Introduction
Ovarian cancer is the leading cause of mortality,
and its morbidity ranks third among gynecological malignancies
(1). Tumour metastasis, which is the
primary cause of cancer-associated mortality, has been closely
associated with the tumour cell microenvironment. The survival of
micrometastases at distal sites to the primary tumour requires an
ability to adapt to different microenvironments (2). In 1889, Stephen Paget's ‘seed and soil’
hypothesis laid the foundation for the concept of the tumour
microenvironment (3). In an
expansion of this hypothesis, Paget accurately predicted that
tumour cells act as ‘seeds’ and are able to settle in a suitable
‘soil’ and grow; when the soil is remote tissues and organs, tumour
cells form synergistic interactions with their microenvironment
(4). A tumour microenvironment
refers to the environment that is created by the tumour and
regulated by tumour-induced interactions (5). It includes tumour, stromal, immune,
inflammatory and endothelial cells, the blood and lymphatic
vascular networks, micro-lymphatics, interstitial fluid and cell
factors of the tumour cell and its microenvironment that co-evolve
through iterative interactions to contribute to tumour progression
(6–9). A previous study demonstrated that the
tumour microenvironment was associated with a more aggressive
cancer phenotype, and that it has a role in tumour progression and
metastatic disease (10). In
addition, it has been suggested that an improved understanding of
the cellular and molecular pathways that operate within the tumour
microenvironment is required in order to develop strategies for
inhibiting tumour metastasis. Therefore, in order to control
malignancy, it may be necessary to control the modifications within
the tumour microenvironment that initiate and promote the growth,
invasion and spread of cancer cells (4,11,12).
Lymphangiogenesis has been shown to be an important
cause of metastasis; the microenvironment provides various
lymphangiogenic factors, including vascular endothelial growth
factor (VEGF)-C and -D, which promote lymphatic vessel formation
(13) and are important for lymph
node metastasis and metastatic tumour spread (6,14).
Previous studies reported that patients with oesophageal squamous
carcinomas and adenocarcinomas are at a higher risk of lymphatic
vessel invasion and lymph node metastasis when they have high
tumour lymphatic vessel densities (15,16).
In order to investigate the effects of the
microenvironment on the lymph node metastasis of malignancies, and
the underlying mechanisms that lead to tumour metastasis via
lymphatic vessels, the present study established a co-culture
system consisting of human lymphatic endothelial cells (HLECs) and
human ovarian carcinoma cells (SKOV3s) with directional high
lymphatic metastasis (SKOV3-PM4s). The SKOV3-PM4s were obtained by
injecting SKOV3s into nude mice to derive a fourth generation
subcell line from metastatic lymph nodes. Alterations in the
biological characteristics of each cell line in different
microenvironments were observed, and involved cell interactive
culture systems containing conditioned media from two types of
cells and an in vitro cell co-culture system. The results of
the present study provided a theoretical basis for the mechanisms
underlying the lymph node metastasis of ovarian cancer.
Materials and methods
Cell lines and plasmids
Human SKOV3-PM4s, human HLECs and the lentiviral
pCDH-COPGFP plasmid, were obtained from the Oncology Laboratory at
the Experimental Center of Guangxi Medical University (Nanning,
China).
Fluorescent-labelled cell lines
The pCDH-COPGFP plasmid with an encoded green
fluorescent protein (GFP gene was transfected into the SKOV3-PM4s,
and SKOV3-PM4s stably expressing GFP were obtained. A stock
solution containing 20 mg/ml fluorescent membrane dye (DiI;
Biotium, Inc., Hayward, CA, USA) in N,N-dimethylformamide (DMF;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) was prepared, and the HLECs were labelled with a final
working dilution of 30 µg/ml DiI in phosphate-buffered saline (PBS;
Beyotime Institute of Biotechnology, Haimen, China) solution.
Preparation of the conditioned culture
media and the establishment of the interactive culture system
Fluorescent-labelled SKOV3-PM4s and HLECs were
initially plated into 75-m2 culture flasks at a density
of 2.5×105 cells/ml. The supernatants were collected
after 48 h, and the cell debris were removed by centrifugation at
1,500 × g for 10 min at 4°C. The supernatants were filtered using
0.22-µm membranes (Beyotime Institute of Biotechnology) and stored
at −20°C until required for further experimentation. The cells were
divided into four groups, as follows: i) SKOV4-PM4s cultured in
RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C with 5% CO2; ii) SKOV3-PM4s cultured in
the supernatant from the HLECs at 37°C with 5% CO2; iii)
HLECs cultured in endothelial cell medium (Sciencell Research
Laboratories, Carlsbad, CA, USA) at 37°C with 5% CO2;
and iv) HLECs cultured in the supernatant from SKOV3-PM4s.
Establishment of the co-culture
system
Fluorescent-labelled SKOV3-PM4 and HLEC cells
(1×105 cells/ml) were added to Transwell®
plates (EMD Millipore, Billerica, MA, USA) and glass-bottomed petri
dishes, respectively, at 200µl, thereby establishing the
fluorescent-labelled SKOV3-PM4-HLEC cell co-culture system.
Observations of cellular
morphology
For groups A, B, C and D, the cell suspensions were
adjusted to a density of 1×104 cells/ml, and 2 ml cell
suspension was added to petri dishes in which coverslips had been
placed. The coverslips were removed following incubation in an
atmosphere containing 5% CO2 for 24 h at 37°C, and were
fixed with 95% ethanol for 30 min and rinsed twice with PBS for
hematoxylin-eosin (HE; Beyotime Institute of Biotechnology)
staining.
Observations of cell proliferation and
metastatic abilities
In order to determine the cell mitotic index of the
SKOV3-PM4s and HLECs, the number of cells in the mitotic phase were
calculated under a light microscope (CKX41-A22PHP; Olympus
Corporation, Tokyo, Japan), based on the appearance of moderate
cellular densities (at least 1,000 cells were counted). The cell
mitotic index was determined using the following equation: Cell
mitotic index (%) = (Number of cells with mitotic figures/total
number of cells counted) × 100.
In order to determine the cell proliferation rate of
the SKOV3-PM4s and HLECs, cell suspensions of groups A, B, C and D
were seeded in 96-well plates (1×104 cells/ml). Once
daily, the cells from three wells of each group underwent the
3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide
colorimetric assay (Beijing Solarbio Science & Technology Co.,
Ltd.), and the results of these assays over seven consecutive days
were used to draw cell growth curves.
The invasion ability of the SKOV3-PM4s and HLECs was
assessed using the Matrigel Invasion Assay (Sigma-Aldrich, St.
