccai GG, Abbate A, Parisi Q,
Agostoni P, Burzotta F, Sandroni C, Zardini P and Biasucci LM: Is
vasopressin superior to adrenaline or placebo in the management of
cardiac arrest? A meta-analysis. Resuscitation. 59:221–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Verstraete M: Value and limitation of
meta-analysis. Pathophysiol Haemost Thromb. 32:278–281. 2002.
View Article : Google Scholar : PubMed/NCBI
|