Introduction
Asthma is a chronic disease characterized by airway
inflammation, airway hyperresponsiveness (AHR) and reversible
airway obstruction. Although the precise mechanism underlying this
response remains to be fully elucidated, it is now generally
believed that asthma arises as a result of dysregulated immune
responses in which T-helper (Th)2 cells have a central role in the
pathogenesis and pathology (1).
Chemokine (C-X-C motif) ligand 12 (CXCL12), also
known as stromal cell-derived factor-1, is a member of the
chemokine family, which consists of low molecular-weight proteins
(8–15 kD) produced by various types of cells involved in allergic
inflammation (2). CXCL12 binds to
chemokine (C-X-C motif) receptor 4 (CXCR4) and attracts a variety
of cells, including resting T lymphocytes, monocytes,
CD34+ stem cells and mature eosinophils (3). CXCL12 and its receptor CXCR4 have been
demonstrated to be involved in Th2-type allergic airway responses,
and inhibition of CXCL12 or CXCR4 leads to reduced airway
inflammation and AHR (4–6). Furthermore, another study demonstrated
that the levels of CXCL12 were significantly higher in
bronchoalveolar lavage fluids (BALF) of asthmatic patients compared
with healthy individuals, and the concentration of CXCL12 was
correlated with inflammatory cell numbers in the BALF (7). These results suggested that CXCL12 may
contribute to inflammatory cell recruitment in asthma.
Interleukin (IL)-17 is a pro-inflammatory cytokine
that is expressed in the airways of patients with asthma (8), and its expression is correlated with
the severity of asthma (9). The
concentration of IL-17 was significantly increased in the BALF,
sputum and blood of patients with asthma (8,10,11).
Furthermore, IL-17 or IL-17R-deficient mice exhibit reduced
allergic airway inflammation (10,12).
These results demonstrate the importance of IL-17 in the induction
of allergic airway inflammation.
IL-17-producing CD4+ T cells (Th17 cells)
are a distinct subset of T helper cells similar to Th1 and Th2
cells (13–16). It has been demonstrated that Th17
cells enhance Th2 cell-mediated eosinophilic airway inflammation in
mice (13). A corresponding subset
of IL-17-secreting CD8+ T cells [T-cytotoxic 17 (Tc17)
cells] similar to the Th17 cells also exists (17). Our previous study demonstrated an
increased proportion of Tc17 cells in the peripheral blood of
patients with asthma compared with healthy controls, as well as in
the spleen cells and lung tissue samples of asthmatic mice
(18). These data support both Th17
and Tc17 cells may have a role in the regulation of allergic airway
inflammation.
Although both experimental and clinical data support
that CXCL12/CXCR4 signaling and Th17/Tc17 cells are involved in the
pathogenesis of asthma, their association during the course of
asthmatic responses however remains unknown. It is proposed that
blockade of CXCR4 may suppress the asthmatic response associated
with the attenuation of Th17 and Tc17 cell infiltration in the
lung. Therefore, the anti-inflammatory effect of a CXCR4
antagonist, AMD3100, on Th2-type cytokines, inflammation cell
infiltration and AHR using an ovalbumin (OVA)-induced murine asthma
model, was investigated. In addition, the effects of AMD3100 on the
percentage of Th17 and Tc17 cells in the lung was investigated.
Materials and methods
Mice
A total of 18 female BALB/c mice (weight, 18–21 g;
age, 5–6 weeks) were obtained from the Laboratory Animal Center of
the Hubei Province (Wuhan, China). All mice were housed in a
specific pathogen-free facility in microisolator cages, an provided
with autoclaved food and acidified water under a 12 h light/dark
cycle. All experimental animal care and treatment protocols
followed the guidelines established by the Institutional Animal
Care and Use Committee of the Tongji Hospital (Wuhan, China). The
ethics committee of Tongii Hospital.
Generation of asthmatic model and
treatment
To generate an asthmatic model, mice were sensitized
and challenged with OVA (Sigma-Aldrich, St. Louis, MO, USA) as
previously reported (19). Briefly,
the mice were sensitized by intraperitoneal injection with 100 µg
OVA and 1 mg aluminium (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) in 200 µl saline on days 0, 7 and 14. In the OVA group,
the sensitized mice were then challenged by intranasal
administration of 1 mg OVA in 50 µl saline on days 21, 22 and 23.
In the control group, mice were challenged with 200 µl saline
alone. In the OVA + AMD3100 group, AMD3100 (Cayman chemical
company, Ann Arbor, MI, USA) was freshly dissolved in saline and
administered intraperitoneally at a dose of 10 mg/kg in 200 µl
saline 1 h prior to every challenge on days 21, 22 and 23.