Louis, MO, USA). Briefly, cells in the logarithmic growth phase
from each of the four groups were adjusted to densities of
5×104 cells/ml. Subsequently, 200-µl cell suspensions
from each group was seeded into the upper chamber of the
Transwell® plate, and the cell supernatants of the other
cells were added to the lower chamber. The membrane filters were
removed following incubation for 16 h at 37°C and 5%
CO2. The Matrigel was wiped with a cotton swab, and the
cells were fixed in methanol for 20 min prior to staining with
HE.
The Transwell migration assay was used to determine
the migratory ability of the SKOV3-PM4s and HLECs. The experimental
procedure was identical to that performed for the Matrigel Invasion
Assay, with the exception that the surface of the polycarbonate
membrane of the upper chamber lacked Matrigel.
Vessel formation assay
Cells from groups C and D were collected at the
logarithmic growth phase and adjusted to a density of
4×105 cells/ml. Subsequently, 50-µl cell suspensions
from each group were seeded into a 24-well plate coated with
Matrigel and incubated at 37°C in an atmosphere containing 5%
CO2. The tube formation abilities were observed under
the microscope (CKX41-A22PHP; Olympus Corporation) between days 4
and 9.
Observations of the cell
interactions
Fluorescent-labelled SKOV3-PM4s and HLECs were
adjusted to densities of 1×105 cells/ml. Each cell type
was plated together in 35-mm glass-bottom petri dishes (Ibidi GmbH,
Planegg, Germany) for confocal microscopy (Nikon, Tokyo, Japan)
analyses. Cell-cell interactions were observed following
co-culturing for 12, 24 and 48 h.
Gelatin zymography method
The cellular supernatants of the
fluorescent-labelled SKOV3-PM4s, HLECs and co-cultured
SKOV3-PM4/HLECs were collected and were cultured in serum-free
medium for 24 h, followed by centrifugation at 1,500 × g for 10 min
at 4°C to remove cell debris. The sample volumes of each group were
adjusted according to protein concentrations, obtained using a BCA
Protein Assay (Beijing Solarbio Science & Technology Co.,
Ltd.). Subsequently, the cell supernatants were mixed with loading
buffer (Beijing Solarbio Science & Technology Co., Ltd.), and
the proteins in the cell supernatants were separated by 10% sodium
dodecyl sulfate-polyacrylamide (Beyotime Institute of
Biotechnology) gel electrophoresis containing 1.0 mg/ml gelatin.
