Introduction
Worldwide, ~170 million individuals are infected
with the hepatitis C virus (HCV) and cirrhosis is estimated to
develop in 5–20% of patients ~20 years after acquiring the
infection (1). In Japan, >70% of
cases of chronic hepatitis C are caused by HCV genotype 1b (HCV-1b)
in high viral loads (>5.0 log IU/ml) (2). A maximum of 50% of patients infected
with HCV-1b attain a sustained viral response (SVR) following
pegylated-interferon (peg-IFN)-α and ribavirin (RBV) combination
therapy (2,3). Telaprevir (TVR), an inhibitor of HCV
NS3/4A protease, has been approved for the treatment of patients
infected with HCV-1b (4–7). Although TVR-based triple therapy,
combining TVR with peg-IFNα and RBV, has improved the SVR rate to
>70%, critical adverse effects, including anemia, skin rashes
and renal dysfunction, may lead to a dose reduction or treatment
discontinuation, resulting in a reduced SVR rate (4–7).
Renal toxicity as a side-effect to medication occurs
through alterations to plasma filtration and the maintenance of
metabolic homeostasis (8). According
to a previous study, the incidence of drug-associated acute tubular
necrosis or acute interstitial nephritis may be as high as 18.3%
(8). In the majority of cases, renal
dysfunction was reversible and the blood test parameters normalized
when the causal drug was discontinued; however, a number of
medications induced chronic renal injury (8). Drugs may affect renal perfusion or
injure vascular, tubular, glomerular and interstitial cells
directly (9). As the TVR-based
triple therapy is administered over a period of 3–6 months, the
authors of the present study hypothesized that the renal damage may
be chronic. However, little is known regarding the mechanism of the
onset and development of renal dysfunction during HCV therapy. The
present study therefore aimed to analyze the pathogenesis of renal
dysfunction in patients infected with HCV-1b during TVR-based
triple therapy. Analysis of clinical parameters associated with
renal injury, including urinary sediment presence, serum creatinine
level, estimated glomerular filtration rate (eGFR), fractional
excretion of sodium (FENa) and β2-microglobulin level,
were examined as potentially important metrics. A knowledge of FENa
levels is useful for the evaluation of acute renal failure
(10); low FENa (<1%) indicates
kidney retention of sodium and exogenous renal dysfunction caused
by pre-renal disease, whilst higher percentages (>2%) suggest
leakage of sodium due to endogenous renal failure. Urinary
β2-microglobulin values were also used to evaluate renal
filtration function.
Materials and methods
Study patients and drug regimen
A standard treatment regimen (5,6) was
adopted for patients infected with HCV-1b at the Kyushu Medical
Center (Fukuoka, Japan) between December 2011 and April 2013. A
12-week triple therapy was administered, including oral TVR (2,250
mg/day; Mitsubishi Tanabe Pharma Corporation, Tokyo, Japan), weekly
subcutaneous peg-IFNα2b (median dose, 1.5 µg/kg; range, 1.3–1.7
µg/kg; MSD Pharmaceuticals, Tokyo, Japan) and oral RBV (600–1,000
mg/day; MSD Pharmaceuticals), followed by a 12-week dual therapy of
peg-IFNα2b and RBV. The dose of RBV was adjusted according to body
weight; 600 mg was administered to patients weighing <60 kg, 800
mg to patients weighing 60–80 kg and 800 mg to patients weighing
>80 kg. Although the standard dose of TVR is 2,250 mg/day, the
dose was reduced to 1,500 mg/day for small patients or aging women.
The present study conformed with the ethical guidelines of the 2013
Declaration of Helsinki and was approved by the Ethics Committee of
the National Hospital Organization (No. 13–72). The patients were
provided with an explanation of the aims and outline of the study,
and written informed consent was obtained from all. A total of 119
patients infected with HCV-1b were enrolled. The patient profiles
and their baseline characteristics are reported in Table I. In all patients, the baseline HCV
RNA levels in serum were >5.0 log IU/ml.
 | Table I.Patient background. |
Table I.
