Introduction
Esophageal squamous cell carcinoma (ESCC) is an
upper gastrointestinal malignancy, characterized by insidious
onset, rapid development and poor prognosis (1). Since the rate of early diagnosis is
poor, the majority of patients are diagnosed while at the advanced
stage of ESCC, and their 5-year survival rates are 10–30%. Invasion
and metastasis of ESCC are the main reasons for poor prognosis
following surgery (2). Early
diagnosis, and anti-invasion and anti-metastasis therapies can
significantly improve the prognosis of ESCC. Therefore, it is
necessary to investigate the pathogenic mechanisms of ESCC.
microRNA (miRNA) is endogenous small non-coding RNA
that modulates gene expression. The abnormal expression of microRNA
plays an important role in tumorigenesis (3). The level of a particular miRNA, miR141,
has been found to be upregulated in breast, lung and stomach tumors
and downregulated in liver and prostate tumors (4–6). miR141
is known to be involved in the proliferation, invasion, apoptosis
and angiogenesis of gastric, liver and pancreatic tumors (7). In the present study, the target gene of
miR141 was predicted to be signal transducer and activator of
transcription 5 (STAT5) using bioinformatic analysis.
STAT5, an important signal transducer and
transcription activator, has an important role in the Janus
kinase/STAT signaling pathway (8).
Its expression level has been shown to be significantly increased
in tumors, where it is directly involved in differentiation,
invasion, proliferation and apoptosis (9–11).
In the present study, the expression of miR141 in
clinical samples of ESCC was detected using reverse transcription
quantitative polymerase chain reaction (RT-qPCR). The levels of
STAT5 were detected in the ESCC tissues by immunohistochemical
staining and western blotting. The effects of miR141 on the
proliferation, migration and invasion of Eca109 human esophageal
epithelial cancer cells were detected with the use of
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT), scratch and Transwell invasion assays, respectively.
Materials and methods
Clinical data of patients
A total of consecutive 45 patients (37 male and 8
female; mean age, 58.30±8.10 years; age range, 42–71 years)
pathologically diagnosed with ESCC were enrolled in the study.
Among these 45 patients, 28 cases had well-differentiated tumors,
14 cases had moderately differentiated tumors and 3 cases had
poorly differentiated tumors. Lymph node metastasis was identified
in 21 cases and the other 24 cases were not found to have lymph
node metastasis. All patients underwent surgical treatment.
Esophageal cancer tissues and peritumoral tissues (≥5 cm away from
the tumor edge) were collected during surgery. Tumor size,
differentiation and invasion were evaluated by pathologists. Lesion
location was identified by endoscopic examination and during
surgery. All tissues were frozen in liquid nitrogen and then stored
at −80°C until further analysis. Information about the patients is
shown in Table I. None of the
patients had a history of chemotherapy or additional tumors. Prior
written and informed consent was obtained from every patient and
the study was approved by the ethics review board of Linyi People's
Hospital (Linyi, China).
 | Table I.Classification of the patients with
esophageal squamous cell carcinoma (n=45). |
Table I.
Classification of the patients with
esophageal squamous cell carcinoma (n=45).
| Classification | No. of cases |
|---|
| Degree of
differentiation |
|
| High
differentiation | 28 |
| Moderate
differentiation | 14 |
| Poor
differentiation | 3 |
| Metastatic
status |
|
| Lymph
node metastasis | 21 |
| Without
lymph node metastasis | 24 |
Bioinformatic analysis
The target gene of miR141 was predicted using
TargetScan (http://www.targetscan.org) and
miRwalk (http://www.umm.uniheidelberg.de/apps/zmf/mirwalk)
databases, and pathway analysis was conducted using Kyoto
Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/) and Gene Ontology (GO;
(http://www.geneontology.org)
databases.
Reagents
Reagents used included TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), rabbit
anti-human STAT5 polyclonal antibody (ab68465; Abcam, Cambridge,
MA, USA), a streptavidin-peroxidase (SP) kit (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd., Beijing, China),
PrimeScript™ RT reagent kit and SYBR® PrimeScript™
real-time PCR kit (both Takara Biotechnology Co., Ltd., Dalian,
China).