Determination of AHR
AHR to inhaled methacholine (Sigma-Aldrich) was
measured using FlexiVent (SCIREQ Scientific Respiratory Equipment,
Inc., Montréal, QC, Canada) (20).
The mice were anesthetized by intraperitoneal injection of
pentobarbital (50 mg/kg) (Wuhan Sanjiang Space Guge Biotech Co.,
Ltd., Wuhan, China), tracheotomized, and connected to the
FlexiVent. Baseline airway resistance was measured in each mouse
following nebulization (SCIREQ Scientific Respiratory Equipment,
Inc.) of phosphate-buffered saline (PBS; vehicle for methacholine)
for 10 sec using an Aeroneb ultrasonic nebulizer (SCIREQ Scientific
Respiratory Equipment, Inc.). Following baseline measurements, the
mice were first exposed to nebulized saline, followed by increasing
doses (3, 6, 12 and 25 mg/ml) of nebulized methacholine for 3 min
each. Breathing indices were read for 3 min following each
nebulization, and the enhanced pause values were determined.
Collection of BALF and histological
analysis
The mice were sacrificed 24 h by cervical
dislocation following the last OVA or saline challenge. The lungs
were lavaged 3 times with 0.8 ml saline, and the collected cells
were centrifuged at 300 × g for 10 min at 4°C. The total number of
cells in BALF was counted by a hemacytometer. Eosinophils,
lymphocytes, neutrophils and macrophages were counted in BALF using
cytospins subjected to Wright-Giemsa staining (Wuhan Sanjiang Space
Guge Biotech Co., Ltd.) at room temperature. Lung tissue samples
were embedded in paraffin, cut into 5 mm sections using a microtome
(Leica RM2016; Leica Biosystems, Wetzlar, Germany) and stained with
hematoxylin and eosin (both purchased from Wuhan Sanjiang Space
Guge Biotech Co., Ltd.). Airway inflammation was assessed using a
light microscopy (BX53; Olympus Corporation, Tokyo, Japan).
Preparation of lung homogenate
The lung tissue suspensions were obtained as
previously described (21). Briefly,
the right lung was dissected prior to being rapidly frozen in
liquid nitrogen and stored at −80°C. Following thawing, the lung
tissue sample was homogenized in PBS and centrifuged at 800 × g for
15 min at 4°C to remove the sediments, and the supernatant was
subsequently used for measurement of IL-17 concentration.
Measurement of cytokines
The levels of IL-4, IL-5 and IL-13 in the BALF, as
well as those of IL-17 in the lung homogenates were determined by
IL-4 (cat. no. 88–7044), IL-5 (cat. no. 88–7054), IL-13 (cat. no.
88–7137) and IL-17 (cat. no. 88–7371) ELISA kits (eBioscience,
Inc., San Diego, CA, USA), according to the manufacturer's
protocol. Briefly, samples were added to 96-well microtiter plates
precoated with monoclonal antibody to mouse IL-4, IL-5 and IL-13 or
IL-17 and incubated for 2 h at room temperature. Subsequently,
96-well plates were washed, and Biotin-Conjugate and
Streptavidin-HRP were added. After washing 6 times with PBS and
0.25% Tween-20 (Wuhan Sanjiang Space Guge Biotech Co., Ltd.), 100
µl TMB Substrate Solution was added to each well. The plate was
incubated for ~10 min in the dark and then 100 µl of Stop Solution
was added into each well. Finally, absorbance was determined at 450
nm using a microplate ELISA reader (ELx800; Bio-Rad Laboratories,
CA, USA). IL-4, IL-5 and IL-13 or IL-17 concentrations were
calculated from a standard curve.
Flow cytometric analysis
The mononuclear cells in the lung tissue samples
were obtained as reported previously (22). The cells collected from the lung
tissue samples were stimulated with phorbol myristate acetate (50
ng/ml; Sigma-Aldrich) and ionomycin (1,000 ng/ml; Sigma-Aldrich)
for 5 h at 37°C in an atmosphere containing 5% CO2, and
bovine serum albumin (50 ng/ml; Sigma-Aldrich) was then added to
block the flow of cytokines from the cytoplasm. Following
stimulation, the cells were stained with phycoerythrin (PE)-cyanine
5-conjugated anti-mouse CD3 antibody (1:20; cat. no. 17A2;
Biolegend, Inc., San Diego, CA, USA) and fluorescein
isothiocyanate-conjugated anti-mouse CD8 antibody (1:50; cat. no.