Patient background.
|
| TVR dose |
|
|---|
|
|
|
|
|---|
| Factor | 2,250 mg/day | 1,500 mg/day | P-value |
|---|
| No. of patients | 20 | 99 |
|
| Gender
(male/female) | 7/13 | 46/53 | NS |
| Age, years | 51.8±97.4 | 60.0±9.5 | 0.0003 |
| History of IFN
therapy | 8/4/8 | 42/18/39 | NS |
| (naive/IFN/IFN + RBV
patients) |
|
|
|
| HCV RNA, log
IU/ml | 6.45±0.61 | 6.27±0.61 | NS |
| IL-28B (rs8099917)
(TT/TG + GG) | 6/14 | 34/65 | NS |
| ITPA (rs1127354)
(CC/CA + AA) | 5/15 | 26/73 | NS |
| Core 70
(WT/mutations) | 11/9 | 65/34 | NS |
| ALT, IU/l | 123.6±110.3 | 86.5±77.0 | NS |
| GGT, IU/l | 98.1±85.6 | 60.3±47.3 | 0.0063 |
| Neutrophils/µl | 2721±1189 | 2254±815 | 0.034 |
| Hemoglobin,
g/dl | 14.4±1.2 | 13.7±1.5 | NS |
| Platelets/µl | 17.1±5.5 | 16.0±6.0 | NS |
| Creatinine,
mg/dl | 0.65±0.15 | 0.70±0.18 | NS |
| eGFR, ml/min/1.73
m2 | 94.7±19.0 | 80.1±15.2 | 0.0003 |
| Uric acid | 5.38±1.42 | 5.69±1.24 | NS |
| TVR dose/body
weight/day | 34.0±6.5 | 24.7±4.6 | <0.0001 |
Laboratory data
Hematological, biochemical, urinary and virological
parameters were determined by the clinical laboratory at Kyushu
Medical Center. Renal dysfunction was defined as elevated serum
creatinine levels (>1.1 and 0.8 mg/dl for men and women,
respectively), or as an eGFR level <60 ml/min/1.73
m2. Severe renal dysfunction was defined as elevated
serum creatinine levels (>1.3 mg/dl) or as decreased eGFR levels
(<40 ml/min/1.73 m2). Serum HCV RNA concentrations
were determined using the COBAS TaqMan HCV test (Roche Diagnostics,
Tokyo, Japan). SVR was defined as no detectable HCV RNA, reported
at weeks 12 and 24 after therapy completion. A number of patients
were subject to genotyping of interleukin-28B (rs8099917) and
inosine triphosphatase (rs1127354) polymorphisms, performed using
TaqMan SNP Genotyping Assays (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) by applying polymerase chain reaction (PCR)-based
restriction fragment length polymorphism assays. To identify amino
acid polymorphisms in the HCV core protein, PCR was conducted using
primers specific to a polymorphism at core 70, in accordance with
the previously reported methodology (11,12).
Briefly, the PCR reaction system (25 µl) consisted of 2X Power SYBR
Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and 2.5 pmol of each primer. The PCR cycling conditions were
as follows: Denaturation at 95°C for 10 min, followed by 40 cycles
at 95°C for 15 sec and 60°C for 1 min. A melting curve analysis was
conducted to confirm their specificity. Peg-IFN, RBV and TVR were
discontinued in various combinations, or their doses were reduced
as required following a decrease in hemoglobin levels, neutrophil
or platelet counts, or the development of other adverse
side-effects. To appropriately evaluate therapeutic effects, SVR
rates were examined with the intention to treat.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were conducted using JMP software, version
8.0.2.2 (SAS Institute Inc., Cary, NC, USA). Differences between
categorical variables were analyzed using Fisher's exact test or a
χ2 test. A Mann-Whitney U test was used to compare
continuous variables. Multivariate analyses were conducted to
identify factors independently associated with renal dysfunction.
The odds ratio (OR) and 95% confidence intervals were also
determined. P<0.05 was considered to indicate a statistically
significant difference.
