Introduction
Concurrent manifestation of congenital absence of
gallbladder and atrial septal defect (ASD) is a rare congenital
organ malformation in clinical practice (1). No apparent association has been
identified between these two congenital organ malformations.
Sufficient attention must be paid to these disorders as they are
often misdiagnosed or overlooked during diagnosis. The incidence of
gallbladder agenesis in the general population is reported to be
13–65/100,000 (2). In clinical
practice, the incidence is 0.007–0.0027%, while in autopsy series
it is 0.04–0.13% (3). Females are
more commonly affected (ratio, 3:1), and >50% of patients with
gallbladder agenesis are symptomatic and require surgical
intervention (4,5). It is crucial that clinicians consider
gallbladder agenesis in cases where the gallbladder appears
abnormal on preoperative imaging studies and cannot be identified
using laparoscopy (6). Preoperative
diagnostic imaging such asmagnetic resonance
cholangiopancreatography (MRCP) and endoscopic ultrasound should be
considered. If such a condition is encountered during surgery,
intraoperative cholangiography and intraoperative ultrasound may be
performed to rule-out agenesis and ectopic gallbladder (7). In the present case report, the
diagnosis and surgical intervention of gallbladder agenesis and ASD
is described.
Case report
A 38-year-old male visited the Affiliated Hospital
of Inner Mongolia Medical University (Hohhot, China) in December
2010 with a 3-year history of recurring upper right abdominal pain,
which had deteriorated in the last week. The pain had no evident
cause. It was accompanied by dyspepsia and gasteremphraxis with
indigestion, and was exacerbated following meals.
Several color Doppler ultrasonography scans were
performed in our hospital, which revealed cholecystitis and
gallbladder stones. In the week prior to hospital admission, the
aforementioned symptoms deteriorated and thus the patient visited
our hospital for treatment. Ultrasound imaging had been previously
performed in the Inner Mongolia Xilingol League Hospital (Xilingol
League, China), and further scans were subsequently conducted on
patient admission to our hospital. Following physical examination,
cardiac murmur was detected. In addition, the color Doppler
ultrasonography scans of the heart were indicative of congenital
heart disease (CHD). The patient was admitted to the Cardiovascular
Surgery Department of our hospital under the diagnosis of CHD with
concomitant congenital ASD and chronic atrophic cholecystitis with
gallbladder stones. During the 34 days of hospitalization, the
patient exhibited occasional palpitations and shortness of breath,
fatigue and cyanosis of the lips.
The medical history of the patient included
self-reported palpitations and shortness of breath following
exercise. In addition, the patient had been prone to
activity-induced fatigue since childhood, and had a history of
susceptibility to respiratory tract infection.
Auscultation of the pulmonary valve revealed
accentuation of the second heart sound at the left side of the
sternum, between the first and second ribs, and grade 3/6 systolic
murmur. Furthermore, a soft abdomen without upper right abdominal
pain on palpation, rebound tenderness, muscle rigidity, a negative
Murphy's sign and borborygmus at a rate of 3/min were
identified.