Cell culture and miRNA
transfection
Eca109 cells (Cell Bank of the Chinese Academy of
Sciences, Shanghai, China) were cultured at a concentration of
2×105 cells/well in 24-well plates with RPMI-1640 (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (Gibco FBS; Thermo Fisher Scientific, Inc.).
When 70–80% confluence was reached, the cells were divided into the
normal control, negative control (NC) and miR141 mimic groups.
Cells in the NC group remained untransfected, while cells in the NC
and miR141 groups were transfected with miR-NC and miR141,
respectively, by lipofection. In brief, 1.5 µl miRNA mimic or
miR-NC (both 20 pmol/µl; Guangzhou RiboBio Co., Ltd., Guangzhou,
China), together with 1 µl Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was added to an EP tube supplemented with
50 µl Opti-MEM medium (Invitrogen; Thermo Fisher Scientific, Inc.),
and allowed to stand undisturbed for 5 min. The mixture was then
added to the cells, and the cells were incubated for 6 h. The
medium was then replaced with RPMI-1640 supplemented with 10% FBS
for further culture. The protein expression level of STAT5 was
detected at 48 h.
Immunohistochemical staining
Tumor tissues were fixed in 10% formaldehyde,
embedded in paraffin and then cut into 4-µm sections. The sections
were dewaxed in graded xylene, dehydrated in graded ethanol, and
then incubated with 3% hydrogen peroxide for 10 min at room
temperature. Antigen retrieval was conducted by incubation with
sodium citrate (pH 6.0) at 95°C for 15 min. Following antigen
retrieval, the sections were treated with anti-STAT5 antibody
(l:200) for 1 h at room temperature, followed by incubation with
biotinylated secondary anti-IgG (PK-4001; 1:200; Beijing Zhongshan
Golden Bridge Biological Technology Co., Ltd.) for 30 min at 37°C.
Subsequently, the sections were stained with 3,3′-diaminobenzidine,
followed by slightly counterstaining with hematoxylin for 30 sec.
Following differentiation with hydrochloric acid and treatment with
xylene, sections sealed in neutral gum were observed under a CX31
microscope (Olympus Corporation, Tokyo, Japan).
Immunohistochemical grading
Brown staining of the cytoplasm or membrane was
considered as STAT5-positive. Five fields of view were randomly
selected. The percentage of positive cells was determined from the
ratio of the number of positive cells relative to the total number
of cells. At least 200 cells were counted.
RT-qPCR
Total RNA was extracted from the tissue samples
using TRIzol reagent and reverse transcribed into cDNA using the
PrimeScript™ RT reagent kit. Completeness of the cDNA was detected
using gel electrophoresis and purity was detected using a
spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE,
USA) to measure the 260/280 absorbance ratio. The levels of miR141
and STAT5 were measured. The forward primer sequence for miR141 was
5′-CACTGTCTGGUAAAGA-3′. The reverse primer sequence for miR141 was
universal. The primer sequences for STAT5 were as follow: Forward,
5′-ATTCAAACACTTGACCCTGA-3′ and reverse, 5′-TGTGTCCTCCAGATCGAAG-3′.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an
internal control and the primer sequences were as follows: Forward,
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse, 5′-GGGGTCGTTGATGGCAACA-3′.
PCR conditions were as follows: Denaturation for 10 min at 95°C,
followed by 40 cycles of annealing for 1 min at 95°C and extension
for 30 sec at 60°C. The dissolution procedure was designed as
follows: 15 sec at 95°C, 30 sec at 60°C and 15 sec at 95°C. The PCR
procedure was conducted using the SYBR® PrimeScript™
real-time PCR kit and a StepOnePlus™ Real-time PCR instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
2﹣ΔΔCq method was used to calculate the relative
expression levels.
Western blot analysis
Proteins were extracted from tissues or cells by
lysis using radioimmunoprecipitation assay (RIPA) buffer (Beyotime
Institute of Biotechnology, Beijing, China). Sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer
was added to the proteins, and the mixture was boiled for 5 min.