Results
Renal dysfunction during TVR-based
triple therapy
Serum creatinine values were markedly elevated in
all patients by ~0.2 mg/dl from the baseline from the third day,
and remained elevated until after week 12 of triple-therapy
administration (Fig. 1A); however,
this difference was not significant (P>0.05). The serum
creatinine concentration in the patients progressively decreased
after week 12, and baseline values were eventually restored
following cessation of TVR (Fig.
1A). The mean eGFR values markedly decreased by 15 ml/min/1.73
m2 during TVR administration, and improved following
cessation of TVR (Fig. 1B); however,
the mean eGFR values were not significantly different from the
baseline throughout the 24-week treatment period (P>0.05).
Treatment was discontinued in 2 patients due to the onset of acute
renal failure. In order to evaluate the underlying mechanism of
renal dysfunction, the presence of urinary sediment, granular casts
and tubular epithelial cells was evaluated, but no abnormal
findings were observed. The FENa values observed in the patients
were ~0.6% at days 3 and 7 of the therapy, when serum creatinine
and eGFR were markedly changed, in the renal dysfunction (n=17) and
normal function groups (n=102) (Fig.
1C). FENa values were also similar between patients treated
with TVR at 2,250 mg/day and those treated with a reduced TVR dose
(1,500 mg/day) (data not shown).
The present study investigated urinary
β2-microglobulin, a marker of tubular damage, at days 3
and 7 of the treatment. Urinary β2-microglobulin was
elevated, but reached abnormal levels (>290 µg/l) in only half
of the patients. Patients with renal dysfunction exhibited
significantly elevated levels of urinary
β2-microglobulin on day 3 of the treatment, as compared
with the baseline and patients without renal dysfunction
(P<0.01; Fig. 1D). However, there
was no significant difference in β2-microglobulin levels
between patients with renal dysfunction and those without renal
dysfunction on day 7 of the treatment (P>0.05; Fig. 1D).
Predictive factors associated with
renal dysfunction
Within the listed factors in Table II, predictive factors associated
with renal dysfunction were examined in patients undergoing triple
therapy. Univariate analysis identified two parameters that were
significantly correlated with renal dysfunction; these were serum
creatinine (P=0.048) and TVR dose (P<0.0001) (Table II). From a multivariate analysis,
TVR dose (2,250 mg/day; OR, 102.6; P<0.0001) and eGFR (<90
ml/min/1.73m2; OR, 30.5; P=0.003) were demonstrated to
be significant contributory factors for renal dysfunction (Table III). Creatinine was not shown to be
a significant contributory factor in this multivariate analysis
(P>0.05).
 | Table II.Factors involved in renal
dysfunction. |
Table II.
Factors involved in renal
dysfunction.
| Factor | Renal
dysfunction(−) | Renal
dysfunction(+) | P-value |
|---|
| No. of
patients | 102 | 17 |
|
| Gender
(male/female), n | 49/53 | 10/7 | NS |
| Age, years | 59.1±9.9 | 56.4±8.1 | NS |
| History of IFN
therapy | 42/20/40 | 8/2/7 | NS |
| (naive/IFN/IFN+RBV
patients), n |
|
|
|
| Staging
(F0/F1/F2/F3/F4), n | 1/12/16/11/20 | 0/1/3/3/0 | NS |
| HCV RNA, log
IU/ml | 6.26±0.60 | 6.58±0.63 | NS |
| IL-28B (rs8099917)
(TT/TG + GG), n | 67/35 | 12/5 | NS |
| ITPA (rs1127354)
(CC/CA + AA), n | 76/26 | 12/5 | NS |
| Core 70
(WT/mutations), n | 66/36 | 10/7 | NS |
| ALT, IU/l | 74.6±86.2 | 81.5±71.5 | NS |
| GGT, IU/l | 67.1±56.4 | 64.2±61.5 | NS |
| Neutrophils/µl | 2302±842 | 2515±1205 | NS |
| Hemoglobin,
g/dl | 13.8±1.5 | 14.0±1.3 | NS |
| Platelets/µl | 16.0±6.0 | 17.2±5.7 | NS |
| Creatinine,
mg/dl | 0.65±0.13 | 0.72±0.16 | 0.048 |
| eGFR, ml/min/1.73
m2 | 85.3±15.4 | 80.1±12.8 | NS |
| Uric acid,
mg/dl | 5.61±1.26 | 5.80±1.40 | NS |
| Total cholesterol,
mg/dl | 141.8±27.4 | 151.5±34.8 | NS |
| Triglycerides,
mg/dl | 89.9±40.3 | 87.9±74.2 | NS |
| Diabetes mellitus,
-/+ | 92/10 | 1/16 | NS |
| TVR dose,
1,500/2,250 mg | 94/8 | 5/12 | <0.0001 |
 | Table III.Multivariate analysis for predictive
factors associated with renal dysfunction. |
Table III.