53–6.7; Biolegend, Inc.) for 30 min at 4°C in the dark. Following
cell-surface staining, the cells were washed twice with washing
buffer (Biolegend, Inc.), fixed and permeabilised with Fix-Perm
solution (BD Biosciences, San Jose, CA, USA) for intracellular
staining with PE-conjugated anti-mouse IL-17 antibody (1:100; cat.
no. TC11-18H10.1; eBioscience) for 30 min at 4°C in the dark. The
stained cells were used for flow cytometric analysis (BD LSR II; BD
Biosciences) in order to determine the number of Th17 and Tc17
lymphocytes. CD8+ T lymphocytes were specifically gated,
the CD3+CD8−IL17+ T lymphocytes
were counted as Th17 cells, and the
CD3+CD8+IL-17+ T lymphocytes were
counted as Tc17 cells.
Statistical analysis
All data were presented as means ± standard error of
the mean. Data analysis was performed using GraphPad Prism version
5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical
differences were assessed by one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
result.
Results
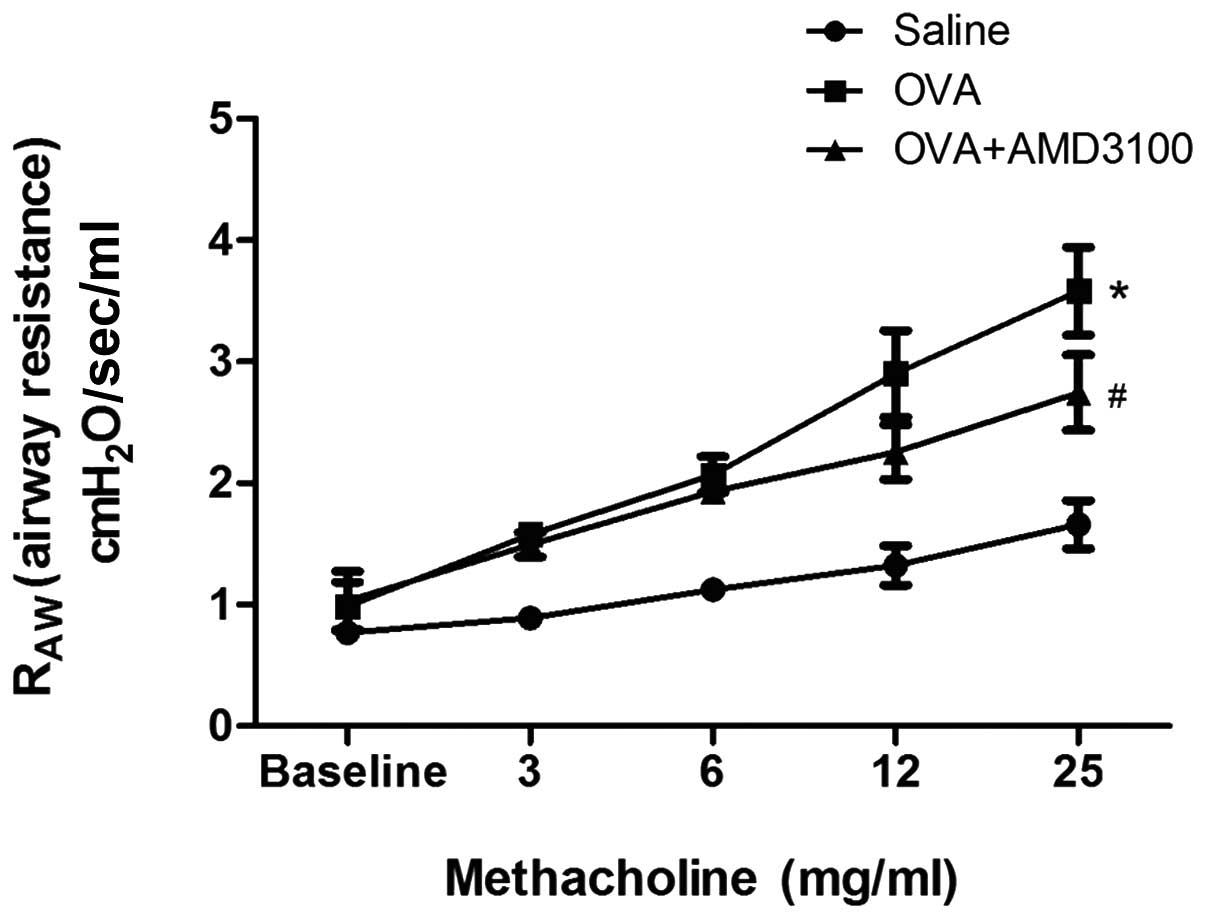
Treatment with AMD3100 reduced AHR in
experimental asthma
To determine the effects of AMD3100 on AHR, airway
resistance was measured 24 h following the last challenge with OVA.
In OVA-challenged mice, airway resistance was increased in a
dose-dependent manner following exposure to methacholine. However,
AMD3100 treatment inhibited the increased airway reactivity to
methacholine induced by OVA (Fig.
1).