Subsequently, 10 µl sample was separated using 10% SDS-PAGE gel
(Beyotime Institute of Biotechnology) at 80 V, followed by
electrophoretic transfer for 2 h at 200 mA to polyvinylidene
difluoride membranes. The membranes were blocked with 50 g/l
skimmed milk for 1 h at room temperature. Rabbit anti-human STAT5
antibody (1:1,000) or rabbit anti-GAPDH antibody (1:5,000;
ab181602; Abcam) was added and the membrane was incubated overnight
at 4°C. Then, goat anti-rabbit (1:2,000; ab6721; Abcam) secondary
antibody was added and the membrane was incubated for 1 h at room
temperature. Protein bands were visualized by
electrochemiluminescence reaction (Beyotime Institute of
Biotechnology). The results were quantified using Quantity One
software, version 4.62 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA
MTT assay
Following transfection, Eca109 cells were cultured
in 96-well plates at a density of 2×103/well for 0, 24, 48 and 72
h. Subsequently 20 µl 5 mg/l MTT was added and the cells were
incubated for 4 h at 37°C. Finally, cell proliferation was
calculated at an absorbance of 490 nm using an iMark absorbance
reader (Bio-Rad Laboratories, Inc.).
Scratch assay
Eca109 cells (5×105) were cultured in
24-well plates for 24 h. A sterile pipette tip was used to draw
straight lines along the longitudinal axis and then the cells were
washed with PBS. The migration of the cells was examined under an
IX83 inverted microscope (magnification, ×10; Olympus Corporation)
at 24 h.
Transwell assay
After thawing overnight at 4°C, Matrigel (dilution,
1:2; BD Biosciences, Franklin Lakes, NJ, USA) was added to the
upper chamber of a Transwell (Corning, Inc., Corning, NY, USA) and
incubated for 60 min at 37°C. Eca109 cells (1×105) and 200 µl
serum-free RPMI-1640 were added to the upper chamber and 500 µl
RPMI-1640 supplemented with 10% FBS was added to the lower chamber
and the Transwell unit was incubated for 24 h. Then, the cells that
had crossed the membrane into the lower chamber were fixed with 4%
formaldehyde for 10 min and stained with Giemsa. The extent of the
cell invasion was determined by counting the transmembranous cells
under a microscope.
Statistical analysis
All data were analyzed using SPSS software, version
11.0. (SPSS, Inc., Chicago, IL, USA). Results are presented as the
mean ± standard deviation. Student's t-test was performed to
compare differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR141 is decreased in
ESCC and in cases with lymph node metastasis
In order to investigate the expression of miR141 in
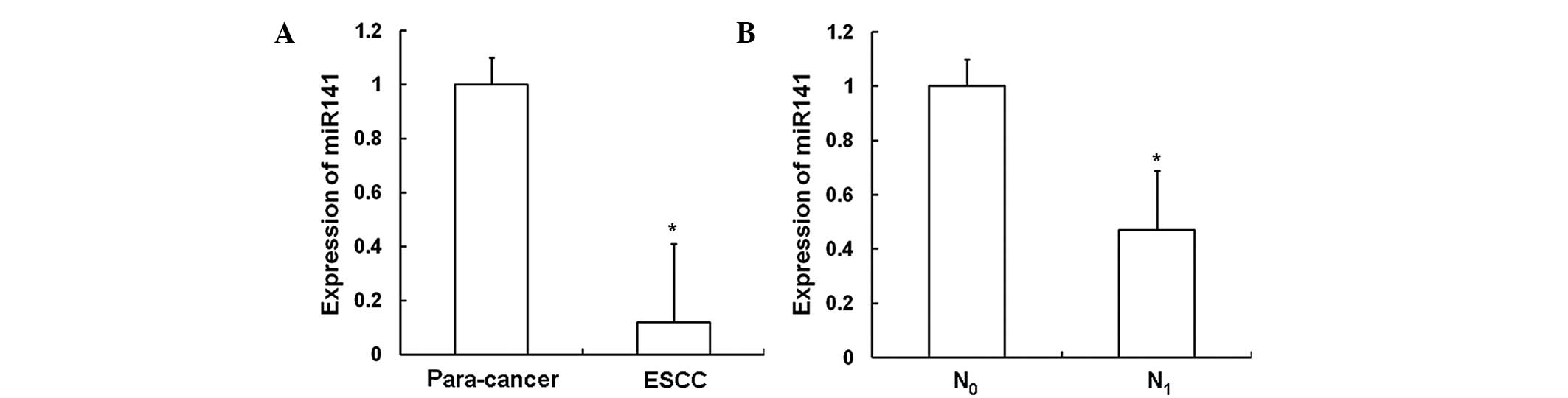
ESCC tissues, RT-qPCR was performed. As shown in Fig. 1A, the expression of miR141 in ESCC
tissues (0.12±0.29) was significantly decreased compared with that
in the adjacent normal tissues (P<0.05). As shown in Fig. 1B, the expression level of miR141 in
the tissues of patients with lymph node metastasis (N1;
0.47±0.22) was significantly decreased compared with that in the
tissues of patients without such metastasis (N0;
P<0.05). These results indicate that the expression of miR141
decreases as ESCC progresses.