Multivariate analysis for predictive
factors associated with renal dysfunction.
| Factors | Odds ratio | 95% confidence
interval | P-value |
|---|
| TVR dose (2,250
mg/day) | 102.6 | 11.3–1650 | <0.0001 |
| eGFR (<90
ml/min/1.73 m2) | 30.5 | 4.2–568 | 0.0003 |
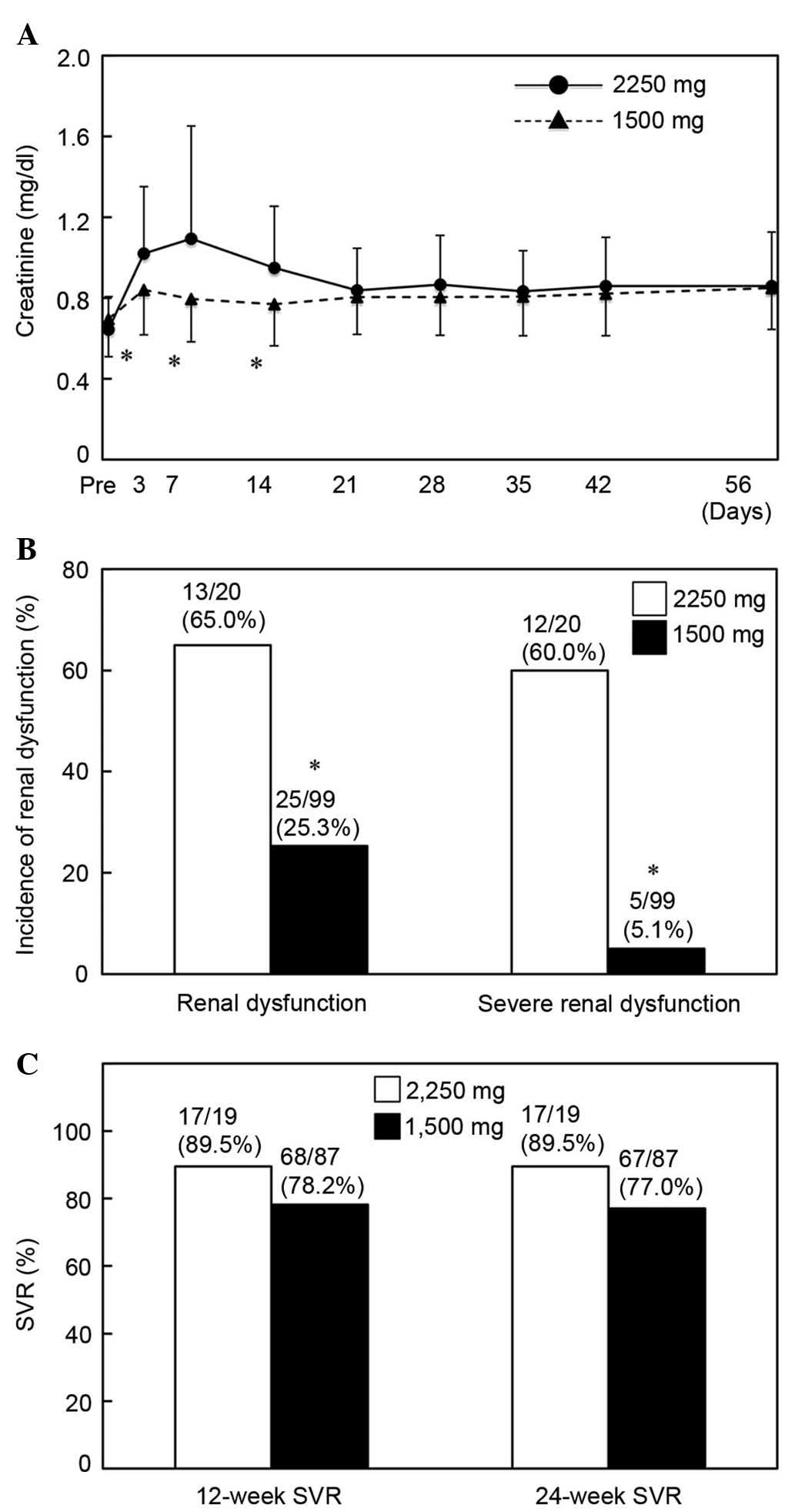
Effect of TVR dose on renal
dysfunction and therapeutic outcome
The present study evaluated the association between
TVR dose and pathogenesis, severity of renal dysfunction and
therapeutic outcome. Although the serum creatinine levels were
increased on day 3 of the treatment and had remained elevated in
patients treated with TVR at 1,500 mg/day, serum creatinine values
were significantly higher in patients treated with TVR doses of
2,250 mg/day at days 3, 7 and 14 of the treatment (P<0.01;
Fig. 2A). Although eGFR depression
was induced by TVR administration (Fig.
1B), the decrease in eGFR was smaller in patients receiving a
reduced TVR dose. However, no significant difference was observed
in the decreasing rates of eGFR between the low- and high-dose
groups (P>0.05; data not shown). Of the 20 patients treated with
TVR at 2,250 mg/day, 13 patients developed renal dysfunction; of
these, 12 patients exhibited severe dysfunction. Patients treated
with TVR at 1,500 mg/day demonstrated a significantly lower
incidence of renal dysfunction (P<0.01; Fig. 2B).
The therapeutic effects of TVR at 2,250 and 1,500
mg/day were assessed. SVR at 12 weeks was achieved in 17 patients
(89.5%) treated with TVR 2,250 mg/day and 68 patients (78.2%)
treated with TVR 1,500 mg/day, but no statistically significant
difference was revealed between the groups (P>0.05; Fig. 2C). Concordantly, the 24-week SVR rate
in patients receiving a reduced dose of TVR was lower than that of