Administration of AMD3100 decreases
airway inflammation
ADM3100 administration significantly reduced total
cell counts and eosinophil counts in the BALF following OVA
sensitization and challenge (Fig.
2A). These changes can also be observed by histological
examination of the lung tissue samples from the treated animals;
after treatment with ADM3100, inflammatory cell infiltration was
inhibited around the airway (Fig.
2B).
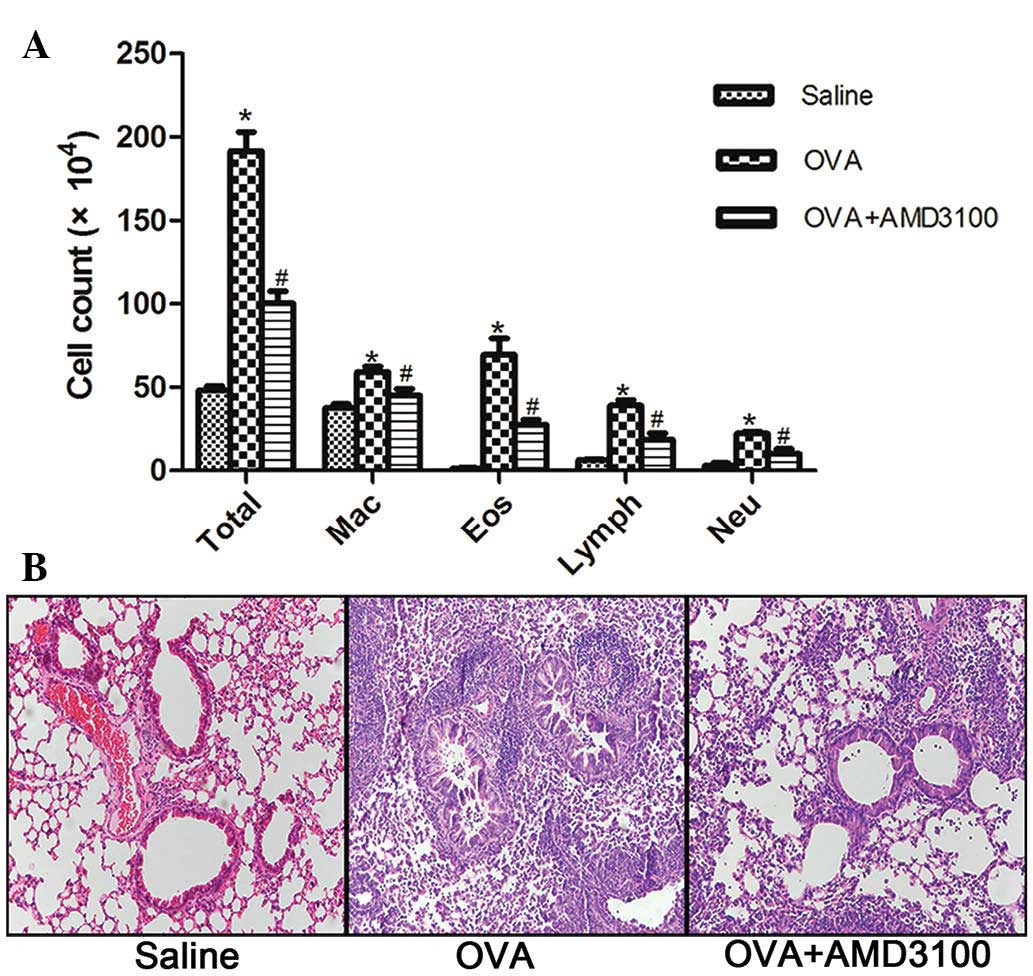 | Figure 2.ADM3100 administration attenuates
airway inflammation following OVA challenge. BALF and lung tissue
samples were collected 24 h following the last challenge with OVA.