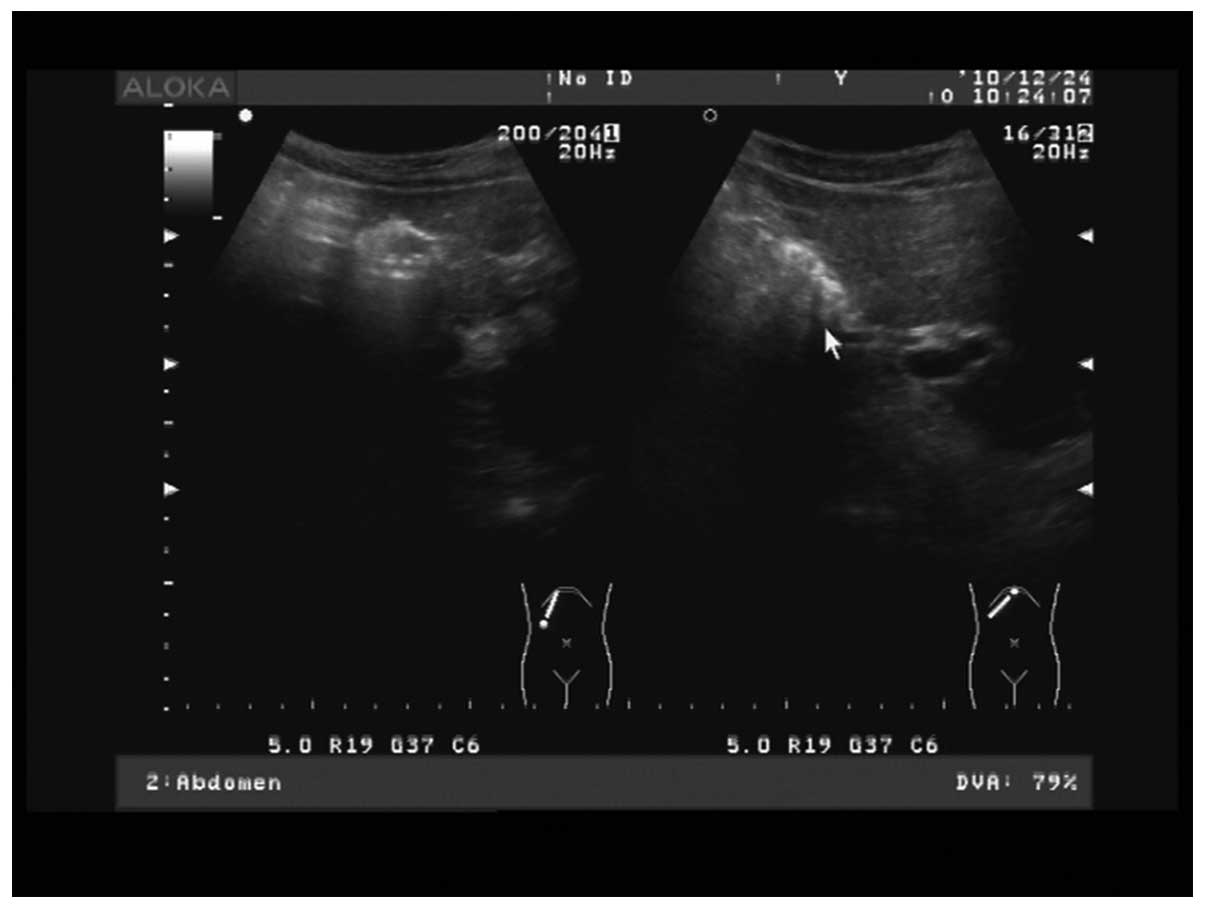
Upon auxiliary examination, color Doppler ultrasound
of the abdomen indicated chronic atrophic cholecystitis with
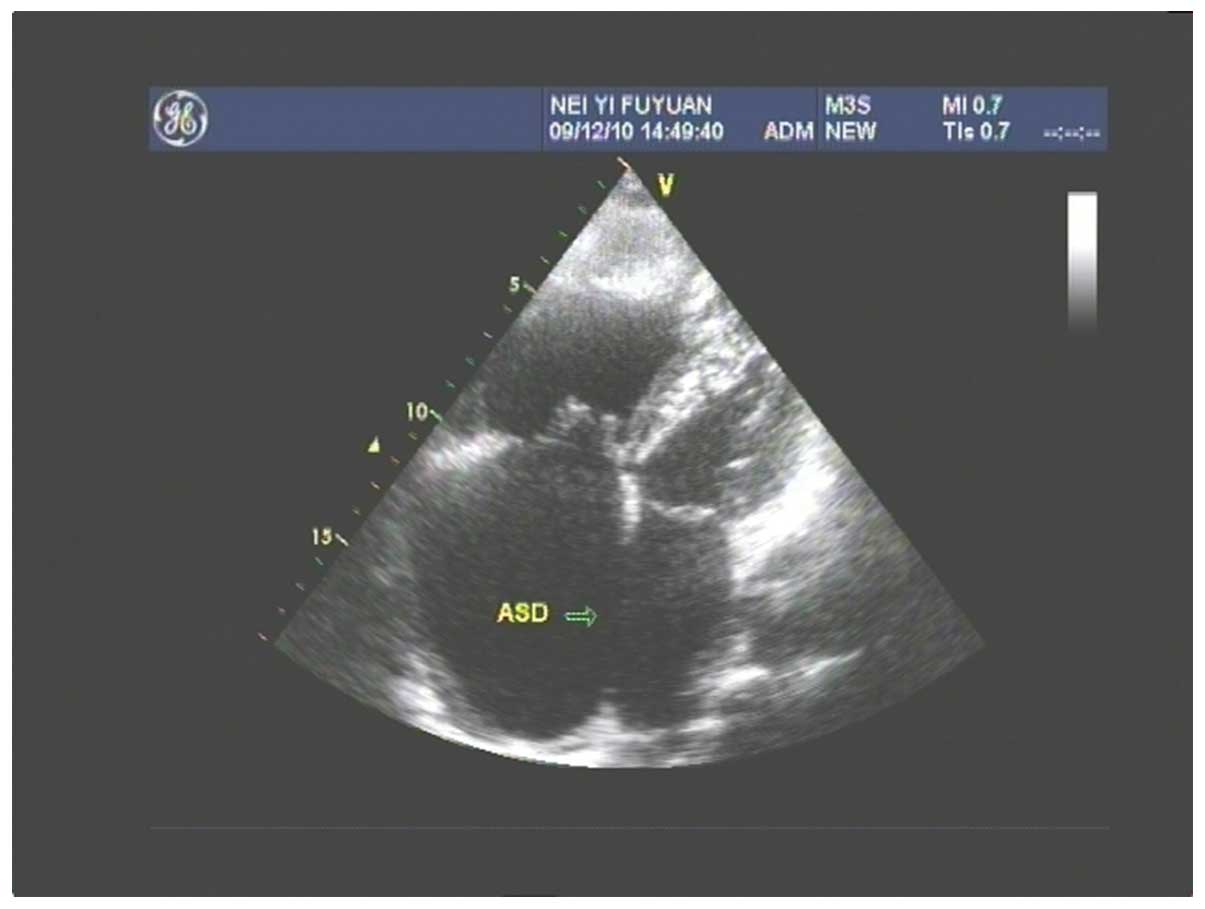
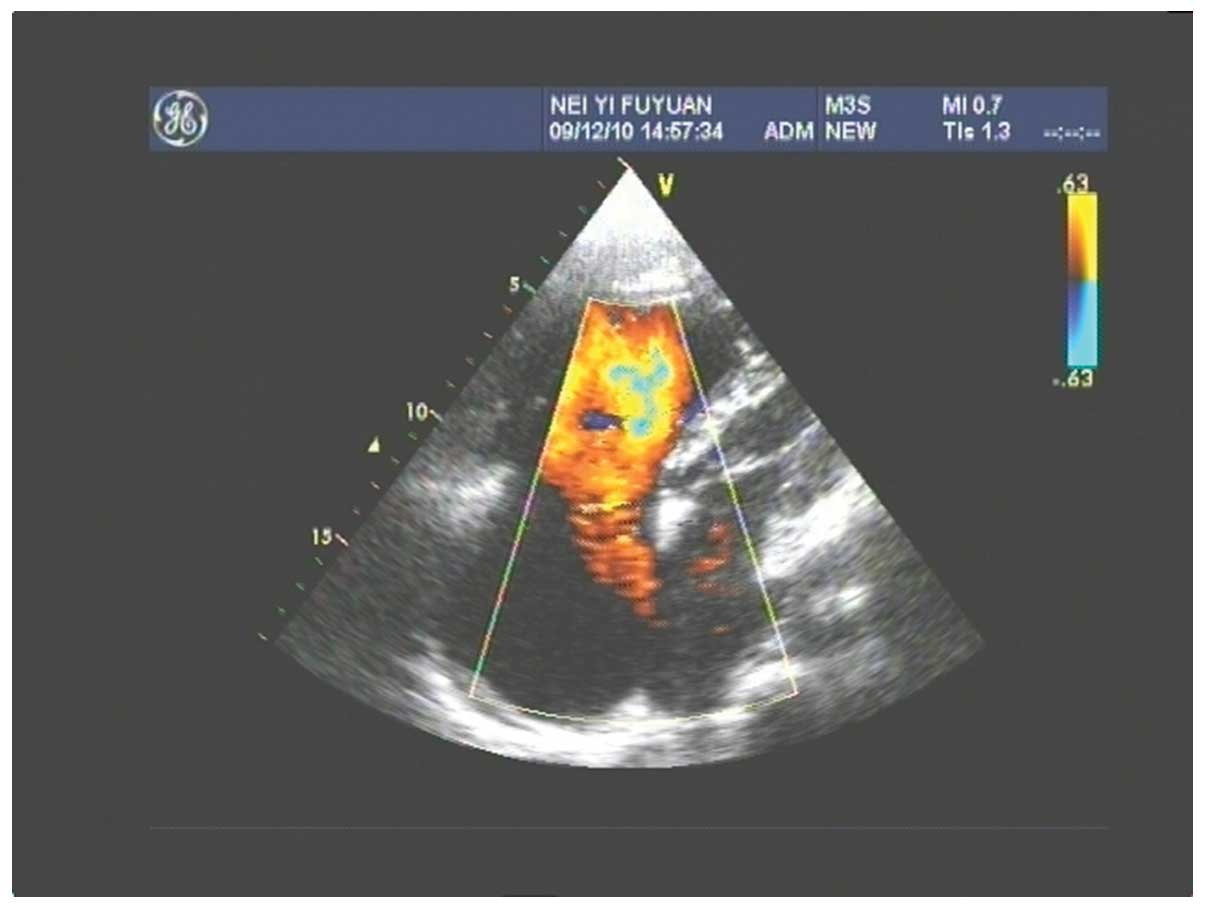
gallbladder stones. Fig. 1 shows a
color Doppler ultrasound of the congenital ASD, atrial septum
consistency degradation, echo loss of 50 mm, large left-to-right
shunt volume and a flow rate of 0.73 m/sec. Figs 2 and 3
show the left anterior descending artery examination results, which
identified that the patient was positive for Hepatitis C virus
antibody, while the results of routine blood test and biochemistry
were normal.
Cardiovascular surgery was performed via a chest
incision, and inter-ASD repair was achieved with extracorporeal
circulation. At 12 days postoperatively, the patient recovered.
Subsequently, a mini-incision cholecystectomy was performed to
confirm the diagnosis of gallbladder agenesis. During surgical
exploration, ~8×3×2-mm soft tissue fragments were found in the
porta hepatis. During the dissection, normal, soft, fat-like tissue
was observed, with no tissue changes in the chorionic and muscular
layer, mucous membrane degradation or changes in the gallbladder
tissue identified. Gallbladder agenesis was considered as a
possible diagnosis during surgery, excluding the possibility of an
outer-ectopic gallbladder. After gradually extending the incision
site and freely dissecting the first part of the choledochus at a
length of ~9 cm, the gallbladder, cystic duct and biliary branches
were not found. The porta hepatis was dissociated from the liver,
but the portal vein and tube wall were found intact.
Despite the dissociation of the porta hepatis from
the liver, the gallbladder and cystic duct were still not found.