patients with TVR at 2,250 mg/day (77.0 and 89.5%, respectively);
however, this difference was not statistically significant
(P>0.05; Fig. 2C). SVR was
undetermined in four patients due to discontinuation of treatment
(2 patients) and hospital visiting (2 patients).
Discussion
In the present study, a combination therapy
comprising peg-IFN, RBV and TVR was demonstrated to cause markedly
increased SVR rates, as compared with a dual therapy of peg-IFN and
RBV. However, critical adverse effects, including anemia and
dermatopathy, have previously been reported (4–7).
Additionally, renal dysfunction is recognized as a significant
adverse effect of TVR-based triple therapy, and may lead to
discontinuation of the treatment (6). IFN is an established causative agent of
renal impairment, including proteinuria, glomerular minimal damage,
cellular hyperplasia and focal segmental glomerulosclerosis, which
are predominantly observed in patients with malignancy. This renal
damage predominantly occurs within the first 4 weeks or several
months of IFN therapy (13–16). In patients with chronic hepatitis C,
renal impairment during dual therapy with peg-IFN and RBV is
relatively rare, and not recognized as a central element of the
adverse event profile (17). In the
present study, deterioration of renal function, which was
recognized only during TVR administration, occurred in all
patients, suggesting that TVR is responsible for renal dysfunction
during the treatment.
Although renal dysfunction has not been listed as a
safety concern in multiple previous clinical trials of TVR and
serious renal adverse events have not been noted, TVR-induced renal
dysfunction has been previously reported (18–23).
Renal dysfunction is now recognized as a critical complication of
TVR-based triple therapy (18–23).