(A) Differential cell counts for macrophages, eosinophils,
lymphocytes and neutrophils were calculated from cytospin
preparations. (B) Lung histopathology was assessed with hematoxylin
and eosin (original magnification, ×200). *P<0.05, vs. the
saline group and #P<0.05, vs. the OVA group. All data
are presented as means ± standard error of the mean for n=5–6
mice/group. OVA, ovalbumin; Mac, macrophages; Eos, eosinophils;
Lymph, lymphocytes; Neu, neutrophils. |
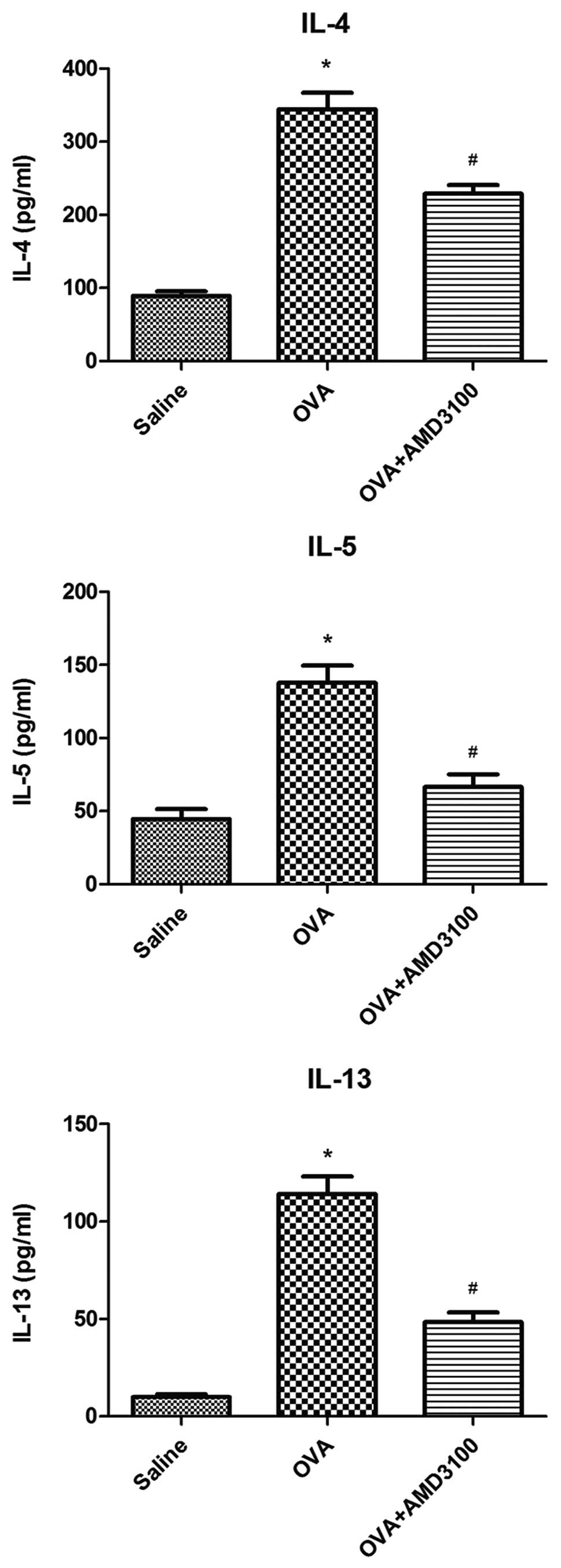
Administration of AMD3100 reduces the
production of Th2 cytokines
As shown in Fig. 3,
the levels of IL-4, IL-5 and IL-13 were significantly increased in
OVA-challenged mice, compared with the controls. Administration of
AMD3100 significantly reduce these increased levels of IL-4, IL-5
and IL-13 in the BALF of OVA-challenged mice.
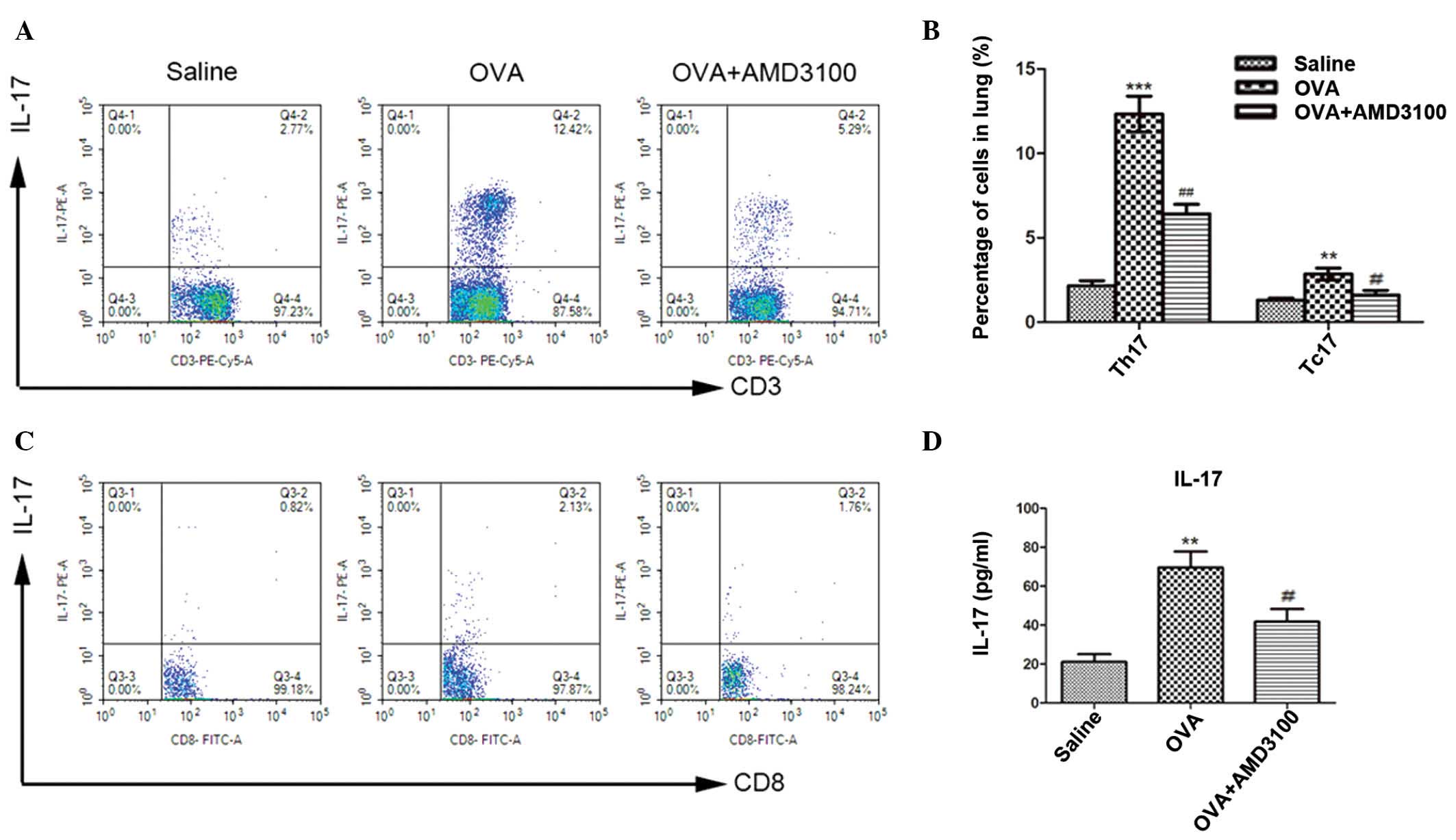
AMD3100 suppresses the development of
Th17 and Tc17 cells in the lung and IL-17 production in lung
homogenates
To investigate the effect of administration of
AMD3100 on the development of Th17 and Tc17 cells,
CD3+CD8− and CD3+CD8+ T
cells were isolated from the lung and gated for expression of IL-17
to compare saline, OVA, and OVA + AMD3100 treatment groups. OVA
sensitization and challenge markedly increased Th17 and Tc17 cells
in the lung compared with the saline-sensitized and challenged
mice. However, this marked increase in Th17 and Tc17 cells was
attenuated by AMD3100 administration, which resulted in a
significant decrease in the percentage of Th17 and Tc17 cells
compared with OVA-challenged mice (Fig.
4A–C). Furthermore, as shown in Fig.
4D, the levels of IL-17 in the lung homogenate were
significantly increased in OVA-challenged mice compared with
negative controls. Administration of AMD3100 significantly
decreased the levels of IL-17 in the lung homogenate of
OVA-challenged mice (P<0.01).
Discussion