Following discussion during the surgery, the surgical exploration
was terminated and the inner liver, posterior pancreatic head and
duodenum were examined using color Doppler ultrasonography. A
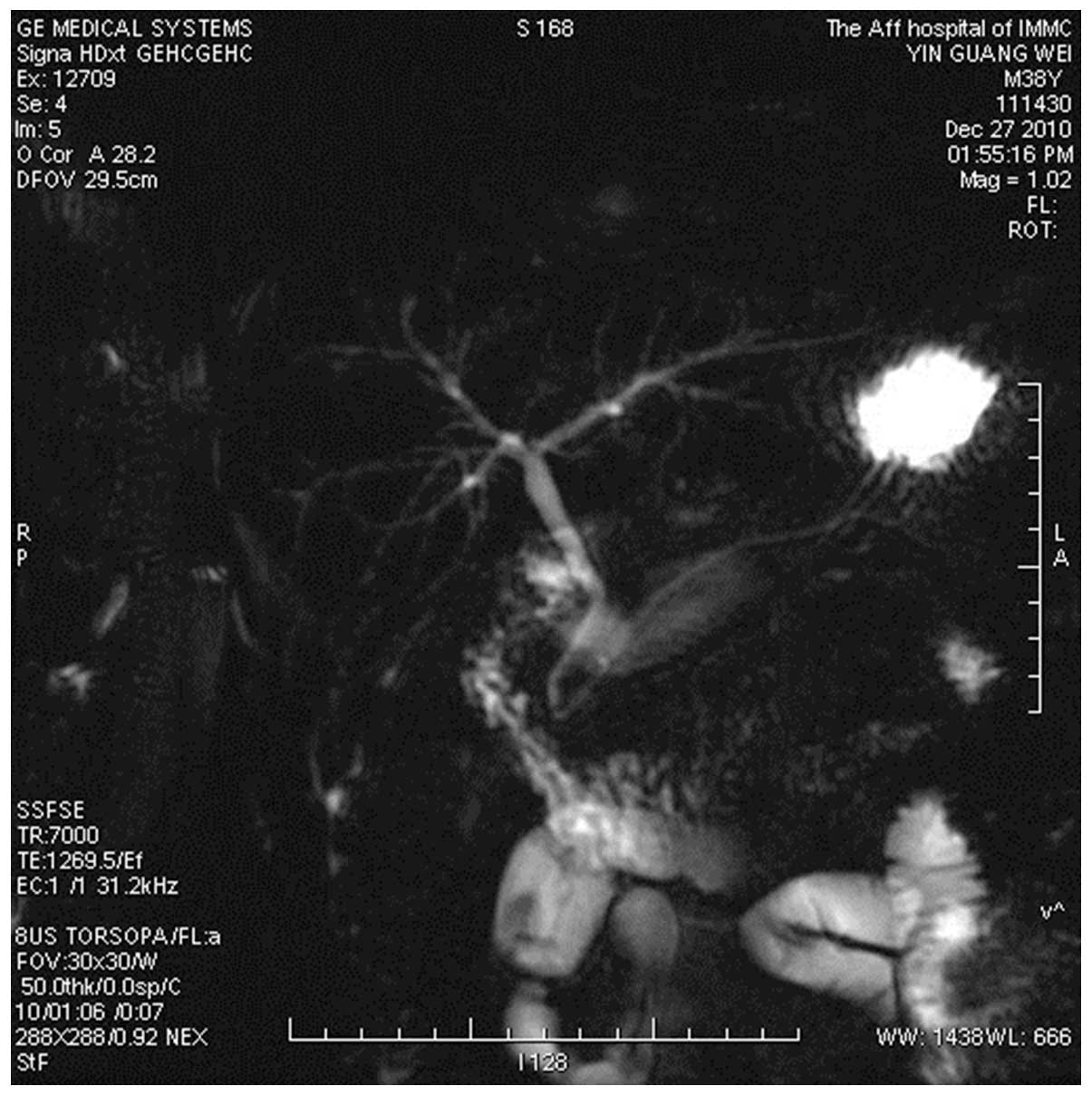
magnetic resonance cholangiopancreatography (MRCP) scan of the
abdomen was performed in order to confirm the diagnosis of
gallbladder and cystic duct hypoplasia (Fig. 4). The patient recovered and was
discharged from the hospital 5 days later, in January 2011.