Higher incidences of serious impairment of eGFR (<60 ml/min)
were previously observed in patients receiving TVR or boceprevir
with peg-IFN and RBV (6.6 and 4.7%, respectively) when compared
with those receiving dual therapy with peg-IFN and RBV only (0.9%)
(19). The risk factors associated
with eGFR <60 ml/min in this previous study were age, increased
baseline serum creatinine level, arterial hypertension and
receiving triple therapy with TVR or boceprevir (19). In the present study, renal
dysfunction was not significantly associated with age, gender,
liver fibrosis, HCV RNA, alanine aminotransferase, uric acid, total
cholesterol or diabetes mellitus. Age and co-morbidity were not
established as significant contributory factors to renal
dysfunction, but the eGFR baseline level, in addition to the TVR
dose, contributed to renal dysfunction during treatment. Typically,
Japanese patients with HCV infection are older, and have a lower
height and body weight compared with patients in the United States
and Europe (24–26). Furthermore, a higher frequency of
treatment discontinuation due to laboratory abnormalities and
adverse side effects has been reported in older patients (24–26). TVR
dose reduction in aging patients or smaller women is a common
practice in the department in which the present study was
conducted; this reduction may have been linked to the difference in
the risk factors reported during TVR treatment. Mauss et al
(19) analyzed the temporal
concentration of eGFR in patients receiving TVR-based triple
therapy; a decrease in eGFR was reported within the first 12 weeks,
which was followed by a marked improvement subsequent to the
termination of TVR treatment in the majority of patients. In the
present study, renal function was also evaluated; the decrease in
eGFR concentration and an increase in serum creatinine were only
observed during the 12 weeks of TVR administration, but these
improved following cessation of TVR treatment. It may, therefore,
be concluded that renal dysfunction during TVR treatment is
reversible in the majority of treated patients.
Carrier et al (23) reported a case of acute renal
insufficiency occurring at week 36 of TVR-based triple therapy at
standard doses. Renal biopsy findings revealed membranous
glomerulonephritis and prominent interstitial fibrosis with tubular
atrophy. The glomerulonephritis was hypothesized to be due to
peg-IFN, while possible involvement of TVR was described in the
interstitial fibrosis. In the present study, 17 patients
experienced severe renal dysfunction; 2 of these had their
treatment discontinued as a result. All patients receiving TVR
demonstrated a decline in renal function, initially occurring at
the first or second week of treatment. These results suggested that
the mechanism of renal impairment may differ from that illustrated
by Carrier et al (23). All
patients in the present study demonstrated low levels of FENa
(<1%) within 7 days of receiving treatment, regardless of renal
function; however, urinary β2-microglobulin was only
significantly elevated in patients with renal dysfunction.
Furthermore, a number of patients with severe renal dysfunction
demonstrated high values of urinary
N-acetyl-β-D-glucosaminidase, a marker of tubular damage,
although no significant difference was found. These results clearly
suggest that renal dysfunction is associated with a pre-renal
mechanism, and additional damage to the renal tubule exacerbates
renal impairment. Although inadequate blood flow into the renal
arterioles and inhibition of renal drug transporters are assumed to
be the origin of renal damage (27),
the pathophysiological mechanism of renal toxicity caused by TVR is
not completely understood, and additional study is necessary.
Multivariate analysis indicated that TVR dose, in
addition to baseline eGFR levels, was a significant contributory
factor to renal dysfunction in the present study. It has been
previously reported that TVR impairs renal function and increases
serum RBV concentration, which exacerbates anemia, emphasizing the
importance of adjusting the dose of TVR in order to avoid an
excessive elevation of serum RBV levels (21,22).