Previous reports have demonstrated that inhibition
of CXCL12/CXCR4 signaling attenuated allergic lung inflammation and
AHR (5,6,23).
However, the exact underlying mechanisms have yet to be fully
understood. The present study investigated whether inhibition of
CXCL12/CXCR4 signaling was able to attenuate OVA-induced airway
inflammation and AHR by decreasing Th17 and Tc17 pro-inflammatory
response. Using a murine model of asthma, the results demonstrated
that administration of AMD3100 attenuated allergic airway
inflammation along and significantly suppressed Th17 and Tc17 cell
infiltration into the lung tissues, as well as decreased IL-17
levels in the lung. These results provide an improved understanding
of the mechanisms underlying CXCL12/CXCR4 signaling in the
pathogenesis of asthma.
AMD3100 is a soluble CXCR4 inhibitor, which inhibits
binding of CXCL12 to CXCR4 and subsequent signal transduction
(24). AMD3100 was shown to
attenuate allergic pulmonary inflammation and AHR (5). In the present study, the results
demonstrated that treatment with AMD3100 prior to allergen
challenge significantly decreased airway inflammatory cell
accumulation and AHR. The results of the present study were
concordant with those of a previous study that demonstrated that
specific inhibition of CXCR4 with AMD3100 reduced the number of
pathological parameters associated with asthmatic-type inflammation
(5). Based on both the results of
the present and previous study (5),
AMD3100 may effectively inhibit experimental allergic asthma in
mice, however, the possible mechanisms underlying this process have
yet to be fully understood.
Th17 cell is a type of CD4+ T cell
subset, and has been defined by its secreted product, IL-17
(25). In addition to Th17 cells,
there are CD8+ T cells named Tc17 cells (26), which also produce IL-17. Th17 cells
and Tc17 cells share many similar characteristics, including
production of IL-17 (27).