The gel was placed in eluent (Beijing Solarbio Science &
Technology Co., Ltd.) containing 2.5% Triton X-100, 50 mmol/l
Tris-HCl, 5 mmol/l CaCl2 and 1 µmol/l ZnCl2
(pH 7.6), and was eluted twice by agitation for 45 min, followed by
twice washing for 20 min with eluent lacking Triton X-100. The gel
was then incubated in incubation buffer (Beijing Solarbio Science
& Technology Co., Ltd.) containing 50 mmol/l Tris-HCl, 5 mmol/l
CaCl2, 1 µmol/l ZnCl2 and 0.02% Brij-35 (pH
7.6) at 37°C for 42 h. The gel was then transferred to 30 ml
developing buffer and incubated for 2 h. Following this, the
developing buffer (Beijing Solarbio Science & Technology Co.,
Ltd.) was replaced with add Coomassie brilliant blue R250 staining
solution (Beijing Solarbio Science & Technology Co., Ltd.) and
incubated for 20–30 min at room temperature. The gel was scanned
using a GS-710 Calibrated Imaging Densitometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and analyzed using Phoretix
1D Pro software (CSL-Cleaver Scientific, Rugby, UK). Negative
staining bands on the blue background were observed.
Statistical analyses
Statistical analyses were conducted using the SPSS
software, version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation of three replicate
assays. The results of cell growth curves for the four groups were
compared using one-way analysis of variance. The mitotic figures
were compared using the χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
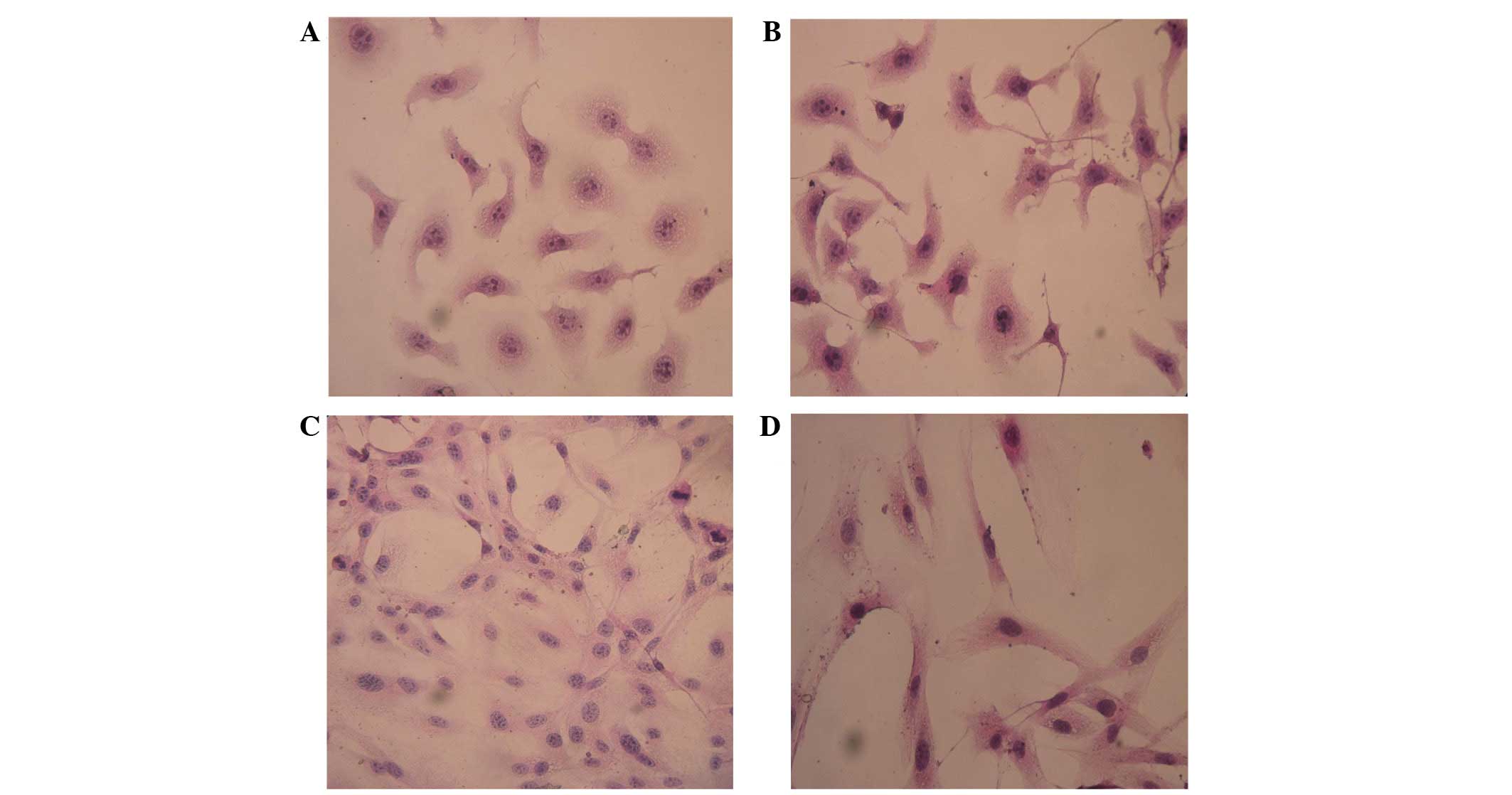
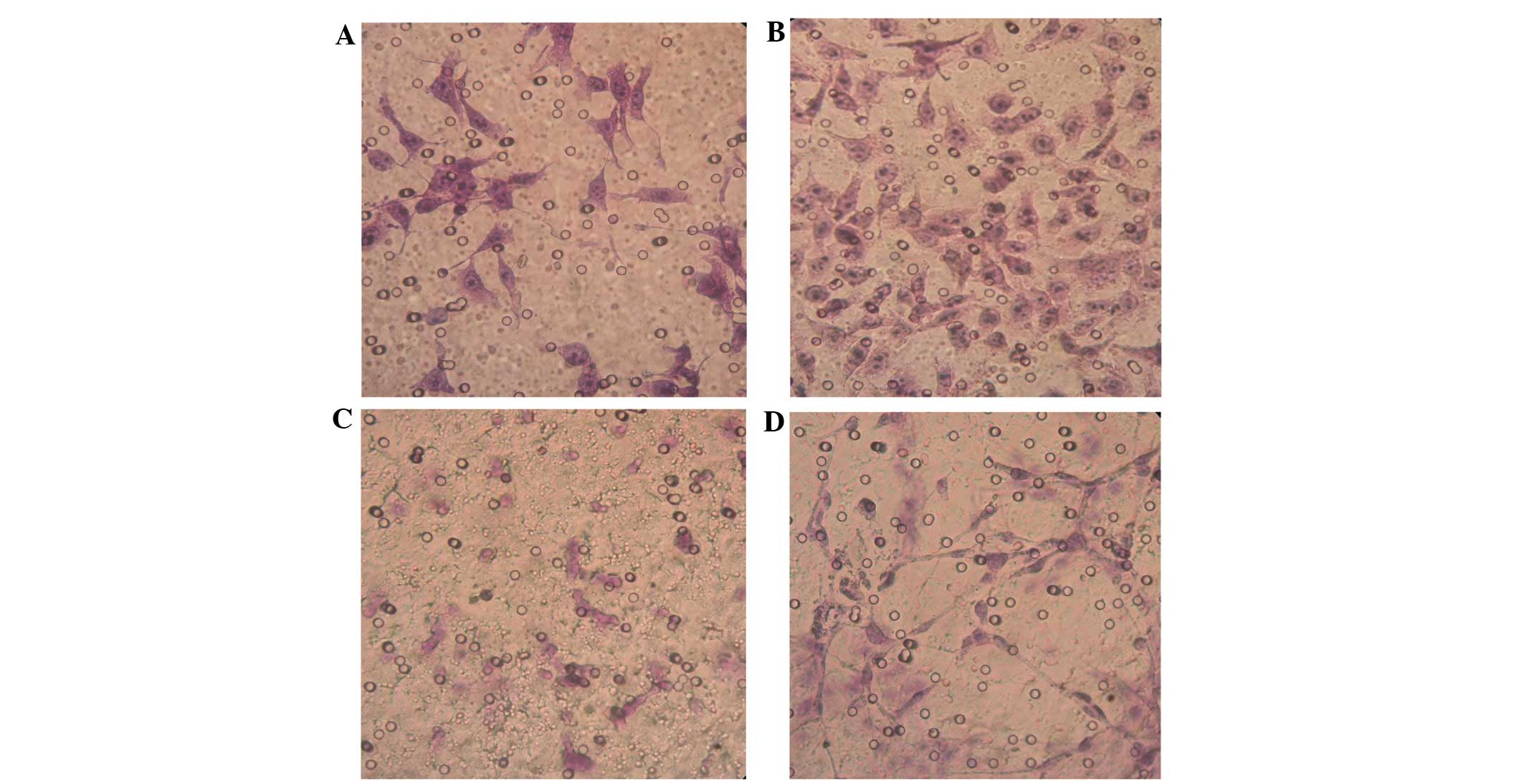
Changes in cell morphology
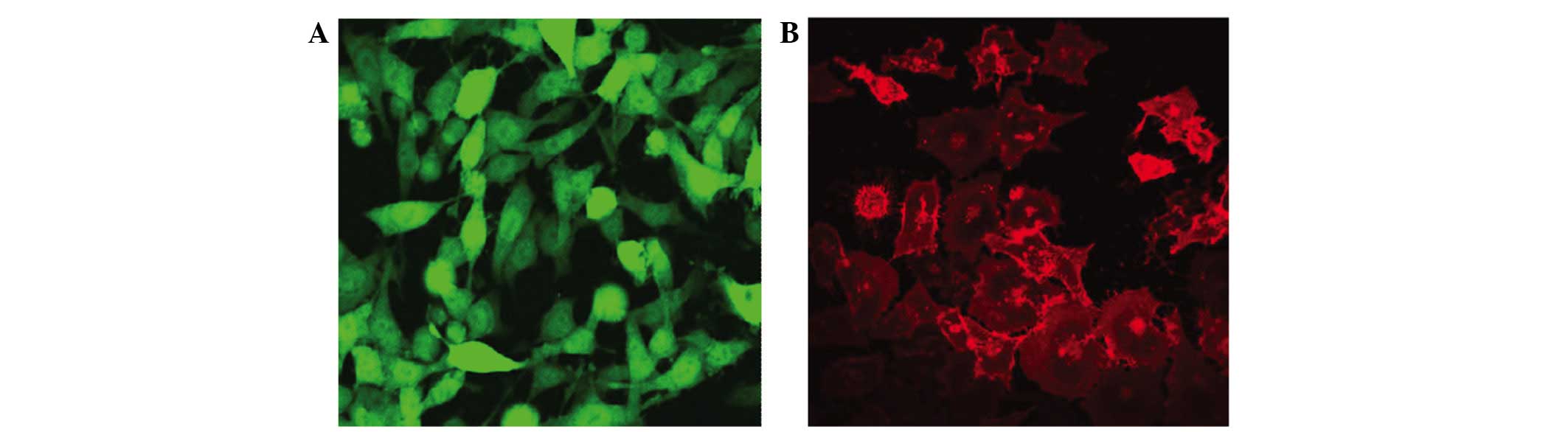
Stably expressing green fluorescent GFP-tagged
SKOV3-PM4s and red fluorescent DiI-labelled HLECs were observed
using an inverted fluorescence microscope (Fig. 1). The SKOV3-PM4s cultured in HLEC
medium exhibited alterations in cell morphology, from immature
round cells to multi-tentacle cells, and the nuclei increased in
size and number. In addition, the number of pseudopodia and mitotic
figures were increased (Fig. 2B). As
compared with the HLECs cultured alone, the cell morphologies of
the HLECs cultured in SKOV3-PM4 medium were oval with short
spindles to fusiform and irregular polygon-shaped. Furthermore, the
cells were larger, the nuclei were changed from small-to-large, and
vacuoles were observed in the cells (Fig. 2D).