Furthermore, dose reduction of TVR also restrains the pathogenesis
of renal damage (21,22). However, it remains unclear whether
TVR dose reduction affects the viral response to treatment. In the
present study, 12- and 24-week SVR rates were lower in patients
treated with 1,500 mg/day TVR than in those treated with 2,250
mg/day TVR, although this difference was not statistically
significant. The TVR 1,500 mg/day group included older patients, a
division established to have a poorer viral response compared with
younger patients (6). It is
therefore possible that the lower SVR rate observed in the present
study does not result from the reduced TVR dose, but that these
rates may be associated with patient age. Univariate and
multivariate analysis indicated that the TVR dose as a proportion
of body weight, in addition to initial TVR dose, was not associated
with SVR in the patients treated with TVR-based triple therapy
(data not shown). For a reliable assessment, additional clinical
data is required to determine the optimum dosage of TVR in order to
achieve a higher SVR rate and a lower incidence of adverse side
effects.
Acknowledgements
The English language of the original manuscript was
reviewed by a general physician and professional medical writer
from the Edanz Group (Fukuoka, Japan). The present study was
supported by the Japan Agency for Medical Research and Development,
Division of Infectious Disease Research (grant no
15fk0210017h0003).
Glossary
Abbreviations
Abbreviations:
|
HCV
|
hepatitis C virus
|
|
HCV-1b
|
HCV genotype 1b
|
|
SVR
|
sustained viral response
|
|
peg-IFN
|
pegylated-interferon
|
|
RBV
|
ribavirin
|
|
TVR
|
telaprevir
|
|
eGFR
|
estimated glomerular filtration
rate
|
|
FENa
|
fractional excretion of sodium
|
References
|
1
|
Alter HJ: HCV natural history: The
retrospective and prospective in perspective. J Hepatol.
43:550–552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumada T, Toyoda H, Honda T, Kuzuya T,
Katano Y, Nakano I and Goto H: Treatment of chronic hepatitis C
with interferon alone or combined with ribavirin in Japan.
Intervirology. 49:112–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aghemo A, Rumi MG and Colombo M: Pegylated
IFN-alpha2a and ribavirin in the treatment of hepatitis C. Expert
Rev Anti Infect Ther. 7:925–935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McHutchison JG, Everson GT, Gordon SC,
Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J and Muir AJ:
PROVE1 Study Team: Telaprevir with peginterferon and ribavirin for
chronic HCV genotype 1 infection. N Engl J Med. 360:1827–1838.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayashi N, Okanoue T, Tsubouchi H, Toyota
J, Chayama K and Kumada H: Efficacy and safety of telaprevir, a new
protease inhibitor, for difficult-to-treat patients with genotype 1
chronic hepatitis C. J Viral Hepat. 19:e134–e142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumada H, Toyota J, Okanoue T, Chayama K,
Tsubouchi H and Hayashi N: Telaprevir with peginterferon and
ribavirin for treatment-naive patients chronically infected with
HCV of genotype 1 in Japan. J Hepatol. 56:78–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rajani AK, Ravindra BK and Dkhar SA:
Telaprevir: Changing the standard of care of chronic hepatitis C. J
Postgrad Med. 59:42–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kleinknecht D, Landais P and Goldfarb B:
Drug-associated acute renal failure. A prospective collaborative
study of 81 biopsied patients. Adv Exp Med Biol. 212:125–128. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choudhury D and Ahmed Z: Drug-associated
renal dysfunction and injury. Nat Clin Pract Nephrol. 2:80–91.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prowle J, Bagshaw SM and Bellomo R: Renal
blood flow, fractional excretion of sodium and acute kidney injury:
Time for a new paradigm? Curr Opin Crit Care. 18:585–592. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamoto S, Kanda T, Yonemitsu Y, Arai M,
Fujiwara K, Fukai K, Kanai F, Imazeki F and Yokosuka O:
Quantification of hepatitis C amino acid substitutions 70 and 91 in
the core coding region by real-time amplification refractory
mutation system reverse transcription-polymerase chain reaction.
Scand J Gastroenterol. 44:872–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kohjima M, Enjoji M, Yoshimoto T, Yada R,
Fujino T, Aoyagi Y, Fukushima N, Fukuizumi K, Harada N, Yada M, et
al: Add-on therapy of pitavastatin and eicosapentaenoic acid
improves outcome of peginterferon plus ribavirin treatment for
chronic hepatitis C. J Med Virol. 85:250–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fahal IH, Murry N, Chu P and Bell GM:
Acute renal failure during interferon treatment. BMJ. 306:9731993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jadoul M, Piessevaux H, Ferrant A, Cosyns
JP and van Ypersele de Strihou C: Renal thrombotic microangiopathy
in patients with chronic myelogenous leukaemia treated with
interferon-alpha 2b. Nephrol Dial Transplant. 10:111–113.