Accumulating data suggests that Th17 or Tc17 cells may have an
important role in the development of various allergic diseases that
have classically been considered to be Th1- or Th2-mediated
disorders (28–30). Our recent study also demonstrated an
increased proportion of Th17 and Tc17 cells in the peripheral blood
of patients with asthma compared with healthy controls, as well as
in the spleen cells and lung tissues of asthmatic mice, which
suggested that a functional disequilibrium between Th17 and Tc17
cell subsets may contribute to the allergic inflammatory process in
asthma (18). Both Th17 and Tc17
cells likely contribute to the immune response in asthma since both
have the ability to produce IL-17.
To determined whether the suppression of allergic
airway responses induced by AMD3100 is associated with the presence
of Th17 and Tc17 cells, the present study investigated the
expression levels of Th17 and Tc17 cells in a murine allergic
asthma model. The results demonstrated that treatment with AMD3100
attenuated allergic airway inflammation and significantly
suppressed Th17 and Tc17 cell recruitment in mice. To the best of
our knowledge, this is the first study to report an association
between CXCL12 signaling and Th17/Tc17 cells. This investigation
provides novel insight into the mechanisms underlying the
involvement of the CXCL12/CXCR4 signaling pathway in asthmatic
responses during the course of disease development.
In conclusion, the data of the present investigation
suggested that inhibition of CXCL12/CXCR4 signaling could suppress
the in vivo development of Th17 and Tc17 cells. These
findings also provide further support for an anti-inflammatory role
of AMD3100 as a CXCR4 inhibitor in the treatment of asthma.
However, further studies are required in order to explore the
precise mechanisms underlying these processes.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81170021 and
30900647).
References
|
1
|
Wills-Karp M, Nathan A, Page K and Karp
CL: New insights into innate immune mechanisms underlying
allergenicity. Mucosal Immunol. 3:104–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shirozu M, Nakano T, Inazawa J, Tashiro K,
Tada H, Shinohara T and Honjo T: Structure and chromosomal
localization of the human stromal cell-derived factor 1 (SDF1)
gene. Genomics. 28:495–500. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McQuibban GA, Butler GS, Gong JH, Bendall
L, Power C, Clark-Lewis I and Overall CM: Matrix metalloproteinase
activity inactivates the CXC chemokine stromal cell-derived
factor-1. J Biol Chem. 276:43503–43508. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daubeuf F, Hachet-Haas M, Gizzi P,
Gasparik V, Bonnet D, Utard V, Hibert M, Frossard N and Galzi JL:
An antedrug of the CXCL12 neutraligand blocks experimental allergic
asthma without systemic effect in mice. J Biol Chem.
288:11865–11876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lukacs NW, Berlin A, Schols D, Skerlj RT
and Bridger GJ: AMD3100, a CxCR4 antagonist, attenuates allergic
lung inflammation and airway hyperreactivity. Am J Pathol.
160:1353–1360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gonzalo JA, Lloyd CM, Peled A, Delaney T,
Coyle AJ and Gutierrez-Ramos JC: Critical involvement of the
chemotactic axis CXCR4/stromal cell-derived factor-1 alpha in the
inflammatory component of allergic airway disease. J Immunol.
165:499–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Negrete-Garcia MC, Velazquez JR,
Popoca-Coyotl A, Montes-Vizuet AR, Juárez-Carvajal E and Teran LM:
Chemokine (C-X-C motif) ligand 12/stromal cell-derived factor-1 is
associated with leukocyte recruitment in asthma. Chest.
138:100–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molet S, Hamid Q, Davoine F, Nutku E, Taha
R, Pagé N, Olivenstein R, Elias J and Chakir J: IL-17 is increased
in asthmatic airways and induces human bronchial fibroblasts to
produce cytokines. J Allergy Clin Immunol. 108:430–438. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chakir J, Shannon J, Molet S, Fukakusa M,
Elias J, Laviolette M, Boulet LP and Hamid Q: Airway
remodeling-associated mediators in moderate to severe asthma:
Effect of steroids on TGF-beta, IL-11, IL-17 and type I and type
III collagen expression. J Allergy Clin Immunol. 111:1293–1298.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakae S, Komiyama Y, Nambu A, Sudo K,
Iwase M, Homma I, Sekikawa K, Asano M and Iwakura Y:
Antigen-specific T cell sensitization is impaired in
IL-17-deficient mice, causing suppression of allergic cellular and
humoral responses. Immunity. 17:375–387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barczyk A, Pierzchala W and Sozanska E:
Interleukin-17 in sputum correlates with airway hyperresponsiveness
to methacholine. Respir Med. 97:726–733. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schnyder-Candrian S, Togbe D, Couillin I,
Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B and
Schnyder B: Interleukin-17 is a negative regulator of established
allergic asthma. J Exp Med. 203:2715–2725. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wakashin H, Hirose K, Maezawa Y, Kagami S,
Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, et
al: IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic
airway inflammation in mice. Am J Respir Crit Care Med.