Comparisons of the mitotic indices and
cell growth curves
The mitotic index is a measure of the percentage of
cells undergoing mitosis. Mitosis is the division of somatic cells,
in which the genetic information from one single cell is equally
dispersed into two daughter cells (17). The mitotic indices of the SKOV3-PM4s
cultured in the HLEC medium were significantly higher, as compared
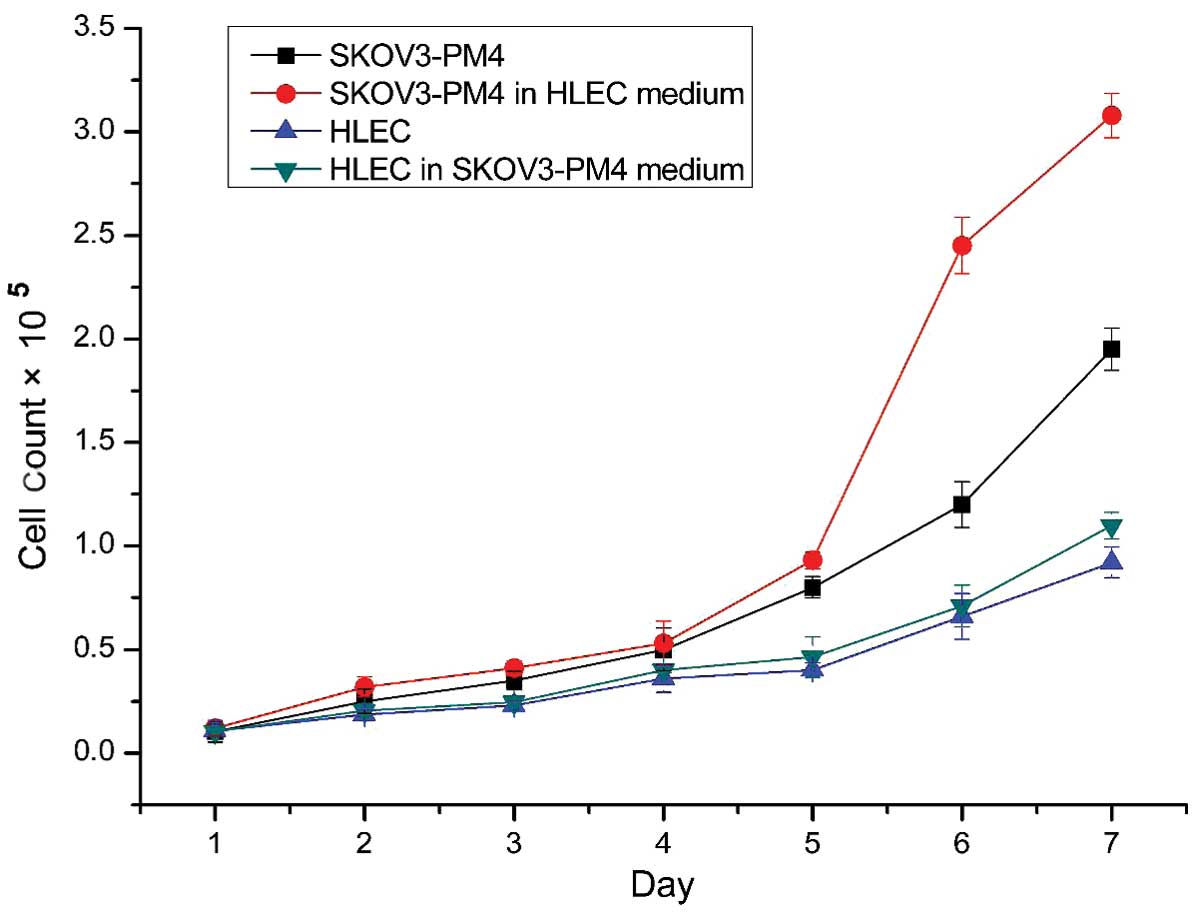
with those cultured in normal medium (P<0.05; Table I). The cell growth curves
demonstrated that the growth rate of the SKOV3-PM4s cultured in the
HLEC medium was accelerated, as compared with the growth of the
SKOV3-PM4s grown in normal medium (Fig.
3). In addition, the growth rate of the HLEC cells cultured in
the SKOV3-PM4s medium was increased marginally, as compared with
the HLECs cultured in the normal medium (Fig. 3).
 | Table I.Mitotic indexes of each group. |
Table I.
Mitotic indexes of each group.