Following surgery, the patient received 6 phone follow-up
consultations (once per month). The date of final follow-up was
August 2015. No new symptoms, further treatment or outcomes
occurred. No upper-right abdominal pain or surgical complications
were reported. Written informed consent was obtained from the
patient.
Discussion
Gallbladder agenesis is a rare gallbladder
malformation caused by the agenesis of the liver diverticulum tail
during the fourth week of the embryonic period (8,9). Lemery
and Bergman first reported this condition in 1701 (10). The incidence rate of gallbladder
agenesis in countries other than China has been found to be
0.013–0.065% (11). The present case
of gallbladder agenesis was confirmed during a surgical
intervention in the patient during his initial visit to our
hospital. Following the retrieval of hospital records, 33,983 cases
of gallbladder removal were identified between 1975 and 2013, which
translates to an incidence rate of 0.0029%, with a rare prevalence
in patients with gallbladder agenesis accompanied by ASD, which is
below the reported rate for countries other than China. Singh et
al (12) classified this
condition into the following 3 types, according to their clinical
features (13): i) Multiple
congenital anomaly (12.8%); ii) asymptomatic (31.6%); and iii)
symptomatic (55.6%). The patient of the current study presented
with a combination of a large ASD and type I congenital gallbladder
agenesis.
Ultrasonography is the preferred imaging modality
for gallbladder diseases. Due to the scarcity of reports on
gallbladder agenesis and the different experiences among
physicians, various confounding diagnoses of gallbladder agenesis
exist in the clinical field. With regard to patients whose
preoperative color Doppler ultrasounds show numerous calculi in the
gallbladder, and gallbladder contraction, imaging practitioners and
surgeons should be aware of this disease. Furthermore, patients
with physical deformities in one organ should be examined for
possible deformities in other organs. Patients with suspicion of
gallbladder agenesis should undergo MRCP or endoscopic retrograde
cholangiopancreatography (4) in
order to confirm the diagnosis.
Surgical intervention is not recommended for
patients who are suspected of having gallbladder agenesis but have
exhibited no acute abdominal symptoms (14). Ectopic gallbladder should be excluded
prior to or during surgery in order to confirm the diagnosis of
gallbladder agenesis. The use of B-mode ultrasonography should be
considered for perioperative diagnosis. Perioperative diagnosis can
avoid the limitation of preoperative percutaneous B-mode
ultrasonography and excessive and blind examination, in order to
prevent an increased risk of iatrogenic damages. With regard to
patients who exhibit local expansion of the hepatoduodenal ligament
during surgery and those highly suspected of having gallbladder
contraction near the common hepatic duct and choledochus, adequate
deboning of the porta hepatis and the adhesion between the liver
surface and transverse colon should be considered. Along the
superior border of the duodenum, the choledochus to porta hepatis
should be dissected, the full length of the outer-liver bile duct
should be identified and the situation of gallbladder requires
confirmation. The diagnosis of gallbladder agenesis should be
definitive and only established after extensive examinations
(6,14).
Previous studies have demonstrated that a definitive
diagnosis of gallbladder agenesis is challenging through ordinary
preoperative examination (4,15). MRCP is a noninvasive and effective
method of biliary tract imaging, and it is not affected by
cholestasis. It can also be used for exclusion of the diagnosis of
ectopic gallbladder. In the present case, the patient was examined
using MRCP following surgery. MRCP showed a good biliary tree image
development, without observation of the gallbladder or ectopic
gallbladder. Although MRCP cannot replace ultrasonography as a
diagnostic modality for gallbladder diseases, it can provide
anatomical information on the gallbladder fossa and has a clear
advantage in the diagnoses of ectopic gallbladder and gallbladder
agenesis (16). If the patient of
the present case had been correctly diagnosed preoperatively, no
unnecessary surgical exploration with a risk of complications would
be required.