Expression of STAT5 is mainly
localized in ESCC tissues
In order to investigate the distribution of STAT5 in
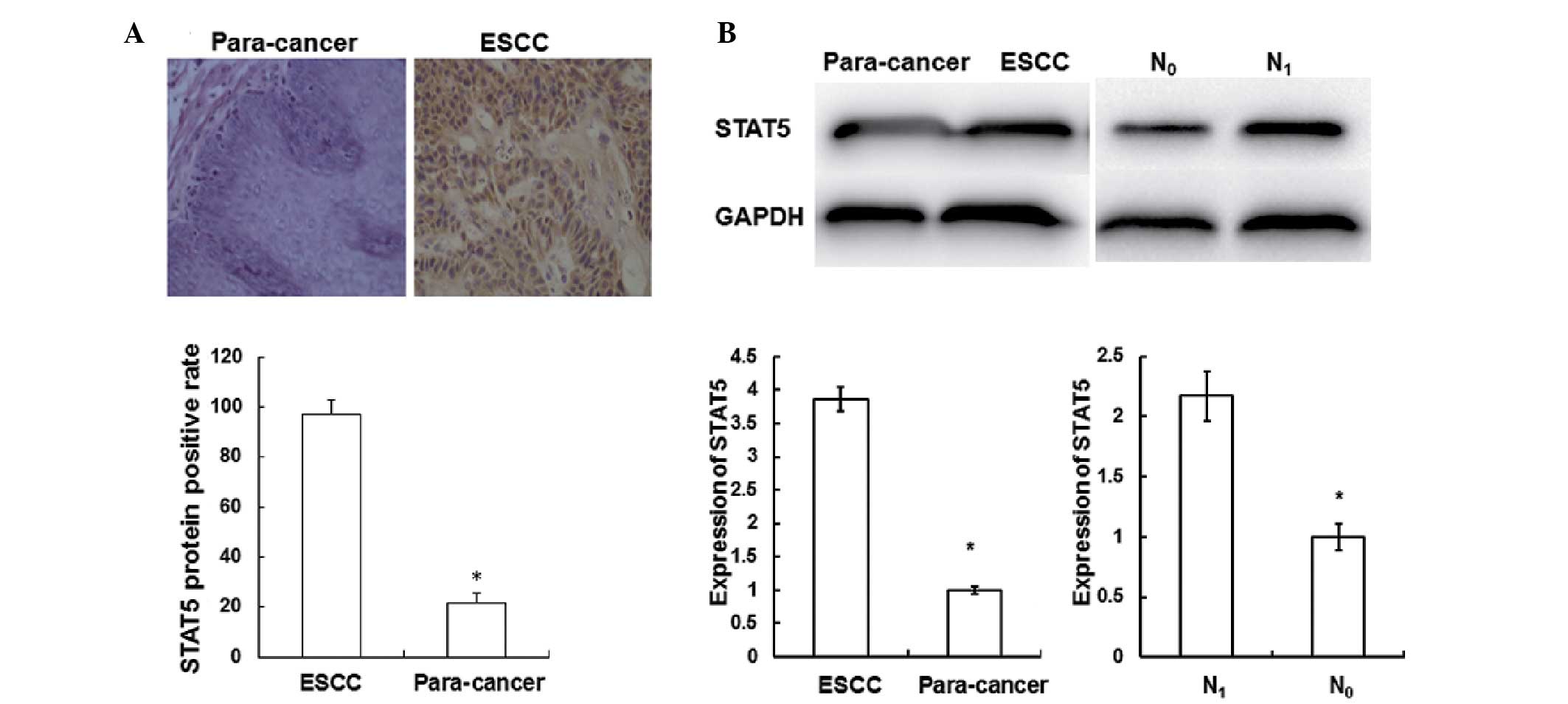
ESCC tissues, immunohistochemical analysis was performed. As shown
in Fig. 2A, STAT5 was predominantly
distributed in the cytoplasm and nuclei of the ESCC tissues. The
percentages of STAT5 in the ESCC tissues were significantly
increased (97.2%) compared with those in the adjacent normal
tissues (21.7%) (P<0.05); however, no statistically significant
differences were observed in miR141 expression according to gender,
age, tumor size, lesion location, differentiation and invasion
(P>0.05). These results indicate that STAT5 is mainly
distributed in ESCC tissues.