|
|
| Occurrence of
mitosis |
|---|
|
|
|
|
|---|
| Group | Cell count | Cell count | Percentage (%) |
|---|
| SKOV3-PM4 | 1,071.33±56.72 | 53.67±2.08 | 5.01±0.17 |
| SKOV3-PM4 in HLEC
medium | 1,045.00±65.85 | 86.00±2.00 |
8.23±0.34a |
| HLEC | 1,073.32±62.69 | 26.65±2.51 | 2.48±0.11 |
| HLEC in SKOV3-PM4
medium | 1,058.50±79.90 | 50.60±1.53 |
4.78±0.12b |
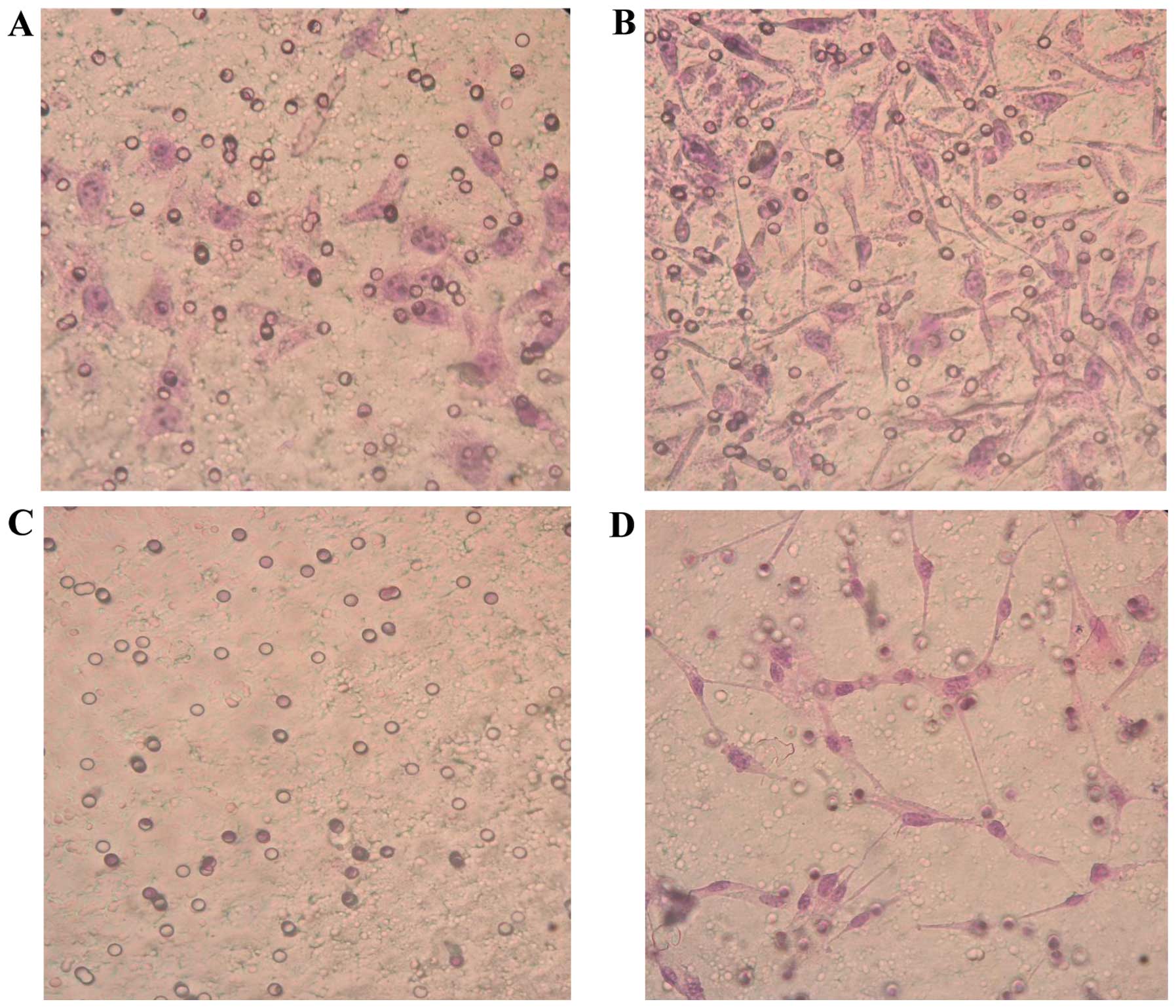
HLECs promote the invasion and
migration of ovarian cancer cells
SKOV3-PM4s were highly invasive and metastatic when
co-cultured with the HLECs for 3 days; thus suggesting that the
highly invasive HLEC cells are able to induce the activation of
SKOV3-PM4s. The invasion ability of the SKOV3-PM4s cultured in
HLECs medium was significantly increased, as compared with the
SKOV3-PM4s cultured in normal medium (P<0.05). The HLECs
cultured in normal medium were unable to cross the Matrigel matrix
membrane to any significant degree; however, the invasion ability
of the HLECs cultured in SKOV3-PM4s medium was significantly
increased (P<0.05; Fig. 4 and
Table II). The migration assay
demonstrated that the transmembrane cell count of the SKOV3-PM4s
cultured in HLECs medium and the HLECs cultured in SKOV3-PM4s
medium were significantly increased, as compared with the cells
cultured in normal medium (P<0.05; Fig. 5 and Table III).
 | Table II.Invasion ability. |
Table II.
Invasion ability.
| Group | Transmembrane cell
count |
|---|
| SKOV3-PM4 |
63.93±13.62 |
| SKOV3-PM4 in HLEC
medium |
106.13±10.34a |
| HLEC | 0 |
| HLEC in SKOV3-PM4
medium |
32.33±4.76b |
 | Table III.Migration ability. |
Table III.
Migration ability.
| Group | Transmembrane cell
count |
|---|
| SKOV3-PM4 | 78.07±6.08 |
| SKOV3-PM4 in HLEC
medium |
110.27±10.92a |
| HLEC | 21.00±3.46 |
| HLEC in SKOV3-PM4
medium |
44.47±5.49b |
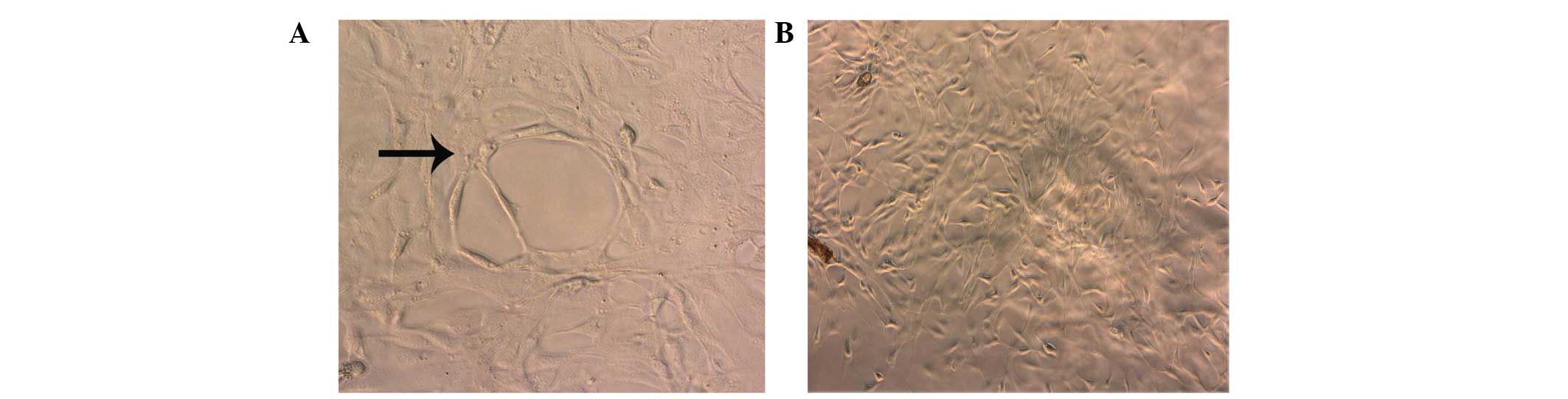
Tube formation assay of the HLECs
cultured in SKOV3-PM4s medium
The HLECs cultured in SKOV3-PM4s medium exhibited
significantly enhanced tube formation abilities, as compared with
the HLECs grown in the normal medium. In addition, after 4 days of
culture, tubular networks were detected in the cultures containing
HLECs and SKOV3-PM4 medium (Fig.