In conclusion, the present case attempted to provide
an improved understanding of gallbladder agenesis, which would
assist diagnosis, as well as the knowledge obtained by various
examination methods. Thus, the diagnosis established should be as
definitive as possible in order to avoid misdiagnosis. Physicians
should be particularly aware of the fact that gallbladder agenesis
tends to be discovered and diagnosed during surgical removal of the
gallbladder. Due to the lack of insight in gallbladder agenesis,
surgeons often conduct an extensive search for the gallbladder and
dissect the bile duct, porta hepatis tissue duodenum and other
parts, which can damage the biliary tract and cause unnecessary
injuries.
Acknowledgements
This study was supported by a grant from the
Ministry of Health of P.R. China (grant no. 201302073).
References
|
1
|
Langer T, Baudenbacher R, Hablützel K and
Kehl O: Gallbladder agenesis: A diagnostic problem? Schweiz Rundsch
Med Prax. 30:646–648. 1989.(In German).
|
|
2
|
Richards RJ, Taubin H and Wasson D:
Agenesis of the gallbladder in symptomatic adults. A case and
review of the literature. J Clin Gastroenterol. 16:231–233. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peloponissios N, Gillet M, Cavin R and
Halkic N: Agenesis of the gallbladder: A dangerously misdiagnosed
malformation. World J Gastroenterol. 11:6228–6231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh BG, Rao KPK, Ghosh SR and Chaudhry
R: Congenital absence of gall bladder. Med J Armed Forces India.
59:152–153. 2003. View Article : Google Scholar
|
|
5
|
Kasi PM, Ramirez R, Rogal SS, Littleton K
and Fasanella KE: Gallbladder agenesis. Case Rep Gastroenterol.
5:654–662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stephenson JA, Norwood M, Al-Leswas D,
Al-Taan O, Beable R, Lloyd DM and Dennison AR: Hepatic haemangioma
masquerading as the gallbladder in a case of gallbladder agenesis:
A case report and literature review. HPB Surg. 2010:9716092010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilson JE and Deitrick JE: Agenesis of the
gallbladder: Case report and familial investigation. Surgery.
99:106–109. 1986.PubMed/NCBI
|
|
8
|
Haddock G, Morran CG and Anderson JR:
Agenesis of the gallbladder. J R Coll Surg Edinb. 31:100–101.
1986.PubMed/NCBI
|
|
9
|
Gotohda N, Itano S, Horiki S, Endo A,
Nakao A, Terada N and Tanaka N: Gallbladder agenesis with no other
biliary tract abnormality: Report of a case and review of the
literature. J Hepatobiliary Pancreat Surg. 7:327–330. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nadeau LA, Cloutier WA, Konecki JT, Morin
G and Taylor RW: Hereditary gallbladder agenesis: Twelve cases in
the same family. J Maine Med Assoc. 63:1–4. 1972.PubMed/NCBI
|
|
11
|
Malde S: Gallbladder agenesis diagnosed
intra-operatively: A case report. J Med Case Rep. 4:2852010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh B, Ramsaroop L, Allopi L, Moodley J
and Satyapal KS: Duplicate gallbladder: An unusual case report.
Surg Radiol Anat. 28:654–657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joliat GR, Shubert CR and Farley DR:
Isolated congenital agenesis of the gallbladder and cystic duct:
Report of a case. J Surg Educ. 70:117–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh B, Moodley J, Haffejee AA and
Rajaruthnam P: Laparoscopic diagnosis of gallbladder agenesis. Surg
Laparosc Endosc. 7:129–132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smadi SI, Naasan WA Alkaabneh and Haddad
JS: Agenesis of Gall Bladder. A Case Report. J Res Med Sci.
12:63–65. 2005.
|
|
16
|
Fiaschetti V, Calabrese G, Viarani S,
Bazzocchi G and Simonetti G: Gallbladder agenesis and cystic duct
absence in an adult patient diagnosed by magnetic resonance
cholangiography: Report of a case and review of the literature.
Case Rep Med. 2009:6747682009.PubMed/NCBI
|