STAT5 protein is upregulated in ESCC
tissues
In order to investigate the STAT5 protein level in
ESCC tissues, western blotting was performed. As shown in Fig. 2B, when compared with the adjacent
normal tissues, the protein level of STAT5 in the ESCC tissues
(3.87±0.18) was significantly increased (P<0.05). The protein
level of STAT5 in the tissues from patients with lymph node
metastasis (2.17±0.21) was significantly increased compared with
that in the tissues from patients without such metastasis
(P<0.05). These results indicate that the expression of STAT5
increases as ESCC progresses.
miR141 inhibits the proliferation,
migration and invasion of Eca109 cells
To investigate the effects of miR141 on ESCC cells,
MTT, scratch and Transwell assays were performed in vitro.
As shown in Fig. 3A, the
proliferation of Eca109 cells in the miR141 mimic group was
significantly decreased compared with that in the NC and the normal
groups (P<0.05). As shown in Fig.
3B, in the scratch assay, the migration of Eca109 cells in the
miR141 mimic group was significantly decreased compared with that
in the NC and the normal groups (P<0.05). Furthermore, as
Fig. 3C and D shows, in the
Transwell assay, the invasion of Eca109 cells in the miR141 mimic
group was significantly decreased compared with that in the NC and
normal groups (P<0.05). These results indicate that miR141 has
an important effect on Eca109 cells, and can significantly inhibit
the proliferation, migration and invasion of ESCC cells.
 | Figure 3.Effects of miR141 on the
proliferation, migration and invasion of Eca109 cells in
vitro. MTT, scratch and Transwell invasion assays were
performed to investigate the effects of miR141 on Eca109 cells. The
experiments were repeated ≥3 times. (A) Graph showing the
proliferation of cells detected using MTT assay. Following
transfection with miR141 mimic, the proliferation of Eca109 cells
was detected at 0, 24, 48 and 72 h. (B) Migration of Eca109 cells
was detected using a scratch assay. Following transfection with
miR141 mimic, scratches were created using a sterile pipette tip
and cells were incubated for 24 h. The cells in the scratch were
then counted under a microscope (magnification, ×10). (C) Images
showing the invasion of Eca109 cells stained with Giemsa in the
Transwell assay (magnification, ×200). (D) Bar graph showing
quantitative results of the Transwell assay. The number of cells
that penetrated through the membrane was counted and compared among
the different groups. *P<0.05 vs. the normal control group;
#P<0.05 vs. the NC group. Error bars show standard
error of mean. miR141, microRNA-141; OD, optical density; NC,
negative control; MTT,
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium
bromide. |
miR141 and STAT5 are negatively
associated in ESCC tissues
In order to investigate the association between
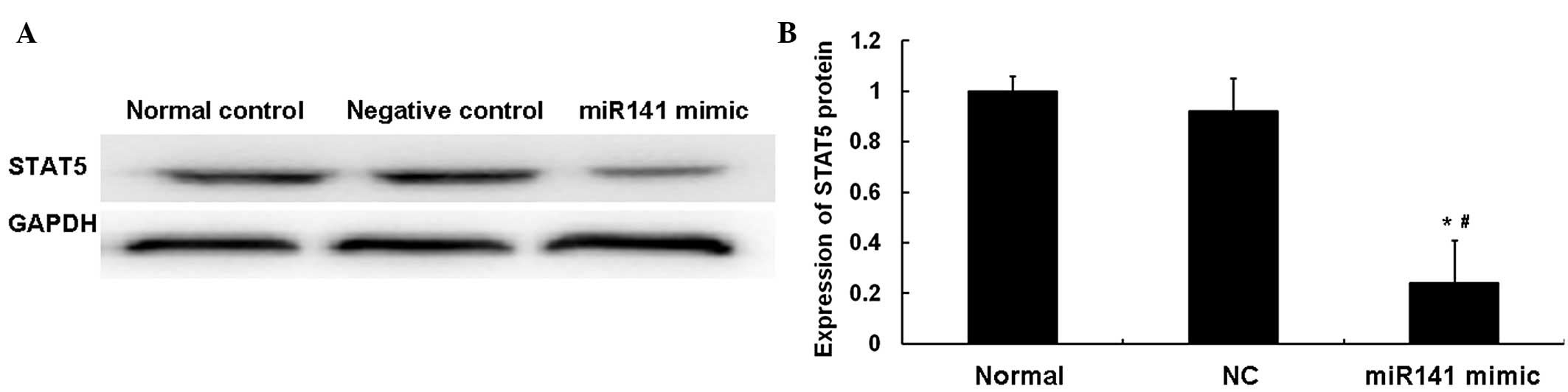
miR141 and STAT5 in ESCC, western blotting was performed. As shown
in Fig. 4, the protein level of
STAT5 in the miR141 mimic group was significantly decreased