6A). Conversely, these tubular structures were not detected in
the cultures containing HLECs and normal mediums (Fig. 6B).
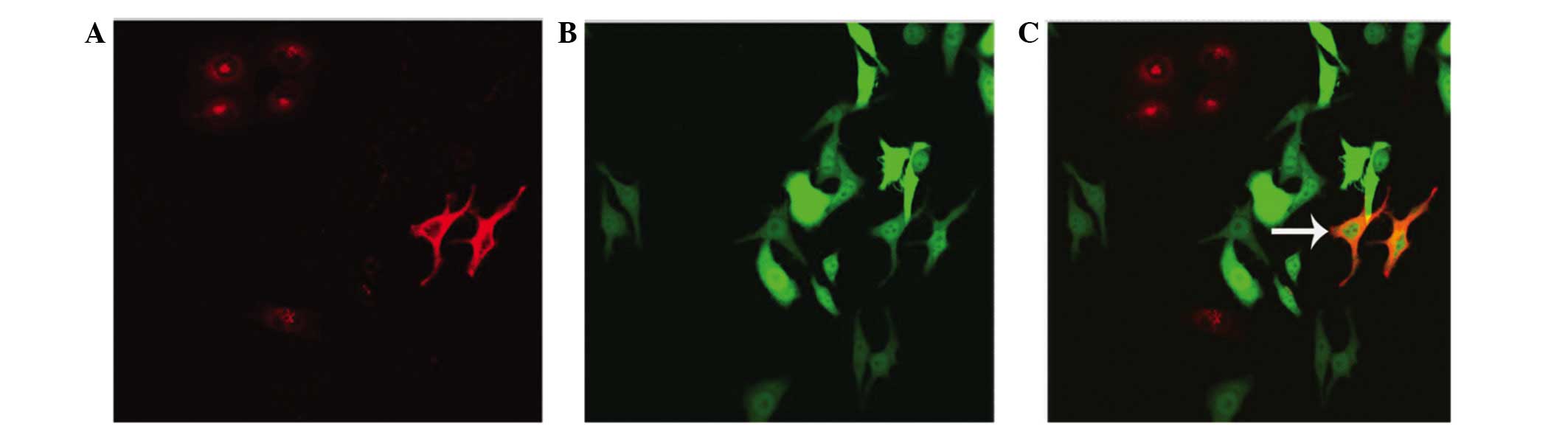
Interactions of the co-culture
cells
The co-cultured cells were observed by laser
confocal microscopy over 48 h. The fluorescent-labelled SKOV3-PM4
and HLECs were co-cultured, and fusion phenomena were observed
after 48 h. The fused cells are indicated by the arrows (Fig. 7).
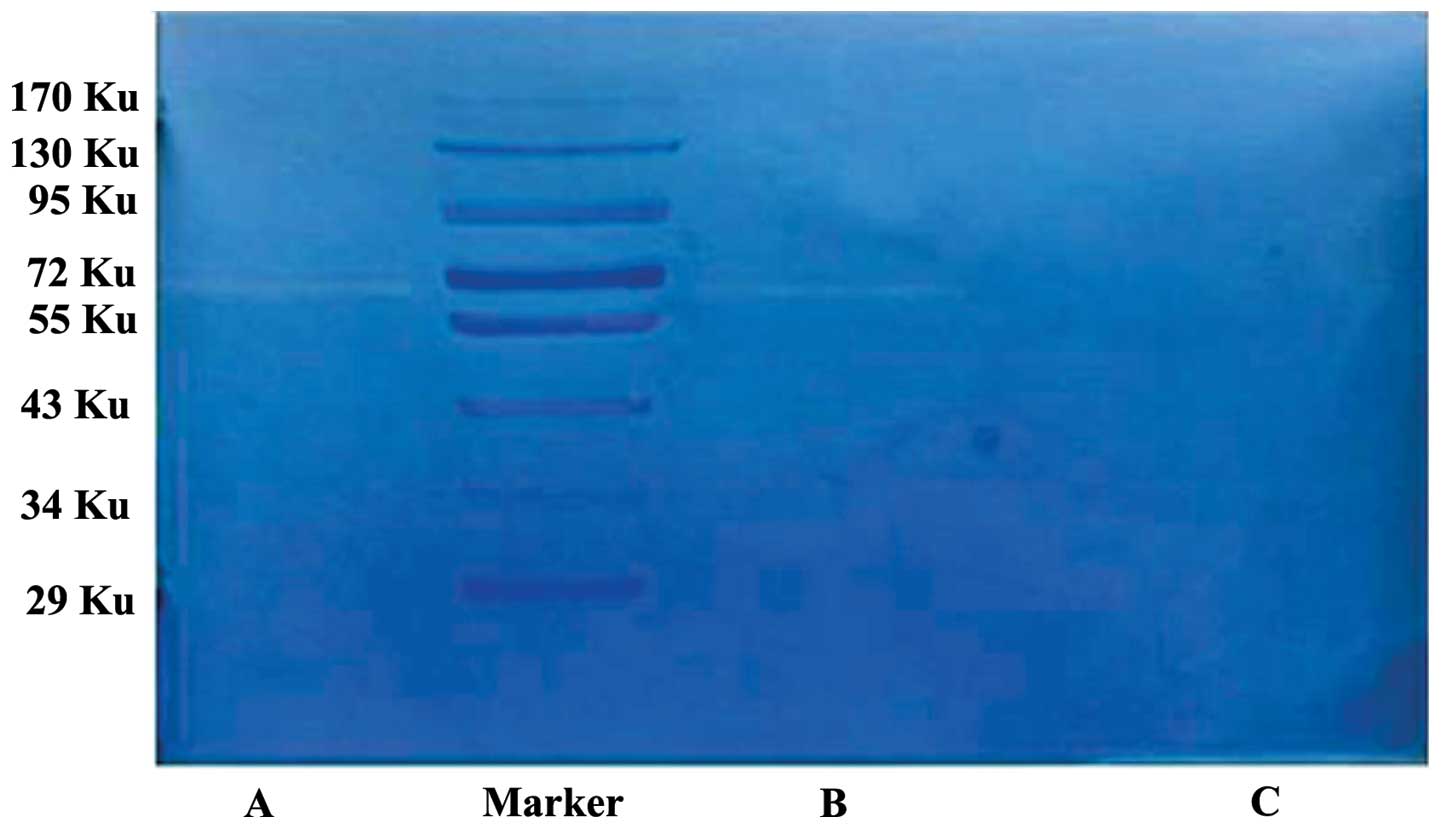
Gelatin is a substrate of MMPs. In the gelatin
zymography assays, gelatin is hydrolysed by MMP-2 and MMP-9, and
negative staining bands appear in the corresponding positions.