178:1023–1032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weaver CT, Harrington LE, Mangan PR,
Gavrieli M and Murphy KM: Th17: An effector CD4 T cell lineage with
regulatory T cell ties. Immunity. 24:677–688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Langrish CL, Chen Y, Blumenschein WM,
Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA and
Cua DJ: IL-23 drives a pathogenic T cell population that induces
autoimmune inflammation. J Exp Med. 201:233–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aggarwal S, Ghilardi N, Xie MH, de Sauvage
FJ and Gurney AL: Interleukin-23 promotes a distinct CD4 T cell
activation state characterized by the production of interleukin-17.
J Biol Chem. 278:1910–1914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huber M, Heink S, Grothe H, Guralnik A,
Reinhard K, Elflein K, Hünig T, Mittrücker HW, Brüstle A, Kamradt T
and Lohoff M: A Th17-like developmental process leads to CD8(+)
Tc17 cells with reduced cytotoxic activity. Eur J Immunol.
39:1716–1725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li K, Wang Z, Cao Y, Bunjhoo H, Zhu J,
Chen Y, Xiong S, Xu Y and Xiong W: The study of the ratio and
distribution of Th17 cells and Tc17 cells in asthmatic patients and
the mouse model. Asian Pac J Allergy Immunol. 31:125–131. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong S, Li J, Ma L, Li K, Zhang L, Wang G,
Liu Y, Ji X, Liu X, Chen P, et al: Blockade of dopamine D1-like
receptor signalling protects mice against OVA-induced acute asthma
by inhibiting B-cell activating transcription factor signalling and
Th17 function. FEBS J. 280:6262–6273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kramer EL, Mushaben EM, Pastura PA,
Acciani TH, Deutsch GH, Khurana Hershey GK, Korfhagen TR, Hardie
WD, Whitsett JA and Le Cras TD: Early growth response-1 suppresses
epidermal growth factor receptor-mediated airway
hyperresponsiveness and lung remodeling in mice. Am J Respir Cell
Mol Biol. 41:415–425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
You QH, Zhang D, Niu CC, Zhu ZM, Wang N,
Yue Y and Sun GY: Expression of IL-17A and IL-17F in
lipopolysaccharide-induced acute lung injury and the counteraction
of anisodamine or methylprednisolone. Cytokine. 66:78–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sauer KA, Scholtes P, Karwot R and Finotto
S: Isolation of CD4+ T cells from murine lungs: A method to analyze
ongoing immune responses in the lung. Nat Protoc. 1:2870–2875.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Daubeuf F, Hachet-Haas M, Gizzi P,
Gasparik V, Bonnet D, Utard V, Hibert M, Frossard N and Galzi JL:
An antedrug of the CXCL12 neutraligand blocks experimental allergic
asthma without systemic effect in mice. J Biol Chem.
288:11865–11876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heesen M, Berman MA, Höpken UE, Gerard NP
and Dorf ME: Alternate splicing of mouse fusin/CXC chemokine
receptor-4: Stromal cell-derived factor-1alpha is a ligand for both
CXC chemokine receptor-4 isoforms. J Immunol. 158:3561–3564.
1997.PubMed/NCBI
|
|
25
|
Weaver CT: Th17: The ascent of a new
effector T-cell subset. Preface. Eur J Immunol. 39:634–636. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yen HR, Harris TJ, Wada S, Grosso JF,
Getnet D, Goldberg MV, Liang KL, Bruno TC, Pyle KJ, Chan SL, et al:
Tc17 CD8 T cells: Functional plasticity and subset diversity. J
Immunol. 183:7161–7168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamashita N and Clement LT: Phenotypic
characterization of the post-thymic differentiation of human
alloantigen-specific CD8+ cytotoxic T lymphocytes. J Immunol.
143:1518–1523. 1989.PubMed/NCBI
|
|
28
|
Zhao Y, Yang J and Gao YD: Altered
expressions of helper T cell (Th)1, Th2, and Th17 cytokines in
CD8(+) and γδ T cells in patients with allergic asthma. J Asthma.
48:429–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oboki K, Ohno T, Saito H and Nakae S: Th17
and allergy. Allergol Int. 57:121–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Y, Balato A, Fishelevich R, Chapoval
A, Mann DL and Gaspari AA: Th17/Tc17 infiltration and associated
cytokine gene expression in elicitation phase of allergic contact
dermatitis. Br J Dermatol. 161:1301–1306. 2009. View Article : Google Scholar : PubMed/NCBI
|