compared with that in the NC group (P<0.05). No statistically
significant difference was observed between the normal and NC
groups (P>0.05). These results indicated that miR141 and STAT5
are negatively associated in ESCC tissues.
Discussion
In the present study, the results indicated that
miR141 functions as a tumor suppressor gene in ESCC, and that
miR141 promotes the proliferation, invasion and metastasis of ESCC
by decreasing the expression of the STAT5 gene.
miR141 has been reported to be abnormally expressed
in various types of tumors, and miR141 has been indicated to be
involved in the regulation of tumor cell proliferation, invasion
and metastasis (12–14). For example, Zhou et al found
that miR141 was downregulated in gastric cancer tissues and
inhibited the proliferation and cell cycle of gastric cancer cells
by interacting with long non-coding RNA MEG3 (12). Abedi et al found that miR141
expression was downregulated in two breast cancer cell lines, and
suggested that miR141 may act through suppressing the expression
and nuclear translocation of β-catenin, which is known to be
important in breast cancer pathogenesis (13). In a study of endometrial carcinomas,
miR141 expression was found to be upregulated, and miR141 exhibited
a close association with the estrogen receptor signaling pathway
(14).
In the present study, the RT-qPCR analysis showed
that the expression of miR141 in the ESCC tissues was significantly
decreased compared with that in the adjacent normal tissues
(P<0.05). In addition, the expression of miR141 in the tissues
from the patients with lymph node metastasis was significantly
decreased compared with that from patients without such metastasis
(P<0.05). The results of immunohistochemical analysis showed
that the percentage of STAT5-positive cells in ESCC tissues was
significantly increased compared with that in the adjacent normal
tissues (P<0.05); however, no statistically significant
differences were observed according to gender, age,
differentiation, tumor size or lesion location (P>0.05). The
results of western blotting showed that the expression level of
STAT5 in the ESCC tissues was significantly increased compared with
that in the adjacent normal tissues (P<0.05). The STAT5
expression level in the tissues from patients with lymph node
metastasis was significantly increased compared with that in
tissues from patients without such metastasis (P<0.05). Results
of in vitro experiments showed that the proliferation,
migration and invasion of Eca109 cells in the miR141 mimic group
were significantly inhibited compared with those in the NC and the
normal groups (P<0.05). Results of western blotting showed that
the expression of STAT5 in the miR141 mimic group was significantly
decreased compared with that in the NC and the normal groups
(P<0.05), but there was no statistically significant difference
between the NC and the normal groups (P>0.05). Furthermore,
miR141 and STAT5 exhibited a negative association in the ESCC
tissues.