Typically, MMP-2 appears at 72 KU, and MMP-9 appears at 92 KU. In
the present study, the HLECs produced no significant bands at 72
KU, whereas the SKOV3-PM4s exhibited a clear band at 72 KU, and the
co-cultured SKOV3-PM4s and HLECs produced a significant band at 72
KU, which was brighter and wider, as compared with that of the
SKOV3-PM4s (Fig. 8).
Discussion
Tumour metastasis, which is responsible for the
majority of cancer-associated mortalities, is a continuous
biological process involving numerous stages and factors, and the
regulation of multiple genes (18,19). The
spread of cancer cells to lymph nodes is an early event in
metastasis, and is regularly used to predict disease outcome and to
guide therapeutic strategies (20,21).
Tumour-associated lymphatic vessels are a key component of
metastatic spread (20). Lymphatic
metastasis is the primary route for the metastasis of ovarian
cancer (22). Previous studies
demonstrated that lymphatic vessel neogenesis had a key role in the
process of tumour lymph node metastasis (23,24).
Lymphatic capillaries are lined by a single layer of endothelial
cells, and a previous study reported that the lymphatic endothelium
may provide a protective microenvironment for long-term tumour cell
survival (23). Therefore, the
present study used HLECs to investigate the molecular mechanisms
underlying the interaction of tumour cells with the lymphatic
endothelium. In the present study, HLECs that were cultured with
the supernatants of SKOV3-PM4s exhibited numerous characteristics,
including irregular nuclei and pseudopodia, that differed from
those of their parent cells. These results suggested that the
supernatant produced by cultured tumour cells was able to induce
alterations in normal endothelial cells. This phenomenon may be
associated with factors in the supernatant that promote lymphatic
growth, including VEGF-C, which may combine with the VEGFR-3
receptors on lymphatic endothelial cells to promote their
proliferation, differentiation, migration and lumen formation
(24–26).
Previous studies reported that ovarian cancer
metastasis is a non-random event that is dependent on the tumour
microenvironment created by the tumour cells themselves, the
specific tissue or organ, and the adaptation of the cells to the
microenvironment (21,27). Peritumoural lymphatic vessels are
predominantly responsible for promoting lymphatic cancer metastasis
(21). The lymph of the tumour is
drained and metastasised by lymph vessels or lymphangiogenesis,
which is mediated by factors that promote lymphatic growth
(28). The new vessels then increase
the lymph tissue, which creates an ideal environment for tumour
growth. Therefore, lymph vessel formation may be considered a
critical aspect of tumour invasion and metastasis (20,21).
Lymphatic endothelial cells surrounding the tumour secrete growth
factors, adhesion factors and chemokines that promote tumour
cellular proliferation, invasion and metastasis (29). In the present study, the SKOV3-PM4s
that were cultured with the supernatant of the HLECs exhibited
increased numbers of pseudopodia, greater alterations in cell
morphology and motility, and enhanced invasion and migratory
abilities.
In the present study, SKOV3-PM4s were transfected
with a lentiviral pCDH-COPGFP plasmid, such that GFP was integrated
into the SKOV3-PM4s, and the HLECs were labelled with DiI, which is
a carbocyanine membrane dye that exhibits enhanced fluorescence
upon insertion of its lipophilic hydrocarbon chains into the lipid
membranes of cells. This enabled the entire cellular profile to be
observed under an inverted fluorescence microscope. Under a laser
confocal microscope, the spontaneous fusion produced by two types
of cells was observed in the SKOV3-PM4 and HLEC co-culture system.
This was consistent with a previous study (30) in which the co-culture of breast
cancer cells and endothelial cells in vitro resulted in the
spontaneous formation of fused cells that expressed parental genes
and protein markers, and thus exhibited the biological
characteristics of endothelial cells. These are involved in the
degradation of the extracellular basement membrane and therefore
the promotion of tumour metastasis (31). The present study hypothesized that
the cytoplasmic connection between the tumour cells and endothelial
cells may be a channel through which signal transduction or
substance circulation is established, and that such connections are
also the material basis of the changes in cell morphology in both
cells.
The MMP family is a group of zinc-dependent
proteolytic enzymes, of which MMP-2 is a glycoprotein that
participates in the degradation of the extracellular matrix,
thereby promoting tumour invasion and metastasis (32). As such, MMP-2 is an important marker
of malignant tumour progression (33,34), and
has been associated with lymphatic invasion and lymph node
metastases (35,36). Previous studies demonstrated that
high expression levels of MMP-2 were associated with the growth,
invasion and metastasis of ovarian cancer, that the expression of
the MMP-2 protein was associated with the progression of ovarian
cancer, and that the survival rates of patients with ovarian cancer
were correlated with MMP-2 expression (37,38). In
the present study, MMP-2 secretion was significantly upregulated in
the SKOV3-PM4-HLEC co-culture system, as compared with the
individually cultured SKOV3-PM4s and HLECs. These results suggested
that various autocrine and paracrine cytokines produced by the
HLECs may have induced increases in the expression levels and
secretion of MMP-2 by the SKOV3-PM4s. This in turn may have caused
the tumour cells to colonize and metastasize.
In conclusion, the results of the present study
suggested that lymphatic endothelial cells cultured in the
supernatants of tumour cells were altered, as compared with normal
lymphatic endothelial cells. In addition, the supernatants of the
HLECs enhanced the invasion and migratory abilities of the
SKOV3-PM4s. These results suggested that tumour cells exposed to
the actions of lymphatic endothelial cells may be more likely to
metastasize in vivo. Therefore, by simulating the
growth-inducing microenvironment of human ovarian carcinoma cells
in vitro by using SKOV3-PM4s and HLECs, the present study
was able to employ genomic and proteomic technology to identify the
key molecules in the tumour microenvironment. Furthermore, the
present study may have elucidated in part the molecular mechanisms
underlying ovarian cancer metastasis and the formation of
tumour-adjacent lymphatic endothelial cells. The results of the
present study may be considered useful for the development of novel
therapeutic strategies that block cancer metastasis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81060218 and
81360502) and the Guangxi Natural Science Foundation (grant nos.