1995.PubMed/NCBI
|
|
15
|
Shah M, Jenis EH, Mookerjee BK, Schriber
JR, Baer MR, Herzig GP and Wetzler M: Interferon-alpha-associated
focal segmental glomerulosclerosis with massive proteinuria in
patients with chronic myeloid leukemia following high dose
chemotherapy. Cancer. 83:1938–1946. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Russo MW and Fried MW: Side effects of
therapy for chronic hepatitis C. Gastroenterology. 124:1711–1719.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willson RA: Nephrotoxicity of interferon
alfa-ribavirin therapy for chronic hepatitis C. J Clin
Gastroenterol. 35:89–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gervasoni C, Milazzo L, Pezzani D, Fucile
S and Cattaneo D: Telaprevir therapy, renal impairment, and their
effects on the pharmacokinetics of tenofovir in HIV/hepatitis C
virus coinfected patients. AIDS. 28:285–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mauss S, Hueppe D and Alshuth U: Renal
impairment is frequent in chronic hepatitis C patients under triple
therapy with telaprevir or boceprevir. Hepatology. 59:46–48. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pariente A, Rémy AJ, Lesgourgues B and
Hagège H: APROVVIE group of the Association Nationale des
Gastroentérologues des Hôpitaux Généraux (ANGH): Risk factors for
severe anaemia during telaprevir-based triple therapy: Is acquired
renal dysfunction the missing link? Liver Int. 34:e163–e164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tempestilli M, Lionetti R, D'Offizi G,
Montalbano M, Giaffreda A, Fazio S and Pucillo LP: Increased plasma
concentration of ribavirin as a result of renal dysfunction in
hepatitis C virus patients treated with telaprevir. Hepatology.
60:1109–1110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karino T, Ozeki I, Hige S, Kimura M,
Arakawa T, Nakajima T, Kuwata Y, Sato T, Ohmura T and Toyota J:
Telaprevir impairs renal function and increases blood ribavirin
concentration during telaprevir/pegylated interferon/ribavirin
therapy for chronic hepatitis C. J Viral Hepat. 21:341–347. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carrier P, Chambaraud T, Vong C,
Guillaudeau A, Debette-Gratien M, Jacques J, Legros R, Sautereau D,
Essig M and Loustaud-Ratti V: Severe renal impairment during triple
therapy with telaprevir. Clin Res Hepatol Gastroenterol.
38:e69–e71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kainuma M, Furusyo N, Kajiwara E,
Takahashi K, Nomura H, Tanabe Y, Satoh T, Maruyama T, Nakamuta M,
Kotoh K, et al: Kyushu University Liver Disease Study Group:
Pegylated interferon α-2b plus ribavirin for older patients with
chronic hepatitis C. World J Gastroenterol. 16:4400–4409. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Honda T, Katano Y, Shimizu J, Ishizu Y,
Doizaki M, Hayashi K, Ishigami M, Itoh A, Hirooka Y, Nakano I, et
al: Efficacy of peginterferon-alpha-2b plus ribavirin in patients
aged 65 years and older with chronic hepatitis C. Liver Int.
30:527–537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furusyo N, Ogawa E, Nakamuta M, Kajiwara
E, Nomura H, Dohmen K, Takahashi K, Satoh T, Azuma K, Kawano A, et
al: Kyushu University Liver Disease Study (KULDS) Group: Telaprevir
can be successfully and safely used to treat older patients with
genotype 1b chronic hepatitis C. J Hepatol. 59:205–212. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kunze A, Huwyler J, Camenisch G and
Gutmann H: Interaction of the antiviral drug telaprevir with renal
and hepatic drug transporters. Biochem Pharmacol. 84:1096–1102.
2012. View Article : Google Scholar : PubMed/NCBI
|