In conclusion, the expression of miR141 was found to
be significantly decreased in the ESCC tissues and to have an
association with lymph node metastasis. miR141 and STAT5 were
negatively associated in the ESCC tissues. The proliferation,
migration and invasion of Eca109 cells were significantly inhibited
by transfection with miR141 mimic in vitro, which indicates
that miR141 may play an important role in the development,
invasion, and metastasis of ESCC cells by regulating the expression
of STAT5.
Acknowledgements
This study was supported by the Science And
Technology Development Project of Linyi City (grant no.
201213022).
References
|
1
|
Baba Y, Watanabe M, Yoshida N and Baba H:
Neoadjuvant treatment for esophageal squamous cell carcinoma. World
J Gastrointest Oncol. 6:121–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roshandel G, Nourouzi A, Pourshams A,
Semnani S, Merat S and Khoshnia M: Endoscopic screening for
esophageal squamous cell carcinoma. Arch Iran Med. 16:351–357.
2013.PubMed/NCBI
|
|
3
|
Gu J, Wang Y and Wu X: MicroRNA in the
pathogenesis and prognosis of esophageal cancer. Curr Pharm Des.
19:1292–1300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roth C, Rack B, Müller V, Janni W, Pantel
K and Schwarzenbach H: Circulating microRNAs as blood-based markers
for patients with primary and metastatic breast cancer. Breast
Cancer Res. 12:R902010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roth C, Kasimir-Bauer S, Pantel K and
Schwarzenbach H: Screening for circulating nucleic acids and
caspase activity in the peripheral blood as potential diagnostic
tools in lung cancer. Mol Oncol. 5:281–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dorris ER, Smyth P, O'Leary JJ and Sheils
O: MIR141 expression differentiates hashimoto thyroiditis from ptc
and benign thyrocytes in irish archival thyroid tissues. Front
Endocrinol (Lausanne). 3:1022012.PubMed/NCBI
|
|
7
|
Sossey-Alaoui K, Bialkowska K and Plow EF:
The miR200 family of microRNAs regulates WAVE3-dependent cancer
cell invasion. J Biol Chem. 284:33019–33029. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Severi I, Senzacqua M, Mondini E, Fazioli
F, Cinti S and Giordano A: Activation of transcription factors
STAT1 and STAT5 in the mouse median eminence after systemic ciliary
neurotrophic factor administration. Brain Res. 1622:217–229. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schafranek L, Nievergall E, Powell JA,
Hiwase DK, Leclercq T, Hughes TP and White DL: Sustained inhibition
of STAT5, but not JAK2, is essential for TKI-induced cell death in
chronic myeloid leukemia. Leukemia. 29:76–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Assefnia S, Kang K, Groeneveld S, Yamaji
D, Dabydeen S, Alamri A, Liu X, Hennighausen L and Furth PA: Trp63
is regulated by STAT5 in mammary tissue and subject to
differentiation in cancer. Endocr Relat Cancer. 21:443–457. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antoon JW, Nitzchke AM, Martin EC, Rhodes
LV, Nam S, Wadsworth S, Salvo VA, Elliott S, Collins-Burow B,
Nephew KP and Burow ME: Inhibition of p38 mitogen-activated protein
kinase alters microRNA expression and reverses
epithelial-to-mesenchymal transition. Int J Oncol. 42:1139–1150.
2013.PubMed/NCBI
|
|
12
|
Zhou X, Ji G, Ke X, Gu H, Jin W and Zhang
G: MiR-141 inhibits gastric cancer proliferation by interacting
with long noncoding RNA MEG3 and down-regulating E2F3 expression.
Dig Dis Sci. 60:3271–3282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abedi N, Mohammadi-Yeganeh S, Koochaki A,
Karami F and Paryan M: miR-141 as potential suppressor of β-catenin
in breast cancer. Tumour Biol. Jul 13–2015.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Y, Si JW, Li WT, Liang L, Zhao J,
Zhou M, Li D and Li T: miR-200a/miR-141 and miR-205 upregulation
might be associated with hormone receptor status and prognosis in
endometrial carcinomas. Int J Clin Exp Pathol. 8:2864–2875.
2015.PubMed/NCBI
|