2012GXNSFAA053157 and 2014GXNSFAA118161).
Glossary
Abbreviations
Abbreviations:
|
HLEC
|
lymphatic endothelial cells
|
|
SKOV3-PM4
|
human ovarian carcinoma cells with
directional high lymphatic metastasis
|
References
|
1
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wood SL, Pernemalm M, Crosbie PA and
Whetton AD: The role of the tumour-microenvironment in lung
cancer-metastasis and its relationship to potential therapeutic
targets. Cancer Treat Rev. 40:558–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paget S: The distribution of secondary
growths in cancer of the breast. Lancet. 133:571–573. 1889.
View Article : Google Scholar
|
|
4
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whiteside TL: The tumour microenvironment
and its role in promoting tumour growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lorusso G and Rüegg C: The tumour
microenvironment and its contribution to tumour evolution toward
metastasis. Histochem Cell Biol. 130:1091–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joyce JA: Therapeutic targeting of the
tumour microenvironment. Cancer Cell. 7:513–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berns A and Pandolfi PP: Tumour
microenvironment revisited. EMBO Rep. 15:458–459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Korkaya H, Liu S and Wicha MS: Breast
cancer stem cells, cytokine networks, and the tumour
microenvironment. J Clin Invest. 121:3804–3809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brauer HA, Makowski L, Hoadley KA,
Casbas-Hernandez P, Lang LJ, Romàn-Pèrez E, D'arcy M, Freemerman
AJ, Perou CM and Troester MA: Impact of tumor microenvironment and
epithelial phenotypes on metabolism in breast cancer. Clin Cancer
Res. 19:571–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lunt SJ, Chaudary N and Hill RP: The
tumour microenvironment and metastatic disease. Clin Exp Metastas.
26:19–34. 2009. View Article : Google Scholar
|
|
12
|
Salo T, Vered M, Bello IO, Nyberg P, Bitu
CC, Hurvitz Zlotogorski A and Dayan D: Insights into the role of
components of the tumour microenvironment in oral carcinoma call
for new therapeutic approaches. Exp cell Res. 325:58–64. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alitalo K and Carmeliet P: Molecular
mechanisms of lymphangiogenesis in health and disease. Cancer Cell.
1:219–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alitalo K, Tammela T and Petrova TV:
Lymphangiogenesis in development and human disease. Nature.
438:946–953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schoppmann SF, Jesch B, Zacherl J, Riegler
MF, Friedrich J and Birner P: Lymphangiogenesis and lymphovascular
invasion diminishes prognosis in esophageal cancer. Surgery.
153:526–534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dutta S, Going J, Crumley A, Mohammed Z,
Orange C, Edwards J, Fullarton G, Horgan P and McMillan D: The
relationship between tumour necrosis, tumour proliferation, local
and systemic inflammation, microvessel density and survival in
patients undergoing potentially curative resection of oesophageal
adenocarcinoma. Br J Cancer. 106:702–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tzur A, Kafri R, LeBleu VS, Lahav G and
Kirschner MW: Cell growth and size homeostasis in proliferating
animal cells. Science. 325:167–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steeg PS: Tumour metastasis: mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valastyan S and Weinberg RA: Tumour
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pepper MS: Lymphangiogenesis and tumour
metastasis: Myth or reality? Clin Cancer Res. 7:462–468.
2001.PubMed/NCBI
|
|
21
|
Tobler NE and Detmar M: Tumour and lymph
node lymphangiogenesis-impact on cancer metastasis. J Leukocyte
Biol. 80:691–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karaman S and Detmar M: Mechanisms of
lymphatic metastasis. J Clin Invest. 124:922–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pepper MS, Tille JC, Nisato R and Skobe M:
Lymphangiogenesis and tumour metastasis. Cell Tissue Res.
314:167–177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He Y, Kozaki K, Karpanen T, Koshikawa K,
Yla-Herttuala S, Takahashi T and Alitalo K: Suppression of tumour
lymphangiogenesis and lymph node metastasis by blocking vascular
endothelial growth factor receptor 3 signaling. J Natl Cancer Inst.
94:819–825. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He Y, Rajantie I, Pajusola K, Jeltsch M,
Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T and
Alitalo K: Vascular endothelial cell growth factor receptor
3-mediated activation of lymphatic endothelium is crucial for
tumour cell entry and spread via lymphatic vessels. Cancer Res.
65:4739–4746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
White EA, Kenny HA and Lengyel E:
Three-dimensional modeling of ovarian cancer. Adv Drug Deliv Rev.
79:184–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng W, Aspelund A and Alitalo K:
Lymphangiogenic factors, mechanisms, and applications. J Clin
Invest. 124:878–887. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gomes FG, Nedel F, Alves AM, Nör JE and
Tarquinio SBC: Tumor angiogenesis and lymphangiogenesis:
tumor/endothelial crosstalk and cellular/microenvironmental
signaling mechanisms. Life Sci. 92:101–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mortensen K, Lichtenberg J, Thomsen PD and
Larsson L: Spontaneous fusion between cancer cells and endothelial
cells. Cell Mol Life Sci. 61:2125–2131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han R, Clark C, Black A, French A, Culshaw
G, Kempson S and Corcoran B: Morphological changes to endothelial
and interstitial cells and to the extra-cellular matrix in canine
myxomatous mitral valve disease (endocardiosis). Vet J.
197:388–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moss LAS, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: Changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koyama S: Enhanced cell surface expression
of matrix metalloproteinases and their inhibitors and
tumour-induced host response in progression of human gastric
carcinoma. Digest Dis Sci. 49:1621–1630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumour microenvironment.
Cell. 141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Langenskiöld M, Holmdahl L, Falk P and
Ivarsson M: Increased plasma MMP-2 protein expression in lymph
node-positive patients with colorectal cancer. Int J Colorectal
Dis. 20:245–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakamura ES, Koizumi K, Kobayashi M and
Saiki I: Inhibition of lymphangiogenesis-related properties of
murine lymphatic endothelial cells and lymph node metastasis of
lung cancer by the matrix metalloproteinase inhibitor MMI270.
Cancer Sci. 95:25–31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuecheng Y and Xiaoyan X: Stromal-cell
derived factor-1 regulates epithelial ovarian cancer cell invasion
by activating matrix metalloproteinase-9 and matrix
metalloproteinase-2. Eur J Cancer Prev. 16:430–435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang KJ and Sui LH: The relevance and
role of vascular endothelial growth factor C, matrix
metalloproteinase-2 and E-cadherin in epithelial ovarian cancer.
Med Oncol. 29:318–323. 2012. View Article : Google Scholar : PubMed/NCBI
|